-
PDF
- Split View
-
Views
-
Cite
Cite
Lewis Radonovich, Michael S Simberkoff, Mary Bessesen, Alexandria C Brown, Derek Cummings, Charlotte Gaydos, Jenna Los, Amanda Krosche, Cynthia Gibert, Geoffrey Gorse, Ann-Christine Nyquist, Nicholas Reich, Maria Rodriguez-Barradas, Connie Price, Trish Perl, 1716. Results of the Respiratory Protection Effectiveness Clinical Trial (ResPECT), Open Forum Infectious Diseases, Volume 5, Issue suppl_1, November 2018, Page S51, https://doi.org/10.1093/ofid/ofy209.122
Close - Share Icon Share
Abstract
Results of the Respiratory Protection Effectiveness Clinical Trial (ResPECT)
Respiratory protection (RP) for healthcare personnel (HCP) is controversial and clinical studies are inconclusive about the effectiveness of N95 respirators (N95) and medical masks (MM) for protecting HCP from workplace viral respiratory infections and illnesses (VRII).
We conducted a cluster-randomized, investigator-blinded, multisite effectiveness study comparing N95 to MM in geographically diverse, high exposure outpatient settings between 2011 and 2016. Each year during VRII season, participants wore assigned devices when within 6 feet of patients with known or suspected respiratory illness. Respiratory swabs were collected from symptomatic and asymptomatic participants. Diaries detailed VRII exposures, influenza vaccination, adherence to RP and hand hygiene, and manifestations of illness. The primary and secondary outcomes were the incidence of laboratory-confirmed influenza (LCI) using polymerase chain reaction (PCR) and hemagglutinin inhibition assays (HAI), and acute respiratory illness (ARI), influenza-like illness (ILI), laboratory-confirmed respiratory illness (LCRI), and laboratory-detected respiratory infection (LDRI) (figure). Intervention protective effects were estimated using unadjusted odds and incidence rate ratios.
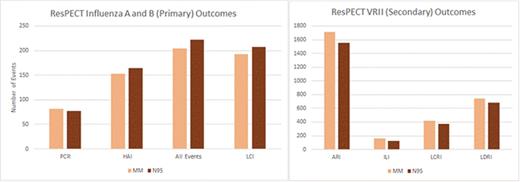
ResPECT Outcomes. (A) Influenza Incidence and Primary Outcomes Panel. (B) Secondary Outcomes
5,180 HCP seasons enrolled and randomized (2,243 to N95 and 2,446 to MM), with 4,689 (91%) completing the study. In the intention-to-treat cohort (ITT), among participants in the N95 and MM groups, respectively, 207 (8.2%) and 193 (7.2%) were diagnosed with LCI (odds ratio [OR] 1.14, 95% confidence interval [CI] 0.93–1.40); 1,556 (61.9%) and 1711 (64.1%) were diagnosed with ARI (relative risk (RR) 0.99, CI 0.92–1.06); 128 (5.1%) and 166 (6.2%) were diagnosed with ILI (RR 0.87, CI 0.68–1.10), 371 (14.8%) and 417 (15.6%) were diagnosed with LCRI (RR 0.97, CI 0.84–1.12); and 679 (27.0%) and 745 (27.9%) were diagnosed with LDRI (RR 0.99, CI 0.89–1.09). The adjusted ITT and per-protocol analyses yielded similar results.
In this outpatient-based, cluster-randomized, controlled trial, neither N95 nor MM resulted in superior protection from LCI or VRII.
C. Gaydos, BioFire: Consultant, Consulting fee. Cepheid: Speaker’s Bureau, Speaker honorarium. Becton Dickinson: Speaker’s Bureau, Speaker honorarium.
Session: 199. Clinical Trials that May Change Your Practice
Saturday, October 6, 2018: 8:45 AM
- polymerase chain reaction
- influenza
- consultation
- exposure
- consultants
- disclosure
- health personnel
- hemagglutinins
- influenza vaccines
- masks
- outpatients
- respiratory tract infections
- workplace
- foot
- washing hands
- viral respiratory infections
- per protocol analysis
- intention to treat
- flu-like illness
- n95 masks
- primary outcome measure
- diaries
- respect
- liver donor risk index





Comments