-
PDF
- Split View
-
Views
-
Cite
Cite
Anirban Basu, Vimalanand S Prabhu, Mary Beth Dorr, Yoav Golan, Erik R Dubberke, Oliver A Cornely, Sebastian M Heimann, Alison Pedley, Ruifeng Xu, Mary E Hanson, Stephen Marcella, Bezlotoxumab Is Associated With a Reduction in Cumulative Inpatient-Days: Analysis of the Hospitalization Data From the MODIFY I and II Clinical Trials, Open Forum Infectious Diseases, Volume 5, Issue 11, November 2018, ofy218, https://doi.org/10.1093/ofid/ofy218
Close - Share Icon Share
Abstract
Patients with recurrent Clostridium difficile infection (rCDI) are more likely to have a hospital readmission and spend increased time in inpatient settings compared with patients with primary CDI. MODIFY I and II demonstrated that bezlotoxumab significantly reduced rCDI vs placebo. A post hoc within-trial analysis assessed whether bezlotoxumab was associated with a reduction in cumulative inpatient-days.
Data were pooled from the MODIFY trials to estimate the cumulative hospitalized days summed over the 84-day follow-up period. We adjusted inpatient use data from pooled MODIFY I and II for survival and censoring to estimate 84-day cumulative inpatient-days, overall and for subgroups. Treatment effects were obtained using recycled predictions based on trial protocol and rCDI risk, and 95% confidence intervals were obtained using 1000 bootstrap replicates.
Mean cumulative inpatient-days were greater in the placebo arm (14.1 days) vs the bezlotoxumab arm (12.1 days) in the overall population. The mean difference between treatment groups was 2.1 days (95% confidence interval, –0.4 to –3.7). This was consistent in participants with risk factors for rCDI: age ≥65 years, compromised immunity, severe CDI, prior CDI, and ribotype 027/078/244 infection. As the number of risk factors increased, bezlotoxumab resulted in greater reductions in the number of inpatient-days compared with placebo (difference: –1.2 days, –2.3 days, –2.5 days, and –3.0 days for 0, 1, 2, and ≥3 risk factors, respectively).
Bezlotoxumab was associated with a reduction in cumulative inpatient-days, suggesting that treatment with bezlotoxumab may substantially reduce rCDI-associated health care resource use.
Trial registrations. MODIFY I (MK-3415A-001, NCT01241552) and II (MK-3415A-002, NCT01513239)
Approximately 25% of patients experience recurrent Clostridium difficile infection (rCDI) after completing initial therapy with metronidazole or vancomycin [1, 2]. The risk of a second recurrence increases to approximately 40% [3] with significantly increasing costs for inpatients with rCDI compared with primary CDI [4]. Factors associated with an increase in rCDI include age ≥65 years [5], inadequate immune response [6], severe CDI [7, 8], prior CDI episode(s) [3], and infection with the B1/NAP1/027 strain [9–12].
Overall, patients with rCDI experience significantly higher rates of hospital readmission vs patients without rCDI (85% vs 41%) [13] and have longer hospital stays (19 days vs 8 days; P < .001) [13]; more than 40% of index hospitalizations associated with CDI result in discharge to long-term care, followed by readmission to the hospital within 90 days (all-cause readmissions) [14]. Recurrent episodes of CDI are associated with excessive costs, and these are attributable to significantly longer hospital stays, including more days in the intensive care unit in tertiary care settings, compared with primary episodes of CDI [4, 15].
The human monoclonal antibody bezlotoxumab is a novel antitoxin agent that, when given as a single intravenous dose during standard of care antibiotic therapy, reduced rCDI compared with antibiotic therapy alone (placebo group) over a 12-week period in 2 independent, phase III, global trials (adjusted difference between groups in rCDI was –10.1% in MODIFY I and –9.9% in MODIFY II; both P < .001) [16]. Furthermore, in a post hoc analysis, the reduction in rCDI was greater in participants at high risk for rCDI (adjusted difference between groups in rCDI was –15.9% (95% confidence interval [CI], –21.6 to –10.2) in participants with ≥1 risk factor for rCDI) [17]. Bezlotoxumab neutralizes C. difficile toxin B. Neutralization of toxin B prevents new symptoms of CDI during the period when there is a high risk of rCDI, thereby preventing clinical relapse.
Bezlotoxumab’s demonstrated efficacy in reducing rCDI over a 12-week period suggests that health care resource utilization and associated costs may also be reduced in patients receiving bezlotoxumab. This post hoc analysis was conducted to assess whether bezlotoxumab reduced the number of days participants spent in the hospital (cumulative inpatient-days) over a 12-week period compared with placebo.
METHODS
MODIFY I (NCT01241552) and II (NCT01513239) were randomized, double-blind, placebo-controlled, multicenter, phase III trials that were conducted from November 2011 through May 2015 at 322 sites in 30 countries. The protocols and all amendments were approved by the institutional review board or independent ethics committee at each study center. Each study was conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. Written informed consent was obtained before study procedures were performed.
Participants
Adults with primary or rCDI receiving oral standard of care antibiotics (metronidazole, vancomycin, or fidaxomicin, chosen by the treating physician) for 10–14 days were included in the trial. CDI was defined as diarrhea (≥3 unformed bowel movements in 24 hours) associated with a positive stool test for toxigenic C. difficile or its toxins. To monitor for new episodes of diarrhea, participants recorded daily loose stool counts in a diary through 12 weeks. Participants included in this analysis received either a single dose of bezlotoxumab 10 mg/kg or placebo (0.9% saline) during antibiotic treatment. Randomization was stratified by oral antibiotic treatment for CDI (metronidazole, vancomycin, or fidaxomicin) and hospitalization status (inpatient or outpatient).
Population, End Points, and Statistical Methods
The analysis population was the modified intent-to-treat (mITT) population (defined as all randomly assigned participants who received study infusion, had a positive stool test for toxigenic C. difficile or its toxins, and began standard of care antibiotic therapy before or within 1 day after receiving the study infusion) in the pooled data set from MODIFY I and MODIFY II. Subgroups were characterized by known risk factors for CDI recurrence that had been prespecified in the protocols: ≥65 years of age, immunocompromised, severe CDI, hypervirulent strain (ribotypes 027, 078, or 244), and/or ≥1 episode of CDI in previous 6 months. Additional subgroups were defined based on the total number of prespecified risk factors: no risk factors, at least 1 risk factor, only 1 risk factor, only 2 risk factors, and ≥3 risk factors. The primary end point for this post hoc analysis was cumulative inpatient-days in any hospital setting during the 84 days following infusion.
We first modeled the probability of surviving each follow-up day, after adjusting for censoring using a standard accelerated failure time model. Then, using the longitudinal data set of up to 84 observations per person, we estimated the probability of spending a day in the hospital using a logistic model. The independent variables for this model included sex, race, age, inpatient status, NAP 1 strain, Charlson Severity Score, history of CDI, being immunocompromised, and region (US vs ex-US). Lin’s (2000) method was applied, where the day-specific probability of spending a day in the hospital among those noncensored was multiplied by the estimated day-specific probability of survival and then summed over all 84 days to obtain the estimate of cumulative inpatient-days, adjusted for censoring [18]. Censoring occurred, as cost data were not available for patients who discontinued from the trial. Lin’s method was adopted to account for censoring by appropriately weighting the uncensored costs with inverse probability of inclusion (survival probability). Lin’s method is an improvement over traditional methods such as analyzing censored cost data by excluding subjects, as these methods do not utilize complete information by ignoring censored subjects and may result in bias. Treatment effects were obtained using recycled predictions by turning on and off the treatment indicator for all participants in our overall sample and for subgroups identified based on trial protocol and risk of rCDI. P values and 95% confidence intervals were obtained using 1000 clustered bootstrap replicates. Statistical analyses were conducted using Stata, version 11.
RESULTS
Study Population
There were 1554 participants (bezlotoxumab group: 781; placebo group: 773) included in the mITT population across both studies. Demographic and clinical characteristics were balanced between the study groups (Table 1) and have been previously reported [16]. The median age (range) was 66 (18–100) years, 86% were white, and 56% were women.
| Characteristic . | Bezlotoxumab (n = 781) . | Placebo (n = 773) . |
|---|---|---|
| Inpatient | 530 (67.9) | 520 (67.3) |
| Female sex | 442 (56.6) | 449 (58.1) |
| Age ≥65 y | 390 (49.9) | 405 (52.4) |
| SOC antibiotic | ||
| Metronidazole | 365 (46.7) | 353 (45.7) |
| Vancomycin | 370 (47.4) | 372 (48.1) |
| Fidaxomicin | 30 (3.8) | 30 (3.9) |
| ≥1 episode of CDI in previous 6 mo | 216 (27.7) | 219 (28.3) |
| ≥2 previous CDI episodes ever | 100 (12.8) | 126 (16.3) |
| Severe CDIa | 122 (15.6) | 125 (16.2) |
| Immunocompromisedb | 178 (22.8) | 153 (19.8) |
| Other antibiotic use during SOC therapyc | 292 (37.4) | 317 (41.0) |
| Other antibiotic use after SOC therapyc | 273 (35.0) | 275 (35.6) |
| Renal impairmentd | 123 (15.7) | 110 (14.2) |
| Hepatic impairmente | 49 (6.3) | 44 (5.7) |
| PCR ribotype | ||
| Participants with positive culture | 490 (62.7) | 486 (62.9) |
| 027, 078, or 244 strainf | 102 (20.8) | 115 (23.7) |
| 027 strainf | 89 (18.2) | 100 (20.6) |
| Characteristic . | Bezlotoxumab (n = 781) . | Placebo (n = 773) . |
|---|---|---|
| Inpatient | 530 (67.9) | 520 (67.3) |
| Female sex | 442 (56.6) | 449 (58.1) |
| Age ≥65 y | 390 (49.9) | 405 (52.4) |
| SOC antibiotic | ||
| Metronidazole | 365 (46.7) | 353 (45.7) |
| Vancomycin | 370 (47.4) | 372 (48.1) |
| Fidaxomicin | 30 (3.8) | 30 (3.9) |
| ≥1 episode of CDI in previous 6 mo | 216 (27.7) | 219 (28.3) |
| ≥2 previous CDI episodes ever | 100 (12.8) | 126 (16.3) |
| Severe CDIa | 122 (15.6) | 125 (16.2) |
| Immunocompromisedb | 178 (22.8) | 153 (19.8) |
| Other antibiotic use during SOC therapyc | 292 (37.4) | 317 (41.0) |
| Other antibiotic use after SOC therapyc | 273 (35.0) | 275 (35.6) |
| Renal impairmentd | 123 (15.7) | 110 (14.2) |
| Hepatic impairmente | 49 (6.3) | 44 (5.7) |
| PCR ribotype | ||
| Participants with positive culture | 490 (62.7) | 486 (62.9) |
| 027, 078, or 244 strainf | 102 (20.8) | 115 (23.7) |
| 027 strainf | 89 (18.2) | 100 (20.6) |
Abbreviations: CDI, Clostridium difficile infection; PCR, polymerase chain reaction; SOC, standard of care.
aSevere infection was defined as a Zar score of 2 or higher. The Zar score ranges from 1 to 8 and is based on the following factors: age greater than 60 years (1 point), body temperature higher than 38.3°C (100°F) (1 point), albumin level lower than 2.5 g per deciliter (1 point), peripheral white cell count higher than 15 000 per cubic millimeter within 48 hours (1 point), endoscopic evidence of pseudomembranous colitis (2 points), and treatment in an intensive care unit (2 points).
bThe determination of whether a participant was immunocompromised was made on the basis of medical history or use of immunosuppressive therapy.
cIncluded are systemic antibiotics other than the standard of care antibiotic that was given to treat C. difficile infection.
dRenal impairment was defined as a serum creatinine level of 1.5 mg per deciliter (133 μmol per liter) or higher.
eHepatic impairment was defined as having 2 or more of the following: an albumin level of 3.1 g per deciliter or lower, an alanine aminotransferase level at least 2 times the upper limit of the normal range, a total bilirubin level at least 1.3 times the upper limit of the normal range, or mild, moderate, or severe liver disease (as reported on the Charlson Index).
fThe denominators used to calculate percentages are the numbers of participants who had a positive culture.
| Characteristic . | Bezlotoxumab (n = 781) . | Placebo (n = 773) . |
|---|---|---|
| Inpatient | 530 (67.9) | 520 (67.3) |
| Female sex | 442 (56.6) | 449 (58.1) |
| Age ≥65 y | 390 (49.9) | 405 (52.4) |
| SOC antibiotic | ||
| Metronidazole | 365 (46.7) | 353 (45.7) |
| Vancomycin | 370 (47.4) | 372 (48.1) |
| Fidaxomicin | 30 (3.8) | 30 (3.9) |
| ≥1 episode of CDI in previous 6 mo | 216 (27.7) | 219 (28.3) |
| ≥2 previous CDI episodes ever | 100 (12.8) | 126 (16.3) |
| Severe CDIa | 122 (15.6) | 125 (16.2) |
| Immunocompromisedb | 178 (22.8) | 153 (19.8) |
| Other antibiotic use during SOC therapyc | 292 (37.4) | 317 (41.0) |
| Other antibiotic use after SOC therapyc | 273 (35.0) | 275 (35.6) |
| Renal impairmentd | 123 (15.7) | 110 (14.2) |
| Hepatic impairmente | 49 (6.3) | 44 (5.7) |
| PCR ribotype | ||
| Participants with positive culture | 490 (62.7) | 486 (62.9) |
| 027, 078, or 244 strainf | 102 (20.8) | 115 (23.7) |
| 027 strainf | 89 (18.2) | 100 (20.6) |
| Characteristic . | Bezlotoxumab (n = 781) . | Placebo (n = 773) . |
|---|---|---|
| Inpatient | 530 (67.9) | 520 (67.3) |
| Female sex | 442 (56.6) | 449 (58.1) |
| Age ≥65 y | 390 (49.9) | 405 (52.4) |
| SOC antibiotic | ||
| Metronidazole | 365 (46.7) | 353 (45.7) |
| Vancomycin | 370 (47.4) | 372 (48.1) |
| Fidaxomicin | 30 (3.8) | 30 (3.9) |
| ≥1 episode of CDI in previous 6 mo | 216 (27.7) | 219 (28.3) |
| ≥2 previous CDI episodes ever | 100 (12.8) | 126 (16.3) |
| Severe CDIa | 122 (15.6) | 125 (16.2) |
| Immunocompromisedb | 178 (22.8) | 153 (19.8) |
| Other antibiotic use during SOC therapyc | 292 (37.4) | 317 (41.0) |
| Other antibiotic use after SOC therapyc | 273 (35.0) | 275 (35.6) |
| Renal impairmentd | 123 (15.7) | 110 (14.2) |
| Hepatic impairmente | 49 (6.3) | 44 (5.7) |
| PCR ribotype | ||
| Participants with positive culture | 490 (62.7) | 486 (62.9) |
| 027, 078, or 244 strainf | 102 (20.8) | 115 (23.7) |
| 027 strainf | 89 (18.2) | 100 (20.6) |
Abbreviations: CDI, Clostridium difficile infection; PCR, polymerase chain reaction; SOC, standard of care.
aSevere infection was defined as a Zar score of 2 or higher. The Zar score ranges from 1 to 8 and is based on the following factors: age greater than 60 years (1 point), body temperature higher than 38.3°C (100°F) (1 point), albumin level lower than 2.5 g per deciliter (1 point), peripheral white cell count higher than 15 000 per cubic millimeter within 48 hours (1 point), endoscopic evidence of pseudomembranous colitis (2 points), and treatment in an intensive care unit (2 points).
bThe determination of whether a participant was immunocompromised was made on the basis of medical history or use of immunosuppressive therapy.
cIncluded are systemic antibiotics other than the standard of care antibiotic that was given to treat C. difficile infection.
dRenal impairment was defined as a serum creatinine level of 1.5 mg per deciliter (133 μmol per liter) or higher.
eHepatic impairment was defined as having 2 or more of the following: an albumin level of 3.1 g per deciliter or lower, an alanine aminotransferase level at least 2 times the upper limit of the normal range, a total bilirubin level at least 1.3 times the upper limit of the normal range, or mild, moderate, or severe liver disease (as reported on the Charlson Index).
fThe denominators used to calculate percentages are the numbers of participants who had a positive culture.
Treatment with bezlotoxumab was associated with a reduction in cumulative inpatient-days compared with placebo (Figure 1) during 84 days of follow-up. In the overall population, participants treated with bezlotoxumab spent an average of 2.1 fewer days (95% CI, –0.4 to –3.7) in the hospital compared with participants who received placebo. Similarly, in all subgroups assessed, including those with no risk factors and those with factors associated with rCDI, bezlotoxumab treatment was associated with a reduction in cumulative inpatient-days compared with those receiving placebo during the 84-day follow-up period. The greatest reduction in inpatient-days was observed in participants with severe CDI at study entry (–3.5 days) (Figure 1). As the number of risk factors for rCDI increased, the reduction in the number of inpatient-days also increased with bezlotoxumab vs placebo (difference: –1.2 days in participants with no risk factors, –2.3 days in those with 1 risk factor, –2.5 days in those with 2 risk factors, and –3.0 days in those with ≥3 risk factors) (Figure 1).
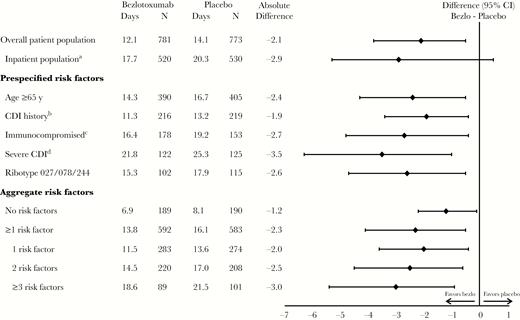
Estimated mean cumulative inpatient-days summed over 84 days in the modified intent-to-treat population. aInpatients at the time of study randomization. bHistory of Clostridium difficile infection recurrence within the previous 6 months. cDefined on the basis of a subject’s medical history or use of immunosuppressive therapy. dZar score ≥2 based on the following: (1) age >60 years (1 point); (2) body temperature >38.3°C (>100°F; 1 point); (3) albumin level <2.5 mg/dL (1 point); (4) peripheral white blood cell count >15 000 cells/mm3 within 48 hours (1 point); (5) endoscopic evidence of pseudomembranous colitis (2 points); and (6) treatment in an intensive care unit (2 points). Abbreviations: CDI, Clostridium difficile infection; CI, confidence interval.
DISCUSSION
We used pooled data from 2 placebo-controlled, multicenter clinical trials to assess the impact of bezlotoxumab treatment on cumulative inpatient-days from any cause over 84 days of follow-up. Our post hoc analysis demonstrated that bezlotoxumab treatment was associated with reduced cumulative inpatient stay of 2.1 days in the overall trial population, with even greater reductions observed in participants with 1 or more risk factors associated with recurrence of CDI. The inpatient subgroup, presumably a sicker subgroup, is also noted to have a larger reduction in cumulative inpatient-days. As the number of risk factors increased, participants treated with bezlotoxumab experienced incrementally greater reductions in the cumulative inpatient-days compared with placebo.
A 2014 model-based estimate of the economic burden of CDI in the United States was substantial at $5.4 billion, mainly attributable to hospitalization [19]. A US MarketScan database study with coverage of more than 45 million members estimated the 6-month health care costs attributable to rCDI at $10 580 (95% CI, $8849 to $12 446), with cumulative hospitalized days attributable to rCDI of 1.95 (95% CI, 1.48 to 2.43) [20], comparable to the study by Dubberke et al. (2014) in which the estimated attributable cost of rCDI was $11 631 (95% CI, $8937 to $14 588) [4]. With the incidence of CDI-associated hospital readmissions rising [13], it is important to find innovative treatment options to reduce rCDI and rCDI-associated hospital admissions. The results of the current analysis are consistent with a related post hoc analysis of the MODIFY trials in the subgroup of participants who were hospitalized at the time of randomization, demonstrating that bezlotoxumab-treated inpatients experienced fewer CDI-associated readmissions during the 30 days after discharge compared with placebo-treated inpatients [21].
The MODIFY trials, which included a prespecified subgroup analysis of participants with risk factors associated with an increase in rCDI or CDI-related adverse outcomes, afforded an opportunity to examine differences in all-cause cumulative inpatient-days in an unbiased manner. In the post hoc analysis at hand, we observed that as the number of risk factors increased, the number of cumulative inpatient-days in the placebo arm also increased, suggesting that the presence of multiple risk factors is associated with an increase in number of days spent in an inpatient facility. This is consistent with another post hoc analysis of the MODIFY trials that demonstrated that the proportion of participants experiencing rCDI in the placebo arm increased as the number of risk factors increased [17]. Regardless of the number of risk factors, treatment with bezlotoxumab was associated with a reduction in cumulative inpatient-days during the 84-day follow-up period compared with placebo. Taken together, bezlotoxumab treatment is consistent with an overall reduction in recurrence, readmissions, and cumulative inpatient-days, suggesting that its use may be an effective treatment for reducing the overall health care resource use associated with CDI.
A cost-effectiveness model showed that bezlotoxumab is cost-effective in the prevention of recurrent CDI compared with placebo among participants receiving standard of care antibiotics for treatment of CDI [22]. With the average cost for 1 inpatient-day in the United States estimated at $2013 in 2015 [23], and an estimated reduction in hospital-days of 2.0 to 3.0 days, depending upon the number of risk factors, the evidence suggests that treatment with bezlotoxumab may substantially reduce direct treatment costs associated with rCDI. A within-trial health-economic evaluation is needed to further assess the value of bezlotoxumab, as bezlotoxumab could potentially be cost-saving among patients with ≥1 risk factor. Further, this work is consistent with the labeled claim for bezlotoxumab and data demonstrating that bezlotoxumab has a higher impact where it matters—among patients who are at risk for rCDI [17].
There are some limitations of this post hoc analysis. First, it was not powered for hypothesis testing. In addition, we did not assess CDI-associated hospital-days; rather we assessed days spent in the hospital for any reason. Therefore, we may be overestimating the specific CDI burden, although the amount of overestimation should be equivalent for both the bezlotoxumab and placebo groups. One population-based, retrospective cohort study among adult inpatients in 29 hospitals found that the risk of readmission for CDI was higher in the first 12 weeks after discharge; after 12 weeks, the risk of readmission dropped to a stable, low level, suggesting that we captured the majority of CDI-associated readmissions during the 84-day follow-up period [24]. Of note, the MODIFY trials may have enrolled a healthier population compared with real-world patients with CDI (despite including a broad population with few exclusion criteria), potentially reducing the estimated number of non-CDI-related hospital-days in this analysis. Another limitation was that we only counted acute care hospitalization–days and did not count days in a long-term care facility or rehabilitation center. As with any clinical trial, there was early loss to follow-up of some patients, which could lead to underestimation of average hospital-days. Our methods directly addressed this censoring using a missing-at-random assumption.
In conclusion, the analysis presented here suggests that bezlotoxumab treatment may result in reduced health care resource utilization, especially among patients at high risk for CDI recurrence.
Acknowledgments
Carol Zecca of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, provided editorial and submission support.
Author contributions. Anirban Basu substantially contributed to the conception, design, or planning of the study, data analysis, interpretation of the results, and drafting of the manuscript; Yoav Golan substantially contributed to the conception, design, or planning of the study, interpretation of the results, and drafting of the manuscript; Erik R. Dubberke substantially contributed to interpretation of the results; Oliver Cornely substantially contributed to the conception, design, or planning of the study, data analysis, interpretation of the results, and drafting of the manuscript; Sebastian M. Heimann substantially contributed to interpretation of the results; Vimalanand Prabhu substantially contributed to the conception, design, or planning of the study, data analysis, interpretation of the results, and drafting of the manuscript; Mary Beth Dorr substantially contributed to acquisition of the data and interpretation of the results; Alison Pedley substantially contributed to the conception, design, or planning of the study, data analysis, and interpretation of the results; Ruifeng Xu substantially contributed to analysis of the data; Mary E. Hanson substantially contributed to the conception, design, or planning of the study, interpretation of the results, and drafting of the manuscript; and Stephen Marcella substantially contributed to the conception, design, or planning of the study, interpretation of the results, and drafting of the manuscript. In addition, all authors critically reviewed or revised the manuscript for important intellectual content, reviewed and approved the version of the manuscript to be submitted, and had access to all the relevant study data and related analyses. Furthermore, all authors vouch for the completeness and accuracy of the data presented and agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey.
Potential conflicts of interest. Anirban Basu consulted with Merck to advise on and supervise the implementation of the methods in this work. He has also consulted with many for-profit and not-for-profit organizations including Pfizer, GSK, Janssen, and Astra Zeneca. Yoav Golan is a scientific advisor for Merck & Co., Inc., Pfizer, Allergan, The Medicines Company, and Seres Pharmaceuticals; a speaker for Merck, Pfizer, Allergan, and The Medicines Company; and has received research grants from Merck and Allergan. Erik R. Dubberke is an Investigator on behalf of Merck & Co., Inc., and Rebiotix, has received grants from Sanofi Pasteur, and is a consultant for Merck & Co., Inc, GSK, Velneva, Rebiotix, and Sanofi Pasteur. Oliver A. Cornely reports research grants from Actelion, Aranis Pharma, Astellas, AstraZeneca, Basilea, Bayer, Cidara, F2G, Gilead, GSK, Leeds University, MedPace, Melinta Therapeutics, Merck & Co., Inc./MSD, Miltenyi, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, Seres Therapeutics, and The Medicine Company; is a consultant to Achaogen, Amplyx, Actelion, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen Pharmaceuticals, Matinas, Menarini Ricerche, Merck & Co., Inc./MSD, Paratek Pharmaceuticals, Scynexis, Seres, Summit, Tetraphase, and Vical; and has received lecture honoraria from Astellas, Basilea, Gilead, Merck & Co., Inc./MSD, and Pfizer outside the submitted work. Sebastian M. Heimann has received research and travel grants from Astellas and Merck & Co., Inc., research grants from Basilea, Gilead, and 3M, travel grants from Pfizer, lecture honoraria from Astellas and Merck & Co., Inc., and is a consultant to Basilea and Gilead. Vimalanand Prabhu, Mary Beth Dorr, Alison Pedley, Ruifeng Xu, Mary E. Hanson, and Stephen Marcella are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, and may own stock or hold stock options in the company. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References





Comments