-
PDF
- Split View
-
Views
-
Cite
Cite
Olivier Van Der Meeren, Mark Hatherill, Videlis Nduba, Robert J Wilkinson, Monde Muyoyeta, Elana Van Brakel, Helen M Ayles, German Henostroza, Friedrich Thienemann, Thomas J Scriba, Andreas Diacon, Gretta L Blatner, Marie-Ange Demoitié, Michele Tameris, Mookho Malahleha, James C Innes, Elizabeth Hellstrom, Neil Martinson, Tina Singh, Elaine J Akite, Aisha K Azam, Anne Bollaerts, Ann M Ginsberg, Thomas G Evans, Paul Gillard, Dereck R Tait, 120. A Randomized Double-blind Trial Assessing the Efficacy of M72/AS01E Vaccine Against Pulmonary Tuberculosis Disease in Adults With Latent Mycobacterium tuberculosis Infection, Open Forum Infectious Diseases, Volume 5, Issue suppl_1, November 2018, Pages S5–S6, https://doi.org/10.1093/ofid/ofy209.011
Close - Share Icon Share
Abstract
An effective tuberculosis (TB) vaccine is urgently needed to support the End TB Strategy to reduce the number of new TB cases by 80% by 2030. M72/AS01E candidate vaccine is an adjuvanted recombinant fusion protein derived from Mycobacterium tuberculosis Mtb32A and Mtb39A proteins.
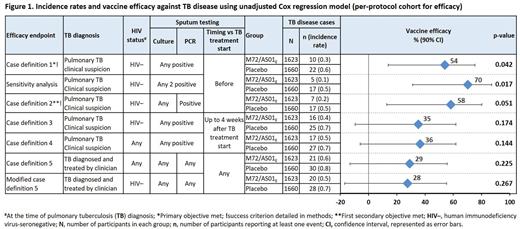
We conducted a randomized, double-blind, placebo-controlled phase 2b trial (NCT01755598) in Southern and Eastern Africa to assess M72/AS01E’s efficacy against bacteriologically confirmed pulmonary TB disease in HIV-seronegative (HIV–) adults aged 18–50 years infected with Mtb (defined by a positive IFN-γ release assay). Participants were randomized 1:1 to receive M72/AS01E or placebo at day D0 and D30. Reactogenicity, safety, immunogenicity were assessed, and incident TB disease measured from D30 postdose 2 up to ≥24 months for this analysis (follow-up ongoing up to 37 months). Efficacy against various TB disease case definitions (Figure 1) was estimated. Primary objective: efficacy against culture- or PCR-confirmed pulmonary TB disease in HIV– adults (case definition 1; success criterion: lower limit of 90% 2-sided CI >0%, power: 80%).
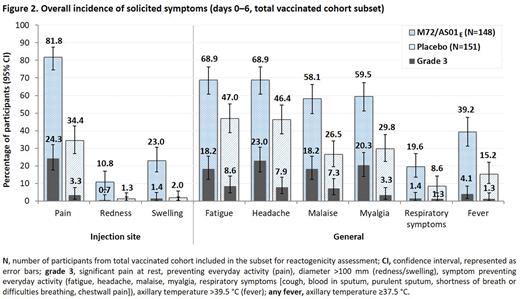
Demographic characteristics were similar between M72/AS01E (1786) and placebo (1787) recipients. Efficacy against pulmonary TB disease case definition 1 was 54.0% (90% CI: 13.9, 75.4; P = 0.042); efficacy against other case definitions and a sensitivity analysis are shown in Figure 1. Leading solicited symptoms were pain, fatigue, and headache (Figure 2). In all recipients, unsolicited symptoms (D0–29) were more frequent after M72/AS01E (67.4%) than placebo (45.4%), mainly attributable to increased injection site reactions and flu-like symptoms. Serious adverse events (D0–month 7) incidences were similar between groups (M72/AS01E: 1.6%; placebo: 1.8%). During the study, 24 adults died (14 due to traumatic events); all deaths were unrelated to the trial.
M72/AS01E presents a clinically acceptable safety profile and significantly reduces bacteriologically confirmed pulmonary TB disease incidence in HIV– adults with latent Mtb infection.
Funding. BMGF; Aeras; DFID UK; DGIS; AusAID; GlaxoSmithKline Biologicals SA.
O. Van Der Meeren, GSK: Employee and Shareholder, Salary. M. Hatherill, Aeras: Investigator, Research grant. R. J. Wilkinson, GSK: Grant Investigator, indirect funding. H. M. Ayles, GSK: Grant Investigator, Research grant. G. Henostroza, Aeras: Investigator, Grant recipient. M. A. Demoitié, GSK: owns stocks and is named inventor on patent applications relating to certain uses of M72/AS01E, Salary. T. Singh, GSK: Employee and Shareholder, Salary. E. J. Akite, GSK: Employee, Salary. A. K. Azam, GSK: Employee, Salary. A. Bollaerts, GSK: Employee, Salary. A. M. Ginsberg, GSK: Collaborator, Research support. BMGF: Grant Investigator, Grant recipient. UK DFID: Grant Investigator, Grant recipient. P. Gillard, GSK: Employee and Shareholder, Salary and stock. D. R. Tait, Aeras: Employee, Salary. GSK: Shareholder, Salary.
Session: 32. Tuberculosis and other Mycobacterial Infections
Thursday, October 4, 2018: 8:45 AM
- polymerase chain reaction
- hiv
- influenza
- pulmonary tuberculosis
- fatigue
- headache
- adult
- africa, eastern
- biological products
- fusion proteins
- demography
- disclosure
- follow-up
- mycobacterium tuberculosis
- pain
- safety
- tuberculosis
- vaccines
- infections
- latent tuberculosis
- sensitivity analysis
- injection site reactions
- adverse event
- patents
- salary and wages
- employee
- immunogenicity





Comments