-
PDF
- Split View
-
Views
-
Cite
Cite
Priya Bhagwat, Shashi N Kapadia, Heather J Ribaudo, Roy M Gulick, Judith S Currier, Racial Disparities in Virologic Failure and Tolerability During Firstline HIV Antiretroviral Therapy, Open Forum Infectious Diseases, Volume 6, Issue 2, February 2019, ofz022, https://doi.org/10.1093/ofid/ofz022
Close - Share Icon Share
Abstract
Racial/ethnic disparities in HIV outcomes have persisted despite effective antiretroviral therapy. In a study of initial regimens, we found viral suppression varied by race/ethnicity. In this exploratory analysis, we use clinical and socioeconomic data to assess factors associated with virologic failure and adverse events within racial/ethnic groups.
Data were from AIDS Clinical Trial Group A5257, a randomized trial of initial regimens with either atazanavir/ritonavir, darunavir/ritonavir, or raltegravir (each combined with tenofovir DF and emtricitabine). We grouped participants by race/ethnicity and then used Cox-proportional hazards regression to examine the impact of demographic, clinical, and socioeconomic factors on the time to virologic suppression and time to adverse event reporting within each racial/ethnic group.
We analyzed data from 1762 participants: 757 self-reported as non-Hispanic black (NHB), 615 as non-Hispanic white (NHW), and 390 as Hispanic. The proportion with virologic failure was higher for NHB (22%) and Hispanic (17%) participants compared with NHWs (9%). Factors associated with virologic failure were poor adherence and higher baseline HIV RNA level. Prior clinical AIDS diagnosis was associated with virologic failure for NHBs only, and unstable housing and illicit drug use for NHWs only. Factors associated with adverse events were female sex in all groups and concurrent use of medications for comorbidities in NHB and Hispanic participants only.
Clinical and socioeconomic factors that are associated with virologic failure and tolerability of antiretroviral therapy vary between and within racial and ethnic groups. Further research may shed light into mechanisms leading to disparities and targeted strategies to eliminate those disparities.
Racial and ethnic disparities in treatment outcomes for HIV have persisted over the course of the epidemic. These outcomes include not only all-cause and HIV-specific mortality, but also measures of treatment such as access to antiretroviral (ARV) therapy, adherence, and viral suppression rates. Observational studies and epidemiologic analyses have shown an increased risk of death from HIV for non-Hispanic blacks (NHBs) compared with non-Hispanic whites (NHWs) in the US population. As of 2016, the age-adjusted HIV mortality rate among the general population was 7.5 per 100 000 for NHBs, compared with 1.7 for Hispanics and 0.8 for NHWs [1]. For HIV-infected individuals, the racial disparity in both mortality and viral suppression had paradoxically widened after the introduction of highly active antiretroviral therapy in 1996, due to differential access to antiretroviral (ARV) therapy [2, 3]. Although efforts to provide comprehensive treatment access, such as the Ryan White program, have helped to alleviate disparities in access to treatment, differences in viral suppression rates across racial groups remain, even within clinical trials [4, 5].
There are multiple mechanisms, apart from access to care, that may give rise to these disparities. Socioeconomic status (SES) has been shown to play a clear role. An analysis using national mortality data concluded that low-SES counties in the HAART era had 2.7 times the incidence of HIV-related mortality than high-SES counties [6], and individual socioeconomic factors have been shown to contribute to both all-cause and HIV/AIDS-related mortality in multiple studies [7]. Related factors, such as unstable housing, less education, nonemployment, and incarceration have also been associated with poor rates of viral suppression [5, 8, 9]. Additionally, the impact of medical and behavioral comorbidities may impact adherence and thereby mediate outcomes, even after ARV prescription. Studies have also suggested that the report of side effects may vary among racial/ethnic groups, though this association may be dependent on the regimen [10, 11]. The relative impact of these mechanisms within racial/ethnic groups is not known, and the potential disparity in ARV toxicity rates has not been well explored.
AIDS Clinical Trial Group (ACTG) A5257 was a multicenter, open-label randomized clinical trial conducted from 2009 to 2011 at 57 sites in the United States and Puerto Rico, which compared 3 ARV regimens, atazanavir/ritonavir, darunavir/ritonavir, and raltegravir, combined with a dual nucleoside backbone in treatment-naïve HIV-1-infected adults followed for 96 weeks after the enrollment of the last participant (maximum follow-up: 213 weeks). The cumulative incidence of virologic failure over 96 weeks was 12.6% for the atazanavir/ritonavir group, 14.9% for the darunavir/ritonavir group, and 9% for the raltegravir group, with confidence intervals that met the criteria for equivalence [12]. There was a significant increase in the incidence of virologic failure across the treatment regimens both for NHBs (hazard ratio [HR], 2.8; 95% confidence interval [CI], 2.0–3.8) and Hispanics (HR, 2.0; 95% CI, 1.4–2.8), compared with NHWs, but there was no evidence of differential treatment effects across these subgroups (Pinteraction > .15) [12, 13]. The cumulative incidence of toxicity-associated discontinuation was significantly higher for atazanavir/ritonavir (12.7%), compared with darunavir/ritonavir (0.9%) and raltegravir (0.9%), though this effect was not significantly different by race/ethnicity group.
In a secondary analysis, adjusting for sociodemographic risk factors did not fully account for the increase in virologic failure disparity for NHB participants [13]. This was true despite a clinical trial setting, with access to antiretroviral therapy (ART) and high-intensity monitoring. Although these analyses evaluated differences between racial/ethnic groups, in the current analysis, we use the data collected during A5257 to determine the factors associated with virologic failure and with ARV toxicity within racial and ethnic groups. We hypothesized that because socioeconomic and clinical factors may contribute to outcomes in fundamentally different ways, an analysis by racial subgroups would provide additional insight into the primary analyses. We were also interested in exploring whether the prevalence of medically comorbid conditions at baseline was associated with virologic failure.
METHODS
Design
This is an exploratory analysis of data from ACTG A5257, described above. The details of ACTG A5257 have been published previously [12].
Population
The population for this analysis consisted of 1762 HIV-1 infected adults aged >18 years who self-identified as non-Hispanic black, non-Hispanic white, or Hispanic at A5257 enrollment. Participants identifying as Asian or Pacific Islander, American Indian or Alaskan Native, and >1 race were not included in the current analysis, as the low number of participants in these groups precluded meaningful comparisons. Of note, to be eligible for A5257, participants could not have received more than 10 days of ARV treatment before enrollment and could not have genotypic resistance to nucleoside reverse transcriptase inhibitors or protease inhibitors. The main A5257 study was reviewed and approved by individual institutional review boards at the study sites; all participants provided written informed consent. The analysis for this study was approved by the institutional review board at the University of California, Los Angeles.
Outcomes
The primary outcome was time to virologic failure, defined as in A5257. Specifically, the time from randomization to the first of 2 consecutive HIV-1 RNA levels >1000 copies/mL if drawn between weeks 16 and 24, or >200 copies/mL if drawn after week 24. Participants who had a single HIV-1 RNA level above these thresholds and discontinued the study before a second confirmatory test was done were considered to have virologic failure. Participants in weeks 4 or 8 who had a single HIV-1 RNA >50 copies/mL with a decrease from baseline of <0.5 or 1.0 log10 copies/mL, respectively, who then discontinued the study before a second HIV-1 RNA, were also considered to have virologic failure.
A secondary adverse event outcome was defined as the time from randomization to the first grade ≥2 adverse event. Adverse events were graded by site investigators using the 2004 Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events [14]. Grade 3 or higher adverse events were recorded in the database throughout the duration of A5257; grade 2 adverse events were only recorded if they occurred during the first 48 weeks of follow-up.
Analyses
Analyses were conducted separately by race/ethnicity to identify within-group factors contributing to outcome differences. Predictors of interest included demographic factors, including continuous age and sex at birth, and HIV-related clinical factors, including randomized treatment regimen, CD4 count, HIV RNA level, AIDS diagnosis at enrollment, and self-reported adherence measured by visual analog scale. Adherence was dichotomized as >90% on the scale or <90%. We also analyzed associations with medical comorbidities, including diabetes, hyperlipidemia, hypertension, mental health conditions, obesity, substance use, and viral hepatitis. Alcohol use was defined categorically based on US Department of Agriculture/Department of Health and Human Services 2010 guidelines [15], and other substance use as any lifetime use. Baseline non-ARV prescription medication use was included. Socioeconomic factors assessed included self-reported education, employment status, housing status, and income. We conducted analysis and modeling within racial/ethnic groups.
After conducting descriptive analyses, the cumulative probability of both outcomes for each racial group overall, and by selected predictor variables, was estimated using the Kaplan-Meier method. Cox proportional hazards modeling was used to estimate hazard ratios of virologic failure within each race/ethnicity group, controlling for treatment group, demographic variables, and clinical and socioeconomic factors at baseline. Differences in risk factor associations across subgroups were assessed based on the magnitude of effects sizes and their associated confidence intervals; formal interaction tests were not performed. This decision was made due to the lack of power in this case, as well as to reduce modeling complexity and assumptions given the number of risk factors under study. Adherence was included as a time-varying covariate. We report multivariate associations in the manuscript. Given the large number of risk factors examined within each racial subgroup, evidence for collinearity was evaluated by examining for excessive shifts in the adjusted estimates compared with their unadjusted counterparts. All analyses were performed using SAS Software, version 9.4, of the SAS System for Windows (SAS Institute Inc., Cary, NC).
RESULTS
Population Characteristics
We analyzed 1762 total participants in this analysis: 615 non-Hispanic white, 757 non-Hispanic black, and 390 Hispanic. Baseline characteristics with respect to clinical and sociodemographic variables, stratified by race/ethnicity, are shown in Table 1. The proportions of participants who experienced virologic failure and who experienced adverse events are shown in Table 2.
Baseline Characteristics of ACTG A5257 Participants Stratified by Race/Ethnicity
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Female sex, No. (%) | 264 (35) | 67 (11) | 99 (25) |
| Median age, y | 36 | 39 | 36 |
| Treatment, No. (%) | |||
| ATV/r | 252 (33) | 212 (35) | 125 (32) |
| RAL | 254 (34) | 212 (35) | 117 (30) |
| DRV/r | 251 (33) | 191 (31) | 148 (38) |
| Median log10 HIV RNA level, log10 copies/mL | 4.53 | 4.67 | 4.75 |
| Mean baseline CD4 cell count, cells/mm3 | 298 | 344 | 270 |
| AIDS diagnosis, No. (%) | 23 (3) | 18 (3) | 18 (5) |
| BMI, No. (%) | |||
| Underweight | 19 (3) | 12 (2) | 7 (2) |
| Normal | 364 (48) | 291 (47) | 193 (50) |
| Overweight | 202 (27) | 203 (33) | 130 (33) |
| Obese | 172 (23) | 109 (18) | 60 (15) |
| Baseline medication use, No. (%) | 257 (34) | 218 (36) | 101 (25) |
| Antihypertensive | 162 (39) | 71 (20) | 37 (23) |
| Hypoglycemic | 31 (7) | 11 (3) | 20 (12) |
| Lipid-lowering | 26 (6) | 56 (16) | 22 (13) |
| Psychotherapeutic | 123 (29) | 146 (41) | 60 (37) |
| Hepatitis B positive, No. (%) | 27 (4) | 12 (2) | 9 (2) |
| Hepatitis C positive, No. (%) | 74 (10) | 41 (7) | 24 (6) |
| Annual income, No. (%) | |||
| Less than $5000 | 195 (31) | 71 (13) | 109 (34) |
| $5000–$19 999 | 207 (33) | 128 (23) | 100 (32) |
| $20 000–$49 999 | 168 (27) | 192 (34) | 84 (27) |
| More than $50 000 | 52 (8) | 177 (31) | 24 (8) |
| Working/in school, No. (%) | 406 (55) | 448 (74) | 237 (62) |
| Lives in owned or rented home, No. (%) | 638 (87) | 556 (9) | 322 (84) |
| Education, No. (%) | |||
| 12th grade or less | 184 (25) | 46 (8) | 154 (40) |
| High school graduate/GED | 192 (25) | 81 (13) | 57 (15) |
| Some college/college graduate | 374 (50) | 486 (79) | 176 (46) |
| Smoking, No. (%) | 450 (60) | 358 (58) | 209 (54) |
| History of illicit drug use, No. (%) | 258 (37) | 314 (56) | 135 (39) |
| Alcohol use, No. (%) | |||
| Abstainer | 231 (33) | 129 (23) | 159 (46) |
| Moderate/heavy drinker | 298 (43) | 275 (50) | 95 (28) |
| Binge drinker | 166 (24) | 154 (28) | 92 (27) |
| Insurance status, No. (%) | |||
| US government funding | 486 (67) | 284 (47) | 278 (75) |
| Private insurance | 182 (25) | 284 (47) | 64 (17) |
| Self-pay, out of pocket | 56 (8) | 41 (7) | 29 (8) |
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Female sex, No. (%) | 264 (35) | 67 (11) | 99 (25) |
| Median age, y | 36 | 39 | 36 |
| Treatment, No. (%) | |||
| ATV/r | 252 (33) | 212 (35) | 125 (32) |
| RAL | 254 (34) | 212 (35) | 117 (30) |
| DRV/r | 251 (33) | 191 (31) | 148 (38) |
| Median log10 HIV RNA level, log10 copies/mL | 4.53 | 4.67 | 4.75 |
| Mean baseline CD4 cell count, cells/mm3 | 298 | 344 | 270 |
| AIDS diagnosis, No. (%) | 23 (3) | 18 (3) | 18 (5) |
| BMI, No. (%) | |||
| Underweight | 19 (3) | 12 (2) | 7 (2) |
| Normal | 364 (48) | 291 (47) | 193 (50) |
| Overweight | 202 (27) | 203 (33) | 130 (33) |
| Obese | 172 (23) | 109 (18) | 60 (15) |
| Baseline medication use, No. (%) | 257 (34) | 218 (36) | 101 (25) |
| Antihypertensive | 162 (39) | 71 (20) | 37 (23) |
| Hypoglycemic | 31 (7) | 11 (3) | 20 (12) |
| Lipid-lowering | 26 (6) | 56 (16) | 22 (13) |
| Psychotherapeutic | 123 (29) | 146 (41) | 60 (37) |
| Hepatitis B positive, No. (%) | 27 (4) | 12 (2) | 9 (2) |
| Hepatitis C positive, No. (%) | 74 (10) | 41 (7) | 24 (6) |
| Annual income, No. (%) | |||
| Less than $5000 | 195 (31) | 71 (13) | 109 (34) |
| $5000–$19 999 | 207 (33) | 128 (23) | 100 (32) |
| $20 000–$49 999 | 168 (27) | 192 (34) | 84 (27) |
| More than $50 000 | 52 (8) | 177 (31) | 24 (8) |
| Working/in school, No. (%) | 406 (55) | 448 (74) | 237 (62) |
| Lives in owned or rented home, No. (%) | 638 (87) | 556 (9) | 322 (84) |
| Education, No. (%) | |||
| 12th grade or less | 184 (25) | 46 (8) | 154 (40) |
| High school graduate/GED | 192 (25) | 81 (13) | 57 (15) |
| Some college/college graduate | 374 (50) | 486 (79) | 176 (46) |
| Smoking, No. (%) | 450 (60) | 358 (58) | 209 (54) |
| History of illicit drug use, No. (%) | 258 (37) | 314 (56) | 135 (39) |
| Alcohol use, No. (%) | |||
| Abstainer | 231 (33) | 129 (23) | 159 (46) |
| Moderate/heavy drinker | 298 (43) | 275 (50) | 95 (28) |
| Binge drinker | 166 (24) | 154 (28) | 92 (27) |
| Insurance status, No. (%) | |||
| US government funding | 486 (67) | 284 (47) | 278 (75) |
| Private insurance | 182 (25) | 284 (47) | 64 (17) |
| Self-pay, out of pocket | 56 (8) | 41 (7) | 29 (8) |
Abbreviations: ATV/r, atazanavir/ritonavir; BMI, body mass index; DRV/r, darunavir/ritonavir; GED, general educational development; RAL, raltegravir.
Baseline Characteristics of ACTG A5257 Participants Stratified by Race/Ethnicity
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Female sex, No. (%) | 264 (35) | 67 (11) | 99 (25) |
| Median age, y | 36 | 39 | 36 |
| Treatment, No. (%) | |||
| ATV/r | 252 (33) | 212 (35) | 125 (32) |
| RAL | 254 (34) | 212 (35) | 117 (30) |
| DRV/r | 251 (33) | 191 (31) | 148 (38) |
| Median log10 HIV RNA level, log10 copies/mL | 4.53 | 4.67 | 4.75 |
| Mean baseline CD4 cell count, cells/mm3 | 298 | 344 | 270 |
| AIDS diagnosis, No. (%) | 23 (3) | 18 (3) | 18 (5) |
| BMI, No. (%) | |||
| Underweight | 19 (3) | 12 (2) | 7 (2) |
| Normal | 364 (48) | 291 (47) | 193 (50) |
| Overweight | 202 (27) | 203 (33) | 130 (33) |
| Obese | 172 (23) | 109 (18) | 60 (15) |
| Baseline medication use, No. (%) | 257 (34) | 218 (36) | 101 (25) |
| Antihypertensive | 162 (39) | 71 (20) | 37 (23) |
| Hypoglycemic | 31 (7) | 11 (3) | 20 (12) |
| Lipid-lowering | 26 (6) | 56 (16) | 22 (13) |
| Psychotherapeutic | 123 (29) | 146 (41) | 60 (37) |
| Hepatitis B positive, No. (%) | 27 (4) | 12 (2) | 9 (2) |
| Hepatitis C positive, No. (%) | 74 (10) | 41 (7) | 24 (6) |
| Annual income, No. (%) | |||
| Less than $5000 | 195 (31) | 71 (13) | 109 (34) |
| $5000–$19 999 | 207 (33) | 128 (23) | 100 (32) |
| $20 000–$49 999 | 168 (27) | 192 (34) | 84 (27) |
| More than $50 000 | 52 (8) | 177 (31) | 24 (8) |
| Working/in school, No. (%) | 406 (55) | 448 (74) | 237 (62) |
| Lives in owned or rented home, No. (%) | 638 (87) | 556 (9) | 322 (84) |
| Education, No. (%) | |||
| 12th grade or less | 184 (25) | 46 (8) | 154 (40) |
| High school graduate/GED | 192 (25) | 81 (13) | 57 (15) |
| Some college/college graduate | 374 (50) | 486 (79) | 176 (46) |
| Smoking, No. (%) | 450 (60) | 358 (58) | 209 (54) |
| History of illicit drug use, No. (%) | 258 (37) | 314 (56) | 135 (39) |
| Alcohol use, No. (%) | |||
| Abstainer | 231 (33) | 129 (23) | 159 (46) |
| Moderate/heavy drinker | 298 (43) | 275 (50) | 95 (28) |
| Binge drinker | 166 (24) | 154 (28) | 92 (27) |
| Insurance status, No. (%) | |||
| US government funding | 486 (67) | 284 (47) | 278 (75) |
| Private insurance | 182 (25) | 284 (47) | 64 (17) |
| Self-pay, out of pocket | 56 (8) | 41 (7) | 29 (8) |
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Female sex, No. (%) | 264 (35) | 67 (11) | 99 (25) |
| Median age, y | 36 | 39 | 36 |
| Treatment, No. (%) | |||
| ATV/r | 252 (33) | 212 (35) | 125 (32) |
| RAL | 254 (34) | 212 (35) | 117 (30) |
| DRV/r | 251 (33) | 191 (31) | 148 (38) |
| Median log10 HIV RNA level, log10 copies/mL | 4.53 | 4.67 | 4.75 |
| Mean baseline CD4 cell count, cells/mm3 | 298 | 344 | 270 |
| AIDS diagnosis, No. (%) | 23 (3) | 18 (3) | 18 (5) |
| BMI, No. (%) | |||
| Underweight | 19 (3) | 12 (2) | 7 (2) |
| Normal | 364 (48) | 291 (47) | 193 (50) |
| Overweight | 202 (27) | 203 (33) | 130 (33) |
| Obese | 172 (23) | 109 (18) | 60 (15) |
| Baseline medication use, No. (%) | 257 (34) | 218 (36) | 101 (25) |
| Antihypertensive | 162 (39) | 71 (20) | 37 (23) |
| Hypoglycemic | 31 (7) | 11 (3) | 20 (12) |
| Lipid-lowering | 26 (6) | 56 (16) | 22 (13) |
| Psychotherapeutic | 123 (29) | 146 (41) | 60 (37) |
| Hepatitis B positive, No. (%) | 27 (4) | 12 (2) | 9 (2) |
| Hepatitis C positive, No. (%) | 74 (10) | 41 (7) | 24 (6) |
| Annual income, No. (%) | |||
| Less than $5000 | 195 (31) | 71 (13) | 109 (34) |
| $5000–$19 999 | 207 (33) | 128 (23) | 100 (32) |
| $20 000–$49 999 | 168 (27) | 192 (34) | 84 (27) |
| More than $50 000 | 52 (8) | 177 (31) | 24 (8) |
| Working/in school, No. (%) | 406 (55) | 448 (74) | 237 (62) |
| Lives in owned or rented home, No. (%) | 638 (87) | 556 (9) | 322 (84) |
| Education, No. (%) | |||
| 12th grade or less | 184 (25) | 46 (8) | 154 (40) |
| High school graduate/GED | 192 (25) | 81 (13) | 57 (15) |
| Some college/college graduate | 374 (50) | 486 (79) | 176 (46) |
| Smoking, No. (%) | 450 (60) | 358 (58) | 209 (54) |
| History of illicit drug use, No. (%) | 258 (37) | 314 (56) | 135 (39) |
| Alcohol use, No. (%) | |||
| Abstainer | 231 (33) | 129 (23) | 159 (46) |
| Moderate/heavy drinker | 298 (43) | 275 (50) | 95 (28) |
| Binge drinker | 166 (24) | 154 (28) | 92 (27) |
| Insurance status, No. (%) | |||
| US government funding | 486 (67) | 284 (47) | 278 (75) |
| Private insurance | 182 (25) | 284 (47) | 64 (17) |
| Self-pay, out of pocket | 56 (8) | 41 (7) | 29 (8) |
Abbreviations: ATV/r, atazanavir/ritonavir; BMI, body mass index; DRV/r, darunavir/ritonavir; GED, general educational development; RAL, raltegravir.
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Virologic failure, No. (%) | 169 (22) | 55 (8) | 65 (17) |
| Grade 2+ adverse event, No. (%) | 558 (74) | 443 (72) | 277 (71) |
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Virologic failure, No. (%) | 169 (22) | 55 (8) | 65 (17) |
| Grade 2+ adverse event, No. (%) | 558 (74) | 443 (72) | 277 (71) |
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Virologic failure, No. (%) | 169 (22) | 55 (8) | 65 (17) |
| Grade 2+ adverse event, No. (%) | 558 (74) | 443 (72) | 277 (71) |
| . | Non-Hispanic Black (n = 757) . | Non-Hispanic White (n = 615) . | Hispanic (n = 390) . |
|---|---|---|---|
| Virologic failure, No. (%) | 169 (22) | 55 (8) | 65 (17) |
| Grade 2+ adverse event, No. (%) | 558 (74) | 443 (72) | 277 (71) |
Factors Associated With Virologic Failure
The factors associated with virologic failure are shown in Figure 1. The most consistent predictors of the cumulative probability of virologic failure were adherence status (>90% vs ≤90%), with >90% being associated with a lower risk of virologic failure for all groups (HR, 0.13–0.15), and higher baseline HIV RNA, which was associated with higher risk for all groups (HR, 1.52–3.02). Underweight body mass index was strongly associated with virologic failure (HR, 2.51–4.44) across all groups, although there was low precision on this estimate across all groups, and confidence intervals contained a hazard ratio of 1. More education was strongly and consistently associated with a lower risk of virologic failure for all 3 groups (HR for “some college” vs “12th grade or less,” 0.21–0.43); this effect was most robust among Hispanic participants, and was only statistically significant for that group.
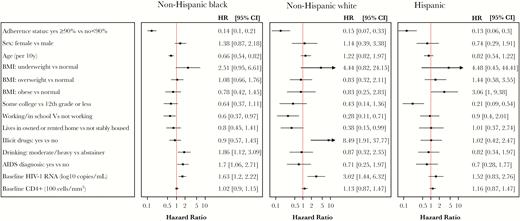
Cox proportional hazards model examining hazard ratios for time to virologic failure across race/ethnicity groups. Hazard ratios >1 reflect greater risk of virologic failure. Models were also adjusted for treatment, adverse event status, income, insurance, smoking history, baseline concomitant medications, fasting non-HDL, fasting glucose, baseline hepatitis B status, and baseline hepatitis C status. Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Certain factors had differing effects among the 3 racial/ethnic groups: A history of illicit drug use was strongly associated with virologic failure among NHWs only (HR, 8.49; 95% CI, 1.91–37.8), whereas moderate to heavy alcohol use was only associated with virologic failure for NHBs (HR, 1.86; 95% CI, 1.12–3.09). Obesity was associated with failure only for Hispanic participants (HR, 3.06; 95% CI, 1–9.38). Older age was associated with lower risk (HR, 0.66; 95% CI, 0.54–0.82 per 10 years older) among NHBs. Among NHWs, older age was associated with a nonsignificant but higher risk of virologic failure (HR, 1.22; 95% CI, 0.82–1.97); the effect among Hispanics was intermediate. A history of AIDS was associated with higher risk (HR, 1.7; 95% CI, 1.06–2.71) for NHBs only. Among socioeconomic variables, being stably housed was associated with lower risk for NHWs only, but not for NHBs or Hispanics (HR, 0.38; 95% CI, 0.15–0.99; vs HR, 0.8 and 1.01, respectively). Being employed or in school was also most strongly associated with virologic failure for NHWs (HR, 0.28; 95% CI, 0.11–0.71). Although this trend was also apparent among NHBs (HR, 0.60; 95% CI, 0.37–0.97), work status was not associated with virologic failure among Hispanics (HR, 0.9).
Factors Associated With Adverse Events
Figure 2 shows the factors associated with reporting of adverse events. Female sex was associated with a higher risk of reporting adverse events in all 3 racial/ethnic groups (HR, 1.19–1.23). Being stably housed was associated with a lower risk of adverse event reporting (HR, 0.85–0.92). Higher educational attainment (HR, 1.21–1.26) was associated with a higher risk of reporting adverse events, but only among NHW participants. The use of concomitant medications at baseline was associated with a higher risk of adverse events; this effect was strongest for Hispanic participants (HR, 1.33; 95% CI, 1.17–1.5).
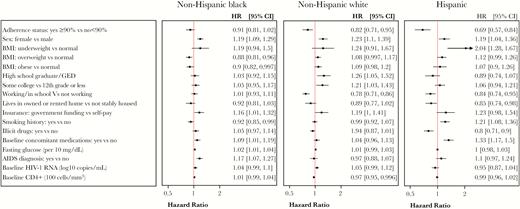
Cox proportional hazards model examining hazard ratios for time to first adverse event across race/ethnicity groups. Hazard ratios >1 reflect greater risk of an adverse event. Models were also adjusted for treatment, income, alcohol use, baseline hepatitis B status, and baseline hepatitis C status. Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
DISCUSSION
Racial/ethnic disparities in HIV treatment outcomes persist despite substantial successful public health efforts to improve care. Differential health outcomes among racial/ethnic groups are often considered to be multifactorial, a combination of socioeconomic, structural, and biologic factors. In this study, we asked if socioeconomic and biologic factors were differentially associated with lower viral suppression rates or with medication intolerance between racial/ethnic groups in a clinical trial population with open access to care. We found that key HIV-related factors, including better adherence and lower baseline HIV RNA level, were associated with better virologic outcome regardless of racial group. The association of other factors, such as history of AIDS-defining illnesses and substance use, appeared to vary by racial/ethnic group. These findings suggest that further exploration of factors specific to racial/ethnic groups may inform strategies to improve successful virologic control and tolerability of HIV treatment. This is particularly true for subpopulations at high risk for virologic failure, such as individuals with suboptimal adherence and/or ongoing substance use.
Regardless of racial/ethnic group, poor self-reported adherence was associated with virologic failure, an expected finding. We also found an association between baseline HIV RNA and treatment outcome, in which higher HIV RNA levels were strongly associated with an increased hazard ratio for virologic failure. Observational studies of antiretroviral therapy have found inconsistent associations between baseline HIV RNA level and failure [16, 17]. An analysis of ACTG trial participants showed that baseline viral load predicted early virologic failure within 6 months, although this was a study of older antiretroviral regimens than the ones studied here [9]. Trials of regimens used in this study have shown this association for darunavir and ritonavir [18, 19], but not for atazanavir, ritonavir, or raltegravir [20, 21]. Similar to other studies, we found an association of illicit substance use with virologic failure [22, 23], even after controlling for regimen and adherence, but this association was significant only in non-Hispanic white participants. This may reflect differing use patterns in clinical trial participants of different races/ethnicities, which then impact treatment response. Although baseline immune status, including CD4 count, has previously been associated with virologic outcomes, in this analysis the effect was strongest for a prior clinical AIDS diagnosis, and only in non-Hispanic black participants. However, the overall rates of prior clinical AIDS diagnoses were low, limiting interpretation of this finding.
We found a higher risk of adverse event reporting for women, regardless of race/ethnicity. This finding is consistent with the literature, which reports more adverse events with antiretroviral therapy in women [24]. Higher education was associated with adverse event reporting in only NHW participants, which may be related to having a lower threshold to report events or a greater knowledge of drug-related adverse events. Concurrent medication use was associated with reporting of adverse events, more strongly in in Hispanic participants than in NHB or NHW participants. There is a known link between polypharmacy and medication adverse effects [25, 26]. In our study, the distribution of comorbidities was different between groups, with NHB and Hispanic participants more likely to be using antihypertensive or hypoglycemic medications and NHW participants more likely to be using psychotherapeutic medications. This may explain the difference in results: Either the presence of certain comorbidities or the specific medications used to treat them may have been associated with an increase in reported adverse events.
There was a noticeably more prominent impact of socioeconomic factors in the NHW subgroup compared with the others. Higher education status, presence of stable housing, and employment were all associated with significantly decreased risks of virologic failure in NHW. In NHBs, these factors were also associated with lower risk of failure but did not meet significance, and the effect was not at all apparent in the Hispanic group. It is possible that among NHW participants, the measured variables reflected underlying socioeconomic subgroups that had differing likelihoods of virologic failure, whereas the other racial groups were more socioeconomically homogenous. Furthermore, other social and economic variables that may impact virologic failure were not measured in our study. For example, the patient–provider relationship, social support, and perception of stigma have been linked to adherence or viral suppression and may impact outcomes differently among racial/ethnic groups [27–30]. As we studied a clinical trial population with good access to ART, we cannot exactly reproduce the extent to which factors such as housing, education, insurance, and income likely affect access to HIV care in practice.
The strengths of our study include the large sample size and systematic collection of exposures and outcomes in conjunction with a multisite clinical trial. This trial collected more detailed socioeconomic and behavioral data about participants than previous ACTG studies, which strengthened our analysis. Participants’ equal access to ART provided by the study eliminated the potential impact of health care access on disparities, which is a common factor in observational settings in the United States. Our study also had several limitations. We had a low rate of events (both virologic failure and severe adverse events), which contributed to lack of precision of some estimates and limited our ability to perform further subgroup analyses or formal interaction testing. The differences in effect estimates observed between groups may also reflect inherent differences in the demographic makeup of these populations. Although there was an overall effect of adherence, education, insurance status, and baseline HIV RNA, upon evaluation of the proportional hazard model assumptions, there was evidence to suggest that these results were driven by early or late effects. The clinical trial population analyzed here may not be representative of the general population. Specifically, our population was younger overall than the HIV-infected US population, and different patterns of comorbidity and medication use are likely to apply in practice. Validation of our findings in nonclinical trial settings is warranted. As discussed above, unmeasured confounders may still exist that were not collected in the study and could impact the observed findings.
In summary, in this exploratory analysis of data from a randomized controlled trial of initial HIV antiretroviral therapy, we found that viral suppression and tolerability within different racial/ethnic groups were largely mediated by similar clinical and socioeconomic factors, but that these factors did not impact all groups equally. Further research on the factors that impacted groups differently, such as drug use for the outcome of virologic failure or comorbidities and concurrent medications for reporting of adverse effects, may help shed light on the mechanisms that give rise to disparities in HIV treatment outcomes and identify key subpopulations in need of treatment support that may differ within racial groups.
Acknowledgments
We would like to acknowledge all study participants and the investigators at each of the study sites.
Financial support. Funding for AIDS Clinical Trial Group (ACTG) A5257 was provided by the National Institute of Allergy and Infectious Diseases (UM1 AI068636, UM1 AI069419, UM1 AI068634), National Center for Advancing Translational Sciences (UL1 RR024996), National Institute of Mental Health, and National Institute of Dental and Craniofacial Research. The protocol received financial support from the ACTG and the Statistics and Data Management Center (UM1 AI68634, UM1 AI68636), the ACTG specialty laboratories, and the 57 clinical research sites. Bristol-Myers Squibb, Merck Inc., Janssen Therapeutics, and Gilead Sciences provided the study medications (atazanavir, raltegravir, darunavir, and tenofovir-emtricitabine, respectively). The analysis of data and preparation of this manuscript were supported by the Postdoctoral HIV Research Training Program for HIV Combination Prevention (T32 MH109205 to Bhagwat) and the Geriatric Mental Health Services Research Fellowship (T32 MH073553 to Kapadia).
Potential conflicts of interest. Dr. Currier received research grants to her institution from Theratechnologies. Dr. Kapadia has received research grants to Weill Cornell from Gilead Sciences. These grants are unrelated to the current study. All other authors report no disclosures. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Equal contribution
- hiv
- acquired immunodeficiency syndrome
- comorbidity
- demography
- ethnic group
- hispanics or latinos
- housing
- ritonavir
- socioeconomic factors
- diagnosis
- blood hiv rna
- anti-retroviral agents
- darunavir
- raltegravir
- health disparity
- aids clinical trial group
- virologic failure
- adverse event
- atazanavir/ritonavir
- racial disparities
- viral suppression





Comments