-
PDF
- Split View
-
Views
-
Cite
Cite
Myrto Eleni Flokas, Nikolaos Andreatos, Michail Alevizakos, Alireza Kalbasi, Pelin Onur, Eleftherios Mylonakis, Inappropriate Management of Asymptomatic Patients With Positive Urine Cultures: A Systematic Review and Meta-analysis, Open Forum Infectious Diseases, Volume 4, Issue 4, Fall 2017, ofx207, https://doi.org/10.1093/ofid/ofx207
Close - Share Icon Share
Abstract
Mismanagement of asymptomatic patients with positive urine cultures (referred to as asymptomatic bacteriuria [ASB] in the literature) promotes antimicrobial resistance and results in unnecessary antimicrobial-related adverse events and increased health care costs.
We conducted a systematic review and meta-analysis of studies that reported on the rate of inappropriate ASB treatment published from 2004 to August 2016. The appropriateness of antimicrobial administration was based on guidelines published by the Infectious Diseases Society of America.
A total of 2142 nonduplicate articles were identified, and among them 30 fulfilled our inclusion criteria. The pooled prevalence of antimicrobial treatment among 4129 cases who did not require treatment was 45% (95% CI, 39–50). Isolation of gram-negative pathogens (odds ratio [OR], 3.58; 95% CI, 2.12–6.06), pyuria (OR, 2.83; 95% CI, 1.9–4.22), nitrite positivity (OR, 3.83; 95% CI, 2.24–6.54), and female sex (OR, 2.11; 95% CI, 1.46–3.06) increased the odds of receiving treatment. The rates of treatment were higher in studies with ≥100 000 cfu/mL cutoff values compared with <10 000 cfu/mL for bacterial growth (P, .011). The implementation of educational and organizational interventions designed to eliminate the overtreatment of ASB resulted in a median absolute risk reduction of 33% (rangeARR, 16–36%, medianRRR, 53%; rangeRRR, 25–80%).
The mismanagement of ASB remains extremely frequent. Female sex and the overinterpretation of certain laboratory data (positive nitrites, pyuria, isolation of gram-negative bacteria and cultures with higher microbial count) are associated with overtreatment. Even simple stewardship interventions can be particularly effective, and antimicrobial stewardship programs should focus on the challenge of differentiating true urinary tract infection from ASB.
A positive urine culture for bacteria or fungi, in the absence of symptoms (reported in the literature simply as asymptomatic bacteriuria [ASB] for reasons of convenience [1]) is a common clinical finding, especially among the inpatient population, the elderly, and patients with indwelling urinary catheters [2]. Antimicrobial therapy has no role in the majority of cases of ASB, and withholding treatment has no effect in mortality or renal function [2, 3]. Moreover, overtreatment of ASB may result in a number of undesirable outcomes, such as disturbance of the intestinal flora that increases the risk for Clostridium difficile infection, antibiotic resistance, and increased health care–associated costs [4, 5]. Furthermore, treatment of ASB may eliminate low-virulence strains that suppress the development of uropathogens, thus counterintuitively promoting the development of symptomatic urinary tract infections (UTIs) [6, 7].
Responding to this challenge, the Infectious Diseases Society of America (IDSA) released evidence-based recommendations to guide health care professionals in their approach to ASB [8]. In spite of these recommendations, ASB remains one of the most common causes of antimicrobial overprescription in both acute [9] and long-term care [10]. For example, in a recent prospective study conducted among hospitalized patients, overtreatment of ASB contributed 17% of total antimicrobial overprescriptions [9]. Nonetheless, the cumulative frequency of ASB overtreatment across the spectrum of care has not been well studied. The aim of this systematic review and meta-analysis was to estimate the overall prevalence of ASB overtreatment. We also identified factors associated with increased odds of overtreatment, as well as quantified the efficacy of initiatives aimed at improving ASB management.
METHODS
This study was conducted according to the PRISMA checklist [11].
Data Sources and Search
Three researchers (MF, AK, PO) searched the PubMed and EMBASE databases from 2004 up to August 24, 2016, using the term “{asymptomatic AND (bacteriuria OR UTI OR (urinary tract infection)]} AND (treatment or therapy or antimicrobial or antibacterial or antibiotic).” We screened citations by abstract and title and only assessed publications in English, French, or Spanish. After the initial selection of eligible studies, their references were also reviewed. We also contacted the study authors as needed to obtain clarifications or additional information that could facilitate our analysis. Case series, case reports, and conference abstracts were not considered for inclusion in our analysis.
Study Selection
We elected to begin our search for eligible studies published in 2004, as this was the year when the IDSA guidelines on ASB management [8] were accepted for publication, a clear indication that at that time enough published evidence existed to support the withholding of treatment in ASB cases. Some of the studies included cases from prior to 2004, and this allowed us to compare the management of ASB before and after the publication of the IDSA guidelines.
To be considered for inclusion, studies had to report the total number of ASB cases that did not warrant antimicrobial treatment according to the IDSA guidelines, as well as the number of cases that were inappropriately treated. Furthermore, we required that eligible studies clarify whether the antimicrobial treatment was specifically administered for the management of ASB and not for a different indication. Studies in which patients with ASB were treated within the context of a research protocol were excluded. Studies performed in populations undergoing surgical procedures with implantation of prostheses, solid organ transplant recipients, and patients with neobladders were also excluded, as no specific recommendations on these subpopulations are offered by the IDSA guidelines [8]. In studies that reported on interventions aimed at reducing ASB overtreatment, only the baseline period before the intervention was considered.
Definitions
Asymptomatic bacteriuria was defined as the isolation of cultivatable microorganisms without the presence of symptoms and signs suggestive of UTI. For convenience, and in accordance with previous reports [1], the term “bacteriuria” was used to describe the presence of bacteria or fungi throughout the manuscript, as funguria cases are commonly included in previously published analyses and could not be studied separately as no distinct rates were provided [1, 12–14]. Instead, as detailed below, we performed a subgroup analysis without the studies that included funguria cases.
We did not employ specific cutoffs for clinically significant growth or impose restrictions on the number of organisms isolated. This broad definition was employed to ensure that inappropriate treatment of ASB was adequately captured, regardless of whether the ASB case in question stemmed from colonization or sample contamination. Patients with bacteriuria and symptoms/signs suggestive of infection were only considered to have ASB when the signs/symptoms could be clearly attributed to a different cause. In line with the IDSA recommendations [8], antimicrobial treatment of ASB was considered appropriate for pregnant patients and patients undergoing urologic procedures with a high likelihood of bleeding. For our secondary analysis, pyuria was defined as the presence of at least 5 white blood cells (WBC)/high-powered field (HPF) whereas hematuria was defined as the presence of at least 10 red blood cells (RBC)/HPF.
Data Extraction and Quality Assessment
Our primary outcome of interest was the rate of inappropriate treatment of ASB and was calculated by dividing the number of ASB cases in which antimicrobial therapy was inappropriately prescribed by the total number of ASB cases in which no treatment was warranted, according to the IDSA guidelines [8]. As a secondary outcome, we sought to identify factors that were associated with ASB overtreatment in the included study cohorts.
Two researchers (MF, AK) extracted data independently; discrepancies between them were resolved by consensus, and data are summarized in Table 1 and Supplementary Table 2. The methodological quality of eligible studies was assessed with the Newcastle–Ottawa Quality Assessment Scale (NOS) (see Supplementary Table 1) [15]. For each study parameter that met adequate quality standards, a star was awarded to the study. Studies that were judged to be of adequate quality in all 6 of the examined study parameters were awarded a maximum of 6 stars. An NOS rating of 5 stars or higher was considered adequate for the purposes of our analysis.
Characteristics of Included Studies: Study Midyear and Country, Design, Number of ASB Cases That Were Treated Inappropriately, Number of ASB Cases That Did Not Require Treatment, Prevalence of Overtreatment, Study Setting, Cutoff Used for Screening, Age and Sex of Participants
| Author (Year) . | Country . | Design . | ASB Treateda . | No. of ASBa . | Rate of Inappropriate Treatment of ASB . | Settingb,c . | Cutoff, cfuc . | Age, yc,d . | Sexc . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chiu [24] (2010) | Canada | Retrospective | 23 | 30 | 76.67 | Inpatients | Tertiary care teaching hospital | None | ≥18 | NR |
| Dalen [25] (2003) | Canada | Prospective | 15 | 29 | 51.72 | Inpatients | 1000-bed teaching hospital, 138-bed hospital | 100 000 000; 1 000 000 for fungi | Mean, 77.8; range, 52–94 | NR |
| Irfan [13] (2012) | Canada | Prospective | 93 | 160 | 58.13 | Inpatients | 2 academic, tertiary acute care hospitals | 100 000 | Mean, 74.1; SD, 12.8 | Female 71.3% |
| Leis [34] (2012) | Canada | Prospective | 12 | 21 | 57.14 | Inpatients | 2 acute care teaching hospitals | NR | ≥18 | NR |
| Leis [33] (2013) | Canada | Prospective | 26 | 57 | 45.61 | Inpatients | Acute care teaching hospital | NR | ≥18 | NR |
| Silver [40] (2006) | Canada | Prospective | 43 | 67 | 64.18 | Inpatients | 1100-bed university-affiliated teaching hospital | 100 000 | ≥18 | Female 55% |
| Winten-berger [42] (2013) | Canada | Prospective | 15 | 31 | 48.39 | Inpatients | Medicine and surgery wards, noncatheterized | NR | ≥18 | NR |
| Al Raiy [44] (2005) | USA | Retrospective | 25 | 43 | 58.14 | Inpatients | ICU, 772-bed teaching hospital | 100 000 | ≥18 | NR |
| Chowdhury [4] (2010) | USA | Retrospective | 30 | 64 | 46.88 | Inpatients and nursing home residents | University-associated hospital | 100 000, 100 for catheterized* | ≥18 | Female 68% |
| Cope [1] (2007) | USA | Retrospective | 53 | 164 | 32.32 | Inpatients | Tertiary care academic hospital, catheterized | 10 000 | Median, 67; range, 21–88 | Male 96% |
| Drekonja [26] (2011) | USA | Prospective | 8 | 45 | 17.78 | Inpatients | Veterans Affairs Medical Center | None | ≥18 | NR |
| Gau [27] (2004) | USA | Retrospective | 25 | 50 | 50.00 | Inpatients | Community hospital | 50 000 | ≥65; mean, 83; SD, 8 | Female 84% |
| Grein [21] (2014) | USA | Retrospective | 62 | 164 | 37.8 | Inpatients | 900-bed tertiary care academic teaching hospital, 550-bed county hospital, 389-bed community hospital | 1000 | >18 | Female 67% |
| Hartley [28] (2008) | USA | Retrospective | 60 | 94 | 63.83 | Inpatients | University-affiliated hospital | NR | ≥18 | NR |
| Heintz [14] (2010) | USA | Retrospective | 33 | 155 | 21.29 | Inpatients | University- affiliated hospital VRE positive | None | >18 | NR |
| Kelley [30] (2012) | USA | Retrospective | 66 | 107 | 61.68 | ED and inpatients | Academic medical center | 10 000 | >18 | Female 78.5% |
| Khair [31] (2011) | USA | Prospective | 46 | 119 | 38.66 | Inpatients | 1250-bed teaching hospital, Enterococci | 50 000; 5000 among catheterized* | Range, 17–96 | NR |
| Khawcha roenporn [32] (2008) | USA | Retrospective | 37 | 184 | 20.11 | ED | University medical center | 10 000 | Median, 59; range, 40–73 | Female 76% |
| Leuck [36] (2009) | USA | Prospective | 39 | 95 | 41.05 | Inpatients | Veterans Affair Medical Center | 100 000/None** | ≥18 | NR |
| Lin [37] (2009) | USA | Retrospective | 60 | 183 | 32.79 | All | 2 tertiary care, academic teaching hospitals Enterococci | 1000 | NR | Male 61% |
| Al Mohajer [38] (2009) | USA | Retrospective | 101 | 326 | 30.98 | All | Veterans Affair Medical Center, S. aureus | 10 000*** | Mean 66.2 | Male >90% |
| Trautner [41] (2011) | USA | Prospective | 167 | 488 | 34.22 | Inpatients | 3 medicine wards and 2 long-term care wards, Veterans Health Care System, catheterized | 1000 | ≥18 | NR |
| Zabarsky [43] (2003) | USA | Prospective | 23 | 34 | 67.65 | Nursing home residents | Veterans Affairs Medical Center, long-term care facility | 100 000 | Median, 70; range, 42–98 | Male 100% |
| Lepeule [35] (2011) | France | Prospective | 19 | 117 | 16.24 | Inpatients | 13 hospitals ESBL positive | NR | Median, 67; range, 1–92 | Female 63% |
| Saurel [39] (2005) | France | Retrospective | 61 | 108 | 56.48 | Inpatients | Medicine and surgery departments, university hospital | 10 000 | ≥18 | Female 63% |
| Pavese [45] (2005) | France | Prospective | 41 | 63 | 65.08 | Inpatients | University-associated hospital | 10 000 | ≥18 | Female 60% |
| Cai [23] (2011) | Italy | Retrospective | 361 | 699 | 51.65 | Outpatients | Department of urology, tertiary care and regional hospitals | 100 000 | ≥18 | Female 100% |
| Hermida Perez [29] (1999) | Spain | Prospective | 44 | 88 | 50 | Outpatients | NR | 100 000 | ≥14 | Female 100% |
| Lee [12] (2011) | Korea | Retrospective | 70 | 219 | 31.96 | Inpatients | 900-bed university-affiliated tertiary care hospital | 100 000 | ≥18 | Female 73.51% |
| Blakiston [22] (2011) | New Zealand | Retrospective | 57 | 125 | 45.6 | Inpatients | Over-65 rehabilitation ward of a secondary level care hospital | 1000 | ≥65 | NR |
| Author (Year) . | Country . | Design . | ASB Treateda . | No. of ASBa . | Rate of Inappropriate Treatment of ASB . | Settingb,c . | Cutoff, cfuc . | Age, yc,d . | Sexc . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chiu [24] (2010) | Canada | Retrospective | 23 | 30 | 76.67 | Inpatients | Tertiary care teaching hospital | None | ≥18 | NR |
| Dalen [25] (2003) | Canada | Prospective | 15 | 29 | 51.72 | Inpatients | 1000-bed teaching hospital, 138-bed hospital | 100 000 000; 1 000 000 for fungi | Mean, 77.8; range, 52–94 | NR |
| Irfan [13] (2012) | Canada | Prospective | 93 | 160 | 58.13 | Inpatients | 2 academic, tertiary acute care hospitals | 100 000 | Mean, 74.1; SD, 12.8 | Female 71.3% |
| Leis [34] (2012) | Canada | Prospective | 12 | 21 | 57.14 | Inpatients | 2 acute care teaching hospitals | NR | ≥18 | NR |
| Leis [33] (2013) | Canada | Prospective | 26 | 57 | 45.61 | Inpatients | Acute care teaching hospital | NR | ≥18 | NR |
| Silver [40] (2006) | Canada | Prospective | 43 | 67 | 64.18 | Inpatients | 1100-bed university-affiliated teaching hospital | 100 000 | ≥18 | Female 55% |
| Winten-berger [42] (2013) | Canada | Prospective | 15 | 31 | 48.39 | Inpatients | Medicine and surgery wards, noncatheterized | NR | ≥18 | NR |
| Al Raiy [44] (2005) | USA | Retrospective | 25 | 43 | 58.14 | Inpatients | ICU, 772-bed teaching hospital | 100 000 | ≥18 | NR |
| Chowdhury [4] (2010) | USA | Retrospective | 30 | 64 | 46.88 | Inpatients and nursing home residents | University-associated hospital | 100 000, 100 for catheterized* | ≥18 | Female 68% |
| Cope [1] (2007) | USA | Retrospective | 53 | 164 | 32.32 | Inpatients | Tertiary care academic hospital, catheterized | 10 000 | Median, 67; range, 21–88 | Male 96% |
| Drekonja [26] (2011) | USA | Prospective | 8 | 45 | 17.78 | Inpatients | Veterans Affairs Medical Center | None | ≥18 | NR |
| Gau [27] (2004) | USA | Retrospective | 25 | 50 | 50.00 | Inpatients | Community hospital | 50 000 | ≥65; mean, 83; SD, 8 | Female 84% |
| Grein [21] (2014) | USA | Retrospective | 62 | 164 | 37.8 | Inpatients | 900-bed tertiary care academic teaching hospital, 550-bed county hospital, 389-bed community hospital | 1000 | >18 | Female 67% |
| Hartley [28] (2008) | USA | Retrospective | 60 | 94 | 63.83 | Inpatients | University-affiliated hospital | NR | ≥18 | NR |
| Heintz [14] (2010) | USA | Retrospective | 33 | 155 | 21.29 | Inpatients | University- affiliated hospital VRE positive | None | >18 | NR |
| Kelley [30] (2012) | USA | Retrospective | 66 | 107 | 61.68 | ED and inpatients | Academic medical center | 10 000 | >18 | Female 78.5% |
| Khair [31] (2011) | USA | Prospective | 46 | 119 | 38.66 | Inpatients | 1250-bed teaching hospital, Enterococci | 50 000; 5000 among catheterized* | Range, 17–96 | NR |
| Khawcha roenporn [32] (2008) | USA | Retrospective | 37 | 184 | 20.11 | ED | University medical center | 10 000 | Median, 59; range, 40–73 | Female 76% |
| Leuck [36] (2009) | USA | Prospective | 39 | 95 | 41.05 | Inpatients | Veterans Affair Medical Center | 100 000/None** | ≥18 | NR |
| Lin [37] (2009) | USA | Retrospective | 60 | 183 | 32.79 | All | 2 tertiary care, academic teaching hospitals Enterococci | 1000 | NR | Male 61% |
| Al Mohajer [38] (2009) | USA | Retrospective | 101 | 326 | 30.98 | All | Veterans Affair Medical Center, S. aureus | 10 000*** | Mean 66.2 | Male >90% |
| Trautner [41] (2011) | USA | Prospective | 167 | 488 | 34.22 | Inpatients | 3 medicine wards and 2 long-term care wards, Veterans Health Care System, catheterized | 1000 | ≥18 | NR |
| Zabarsky [43] (2003) | USA | Prospective | 23 | 34 | 67.65 | Nursing home residents | Veterans Affairs Medical Center, long-term care facility | 100 000 | Median, 70; range, 42–98 | Male 100% |
| Lepeule [35] (2011) | France | Prospective | 19 | 117 | 16.24 | Inpatients | 13 hospitals ESBL positive | NR | Median, 67; range, 1–92 | Female 63% |
| Saurel [39] (2005) | France | Retrospective | 61 | 108 | 56.48 | Inpatients | Medicine and surgery departments, university hospital | 10 000 | ≥18 | Female 63% |
| Pavese [45] (2005) | France | Prospective | 41 | 63 | 65.08 | Inpatients | University-associated hospital | 10 000 | ≥18 | Female 60% |
| Cai [23] (2011) | Italy | Retrospective | 361 | 699 | 51.65 | Outpatients | Department of urology, tertiary care and regional hospitals | 100 000 | ≥18 | Female 100% |
| Hermida Perez [29] (1999) | Spain | Prospective | 44 | 88 | 50 | Outpatients | NR | 100 000 | ≥14 | Female 100% |
| Lee [12] (2011) | Korea | Retrospective | 70 | 219 | 31.96 | Inpatients | 900-bed university-affiliated tertiary care hospital | 100 000 | ≥18 | Female 73.51% |
| Blakiston [22] (2011) | New Zealand | Retrospective | 57 | 125 | 45.6 | Inpatients | Over-65 rehabilitation ward of a secondary level care hospital | 1000 | ≥65 | NR |
Abbreviations: ASB, asymptomatic bacteriuria; ED, emergency department; ESBL, extended-spectrum beta-lactamases; ICU, intensive care unit; NR, not reported; S. aureus, Staphylococcus Aureus; UTI: irinary tract infections; VRE: vancomycin-resistant enterococcus. *The lower count was used as a cut-off for the purposes of the respective sub-analysis. **Only the portion of the cohort that used the 100 000 cfu/mL cut-off was included in the respective sub-analysis. In all other cases, the full cohort was used. ***The majority of the cohort used this cut-off, so it was employed for the respective sub-analysis.
aAsymptomatic bacteriuria cases (not requiring treatment).
bED, ESBL, ICU, S. aureus, UTI, VRE.
cNot reported.
dStandard deviation.
Characteristics of Included Studies: Study Midyear and Country, Design, Number of ASB Cases That Were Treated Inappropriately, Number of ASB Cases That Did Not Require Treatment, Prevalence of Overtreatment, Study Setting, Cutoff Used for Screening, Age and Sex of Participants
| Author (Year) . | Country . | Design . | ASB Treateda . | No. of ASBa . | Rate of Inappropriate Treatment of ASB . | Settingb,c . | Cutoff, cfuc . | Age, yc,d . | Sexc . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chiu [24] (2010) | Canada | Retrospective | 23 | 30 | 76.67 | Inpatients | Tertiary care teaching hospital | None | ≥18 | NR |
| Dalen [25] (2003) | Canada | Prospective | 15 | 29 | 51.72 | Inpatients | 1000-bed teaching hospital, 138-bed hospital | 100 000 000; 1 000 000 for fungi | Mean, 77.8; range, 52–94 | NR |
| Irfan [13] (2012) | Canada | Prospective | 93 | 160 | 58.13 | Inpatients | 2 academic, tertiary acute care hospitals | 100 000 | Mean, 74.1; SD, 12.8 | Female 71.3% |
| Leis [34] (2012) | Canada | Prospective | 12 | 21 | 57.14 | Inpatients | 2 acute care teaching hospitals | NR | ≥18 | NR |
| Leis [33] (2013) | Canada | Prospective | 26 | 57 | 45.61 | Inpatients | Acute care teaching hospital | NR | ≥18 | NR |
| Silver [40] (2006) | Canada | Prospective | 43 | 67 | 64.18 | Inpatients | 1100-bed university-affiliated teaching hospital | 100 000 | ≥18 | Female 55% |
| Winten-berger [42] (2013) | Canada | Prospective | 15 | 31 | 48.39 | Inpatients | Medicine and surgery wards, noncatheterized | NR | ≥18 | NR |
| Al Raiy [44] (2005) | USA | Retrospective | 25 | 43 | 58.14 | Inpatients | ICU, 772-bed teaching hospital | 100 000 | ≥18 | NR |
| Chowdhury [4] (2010) | USA | Retrospective | 30 | 64 | 46.88 | Inpatients and nursing home residents | University-associated hospital | 100 000, 100 for catheterized* | ≥18 | Female 68% |
| Cope [1] (2007) | USA | Retrospective | 53 | 164 | 32.32 | Inpatients | Tertiary care academic hospital, catheterized | 10 000 | Median, 67; range, 21–88 | Male 96% |
| Drekonja [26] (2011) | USA | Prospective | 8 | 45 | 17.78 | Inpatients | Veterans Affairs Medical Center | None | ≥18 | NR |
| Gau [27] (2004) | USA | Retrospective | 25 | 50 | 50.00 | Inpatients | Community hospital | 50 000 | ≥65; mean, 83; SD, 8 | Female 84% |
| Grein [21] (2014) | USA | Retrospective | 62 | 164 | 37.8 | Inpatients | 900-bed tertiary care academic teaching hospital, 550-bed county hospital, 389-bed community hospital | 1000 | >18 | Female 67% |
| Hartley [28] (2008) | USA | Retrospective | 60 | 94 | 63.83 | Inpatients | University-affiliated hospital | NR | ≥18 | NR |
| Heintz [14] (2010) | USA | Retrospective | 33 | 155 | 21.29 | Inpatients | University- affiliated hospital VRE positive | None | >18 | NR |
| Kelley [30] (2012) | USA | Retrospective | 66 | 107 | 61.68 | ED and inpatients | Academic medical center | 10 000 | >18 | Female 78.5% |
| Khair [31] (2011) | USA | Prospective | 46 | 119 | 38.66 | Inpatients | 1250-bed teaching hospital, Enterococci | 50 000; 5000 among catheterized* | Range, 17–96 | NR |
| Khawcha roenporn [32] (2008) | USA | Retrospective | 37 | 184 | 20.11 | ED | University medical center | 10 000 | Median, 59; range, 40–73 | Female 76% |
| Leuck [36] (2009) | USA | Prospective | 39 | 95 | 41.05 | Inpatients | Veterans Affair Medical Center | 100 000/None** | ≥18 | NR |
| Lin [37] (2009) | USA | Retrospective | 60 | 183 | 32.79 | All | 2 tertiary care, academic teaching hospitals Enterococci | 1000 | NR | Male 61% |
| Al Mohajer [38] (2009) | USA | Retrospective | 101 | 326 | 30.98 | All | Veterans Affair Medical Center, S. aureus | 10 000*** | Mean 66.2 | Male >90% |
| Trautner [41] (2011) | USA | Prospective | 167 | 488 | 34.22 | Inpatients | 3 medicine wards and 2 long-term care wards, Veterans Health Care System, catheterized | 1000 | ≥18 | NR |
| Zabarsky [43] (2003) | USA | Prospective | 23 | 34 | 67.65 | Nursing home residents | Veterans Affairs Medical Center, long-term care facility | 100 000 | Median, 70; range, 42–98 | Male 100% |
| Lepeule [35] (2011) | France | Prospective | 19 | 117 | 16.24 | Inpatients | 13 hospitals ESBL positive | NR | Median, 67; range, 1–92 | Female 63% |
| Saurel [39] (2005) | France | Retrospective | 61 | 108 | 56.48 | Inpatients | Medicine and surgery departments, university hospital | 10 000 | ≥18 | Female 63% |
| Pavese [45] (2005) | France | Prospective | 41 | 63 | 65.08 | Inpatients | University-associated hospital | 10 000 | ≥18 | Female 60% |
| Cai [23] (2011) | Italy | Retrospective | 361 | 699 | 51.65 | Outpatients | Department of urology, tertiary care and regional hospitals | 100 000 | ≥18 | Female 100% |
| Hermida Perez [29] (1999) | Spain | Prospective | 44 | 88 | 50 | Outpatients | NR | 100 000 | ≥14 | Female 100% |
| Lee [12] (2011) | Korea | Retrospective | 70 | 219 | 31.96 | Inpatients | 900-bed university-affiliated tertiary care hospital | 100 000 | ≥18 | Female 73.51% |
| Blakiston [22] (2011) | New Zealand | Retrospective | 57 | 125 | 45.6 | Inpatients | Over-65 rehabilitation ward of a secondary level care hospital | 1000 | ≥65 | NR |
| Author (Year) . | Country . | Design . | ASB Treateda . | No. of ASBa . | Rate of Inappropriate Treatment of ASB . | Settingb,c . | Cutoff, cfuc . | Age, yc,d . | Sexc . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chiu [24] (2010) | Canada | Retrospective | 23 | 30 | 76.67 | Inpatients | Tertiary care teaching hospital | None | ≥18 | NR |
| Dalen [25] (2003) | Canada | Prospective | 15 | 29 | 51.72 | Inpatients | 1000-bed teaching hospital, 138-bed hospital | 100 000 000; 1 000 000 for fungi | Mean, 77.8; range, 52–94 | NR |
| Irfan [13] (2012) | Canada | Prospective | 93 | 160 | 58.13 | Inpatients | 2 academic, tertiary acute care hospitals | 100 000 | Mean, 74.1; SD, 12.8 | Female 71.3% |
| Leis [34] (2012) | Canada | Prospective | 12 | 21 | 57.14 | Inpatients | 2 acute care teaching hospitals | NR | ≥18 | NR |
| Leis [33] (2013) | Canada | Prospective | 26 | 57 | 45.61 | Inpatients | Acute care teaching hospital | NR | ≥18 | NR |
| Silver [40] (2006) | Canada | Prospective | 43 | 67 | 64.18 | Inpatients | 1100-bed university-affiliated teaching hospital | 100 000 | ≥18 | Female 55% |
| Winten-berger [42] (2013) | Canada | Prospective | 15 | 31 | 48.39 | Inpatients | Medicine and surgery wards, noncatheterized | NR | ≥18 | NR |
| Al Raiy [44] (2005) | USA | Retrospective | 25 | 43 | 58.14 | Inpatients | ICU, 772-bed teaching hospital | 100 000 | ≥18 | NR |
| Chowdhury [4] (2010) | USA | Retrospective | 30 | 64 | 46.88 | Inpatients and nursing home residents | University-associated hospital | 100 000, 100 for catheterized* | ≥18 | Female 68% |
| Cope [1] (2007) | USA | Retrospective | 53 | 164 | 32.32 | Inpatients | Tertiary care academic hospital, catheterized | 10 000 | Median, 67; range, 21–88 | Male 96% |
| Drekonja [26] (2011) | USA | Prospective | 8 | 45 | 17.78 | Inpatients | Veterans Affairs Medical Center | None | ≥18 | NR |
| Gau [27] (2004) | USA | Retrospective | 25 | 50 | 50.00 | Inpatients | Community hospital | 50 000 | ≥65; mean, 83; SD, 8 | Female 84% |
| Grein [21] (2014) | USA | Retrospective | 62 | 164 | 37.8 | Inpatients | 900-bed tertiary care academic teaching hospital, 550-bed county hospital, 389-bed community hospital | 1000 | >18 | Female 67% |
| Hartley [28] (2008) | USA | Retrospective | 60 | 94 | 63.83 | Inpatients | University-affiliated hospital | NR | ≥18 | NR |
| Heintz [14] (2010) | USA | Retrospective | 33 | 155 | 21.29 | Inpatients | University- affiliated hospital VRE positive | None | >18 | NR |
| Kelley [30] (2012) | USA | Retrospective | 66 | 107 | 61.68 | ED and inpatients | Academic medical center | 10 000 | >18 | Female 78.5% |
| Khair [31] (2011) | USA | Prospective | 46 | 119 | 38.66 | Inpatients | 1250-bed teaching hospital, Enterococci | 50 000; 5000 among catheterized* | Range, 17–96 | NR |
| Khawcha roenporn [32] (2008) | USA | Retrospective | 37 | 184 | 20.11 | ED | University medical center | 10 000 | Median, 59; range, 40–73 | Female 76% |
| Leuck [36] (2009) | USA | Prospective | 39 | 95 | 41.05 | Inpatients | Veterans Affair Medical Center | 100 000/None** | ≥18 | NR |
| Lin [37] (2009) | USA | Retrospective | 60 | 183 | 32.79 | All | 2 tertiary care, academic teaching hospitals Enterococci | 1000 | NR | Male 61% |
| Al Mohajer [38] (2009) | USA | Retrospective | 101 | 326 | 30.98 | All | Veterans Affair Medical Center, S. aureus | 10 000*** | Mean 66.2 | Male >90% |
| Trautner [41] (2011) | USA | Prospective | 167 | 488 | 34.22 | Inpatients | 3 medicine wards and 2 long-term care wards, Veterans Health Care System, catheterized | 1000 | ≥18 | NR |
| Zabarsky [43] (2003) | USA | Prospective | 23 | 34 | 67.65 | Nursing home residents | Veterans Affairs Medical Center, long-term care facility | 100 000 | Median, 70; range, 42–98 | Male 100% |
| Lepeule [35] (2011) | France | Prospective | 19 | 117 | 16.24 | Inpatients | 13 hospitals ESBL positive | NR | Median, 67; range, 1–92 | Female 63% |
| Saurel [39] (2005) | France | Retrospective | 61 | 108 | 56.48 | Inpatients | Medicine and surgery departments, university hospital | 10 000 | ≥18 | Female 63% |
| Pavese [45] (2005) | France | Prospective | 41 | 63 | 65.08 | Inpatients | University-associated hospital | 10 000 | ≥18 | Female 60% |
| Cai [23] (2011) | Italy | Retrospective | 361 | 699 | 51.65 | Outpatients | Department of urology, tertiary care and regional hospitals | 100 000 | ≥18 | Female 100% |
| Hermida Perez [29] (1999) | Spain | Prospective | 44 | 88 | 50 | Outpatients | NR | 100 000 | ≥14 | Female 100% |
| Lee [12] (2011) | Korea | Retrospective | 70 | 219 | 31.96 | Inpatients | 900-bed university-affiliated tertiary care hospital | 100 000 | ≥18 | Female 73.51% |
| Blakiston [22] (2011) | New Zealand | Retrospective | 57 | 125 | 45.6 | Inpatients | Over-65 rehabilitation ward of a secondary level care hospital | 1000 | ≥65 | NR |
Abbreviations: ASB, asymptomatic bacteriuria; ED, emergency department; ESBL, extended-spectrum beta-lactamases; ICU, intensive care unit; NR, not reported; S. aureus, Staphylococcus Aureus; UTI: irinary tract infections; VRE: vancomycin-resistant enterococcus. *The lower count was used as a cut-off for the purposes of the respective sub-analysis. **Only the portion of the cohort that used the 100 000 cfu/mL cut-off was included in the respective sub-analysis. In all other cases, the full cohort was used. ***The majority of the cohort used this cut-off, so it was employed for the respective sub-analysis.
aAsymptomatic bacteriuria cases (not requiring treatment).
bED, ESBL, ICU, S. aureus, UTI, VRE.
cNot reported.
dStandard deviation.
Data Analysis
The meta-analysis was conducted using the random-effects model, proposed by DerSimonian and Laird, to estimate the pooled rates and 95% confidence intervals of the primary outcome [16]. The Freeman-Tukey arcsine transformation was employed to ensure the inclusion of studies that reported extreme rates (rates near 0 or 1) [17]. The tau-squared statistic was calculated to assess the heterogeneity between the studies, and possible sources of heterogeneity were further explored by meta-regression analysis (Knapp and Hartung model) [18]. Using this methodology, we performed a temporal sensitivity analysis that included only the studies performed in 2006 and after to assess the effect of the introduction of the IDSA guidelines on the reported rates. A subgroup analysis without the studies that included funguria cases was also conducted. We used Egger’s regression test (ET) as an indicator of small study effect [19]. A time-trend analysis was performed by transforming the model coefficients to rates and plotting them against the median year along with the reported prevalence rates [20].
We also performed a secondary analysis of the studies that reported on factors associated with ASB treatment. This secondary analysis only included factors that were assessed by a minimum of 3 studies [20]. Pooled relative effects were reported as unadjusted odds ratios (ORs) and 95% confidence intervals, calculated with the aid of the random-effects model. The heterogeneity among studies was further assessed with the tau-squared statistic. In studies that implemented interventions aimed at reducing ASB overtreatment, the absolute risk reduction (ARR) and relative risk reduction (RRR) that followed the intervention were calculated for each site and then summarized into median and range. To facilitate the presentation of our results, all percentages reported in the text were rounded up to the nearest integer.
Statistical analysis was conducted using the STATA v. 13 software package (STATA Corporation, College Station, TEXAS). Statistical significance was defined as a P value of less than .05.
RESULTS
A total of 2848 articles published from January 1, 2004, to August 24, 2016, were identified. Thirty studies were considered eligible and were incorporated into our analysis after quality evaluation. The selection process is depicted in Figure 1. The 30 studies included in our analysis reported on a total of 4129 ASB cases (Table 1) [1, 4, 12–14, 21–45]. The majority of studies were performed in North America (16 in the United States [1, 4, 14, 21, 26–28, 30–32, 36–38, 41, 43, 44] and 7 in Canada [13, 24, 25, 33, 34, 40, 42]) and Europe (3 in France [35, 39, 45], 1 in Italy [23], and 1 in Spain [29]). Finally, 1 study each was performed in New Zealand [22] and South Korea [12]. Contact with study authors of potentially relevant articles for additional data and a systematic search of article references failed to yield additional data and studies.
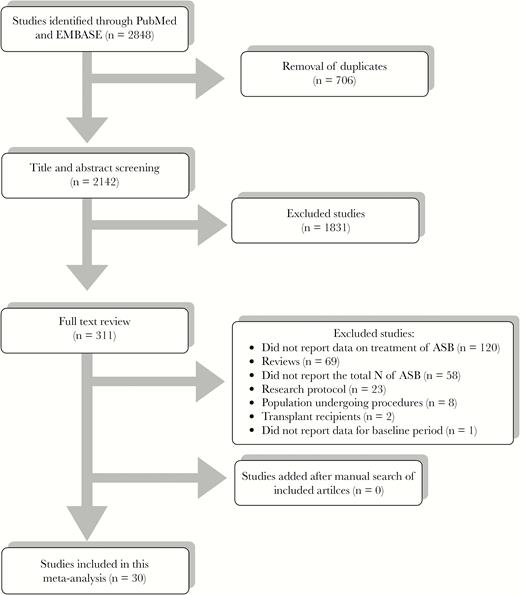
PRISMA flow diagram. Abbreviation: ASB, asymptomatic bacteriuria.
Remarkably, the pooled rate of inappropriate treatment was 45% (95% CI, 39–50; τ2, 0.08). Specifically, the rate of inappropriate treatment was 45% in North America (95% CI, 38–51; 40% in the United States, 58% in Canada) and 47% in Europe (95% CI, 32–62) (Figure 2). No small study effect across the included studies was detected (ΕΤ, 1.01; PET, 0.161). Twenty-two out of the 30 studies were performed in inpatient populations and had a rate of inappropriate treatment of 45% (95% CI, 38–51) [1, 12–14, 21, 22, 24–28, 31, 33–36, 39–42, 44, 45]. Two were performed in outpatient settings [23, 29], 1 in the emergency department [32], 1 among nursing home residents [43], and 4 in a mixture of settings [4, 30, 37, 38]. Five of the studies reported rates of inappropriate treatment among patients with indwelling urinary catheters, and their pooled prevalence was similar to the overall rate (44%; 95% CI, 34–55) [1, 24, 25, 36, 41].
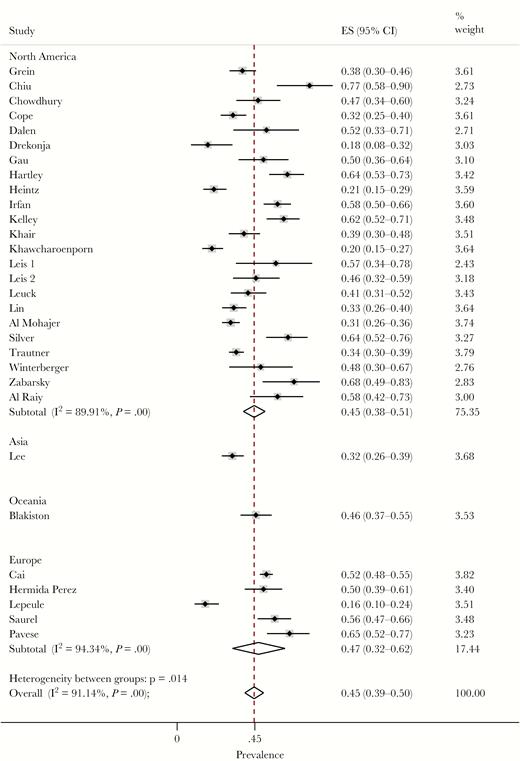
Forest plot of included studies. Rates of overtreatment of asymptomatic bacteriuria stratified by region. ES, effect size.
In a sensitivity analysis, we pooled the rates from studies that were performed in 2006 or later to estimate the effect of the introduction of the IDSA guidelines on inappropriate treatment rates. For the 23 studies that were performed in the years 2006–2014 [1, 4, 12–14, 21–24, 26, 28, 30–38, 40, 41], the pooled rate was 41% (95% CI, 35–47; τ2, 0.08), whereas for the 7 studies performed from 1996 to 2005 [25, 27, 29, 39, 43–45], it was 56% (95% CI, 52–61; τ2 < 0.001). This difference was statistically significant (coeff, 0.31; P, .028), however, we noted no significant time trend in the subanalysis for the years 2006–2014 (P, .885), indicating that the potential beneficial effect from the publication of structured guidelines might have reached a plateau.
As discussed in the “Methods,” we explored the impact of factors potentially associated with inappropriate ASB treatment. Our analysis demonstrated that patients with gram-negative isolates had a higher chance of receiving inappropriate treatment (OR, 3.58; 95% CI, 2.12–6.06; τ2, 0.08; ΕΤ, 9.48; PET, 0.131), based on 3 studies with 547 patients [1, 12, 21]. This was also the case for female patients (OR, 2.11; 95% CI, 1.46–3.06; τ2 < 0.001; ΕΤ, 0.9; PET, 0.608), based on 5 studies with 891 participants [1, 12, 13, 21, 32]. Patients with pyuria were also more likely to be treated inappropriately, based on 5 studies involving 865 participants (OR, 2.83; 95% CI, 1.9–4.22; τ2, 0.06; ΕΤ, 5.26; PET, 0.252) [12, 13, 31, 32, 37].
Regarding the impact of cutoffs used for detecting bacteriuria in the rate of inappropriate treatment, among the 30 included studies, 9 used a cutoff of ≥100 000 cfu/mL or higher, and these studies had a pooled prevalence of inappropriate treatment of 53% (95% CI, 46–61) [12, 13, 23, 25, 29, 36, 40, 43, 44]. Studies that used lower cutoffs (and thus lower bacterial counts) reported lower prevalence of inappropriate ASB treatment. Specifically, 7 studies that used cutoffs between 10 000 and 100 000 cfu/mL had a pooled prevalence of 44% (95% CI, 32–57) [1, 27, 30, 32, 38, 39, 45], while 6 studies that used a cutoff of <10 000 cfu/mL had a pooled prevalence of 38% (95% CI, 34–42) [4, 21, 22, 31, 37, 41]. Notably, the rate was significantly lower among studies that utilized lower cutoff values to define bacteriuria (coeff, –0.15; P, .035). A significant difference was noted while comparing the rates reported between studies using cut-offs <10 000 cfu/mL vs those using ≥100 000 cfu/mL (coeff, –0.29; P, .011). A sensitivity analysis that recalculated the rate excluding studies with funguria cases [1, 12–14, 25, 36] did not result in any difference (45%; 95% CI, 39–51; τ2, 0.09).
Based on 4 studies with 727 patients, positivity for nitrites in urinalysis also increased the risk of inappropriate administration of antimicrobial therapy (OR, 3.83; 95% CI, 2.24–6.54; τ2, 0.16; ΕΤ, –1.93; PET, 0.861) [12, 13, 21, 32]. Interestingly, the risk of inappropriate ASB treatment appeared similar among patients in surgical and nonsurgical units (OR, 1.04; 95% CI, 0.67–1.55) [12, 13, 21, 40], as well as among patients admitted to intensive care units (ICUs) and those in general hospital wards (OR, 0.67; 95% CI, 0.33–1.35) [12, 13, 21, 40]. Furthermore, no difference in the risk of inappropriate treatment was reported among diabetic vs nondiabetic patients (OR, 0.69; 95% CI, 0.39–1.22) [12, 13, 32], patients with indwelling urinary catheters vs noncatheterized patients (OR, 0.95; 95% CI, 0.58–1.54) [12, 13, 21, 22, 33, 44] or patients with hematuria vs those without hematuria (OR, 1.98; 95% CI, 0.76–5.16) [12, 13, 37].
Finally, among the 30 included studies, 8 reported on interventions to reduce the rate of inappropriate treatment of ASB from 2002 to 2013 (Supplementary Table 2). Five of these studies were performed in inpatient settings [13, 33, 41, 42, 45], 1 was performed in a nursing home [43], and 2 involved different settings [4, 30]. The duration of the baseline period varied from 1 to 12 months (treatment rates, 38–74%), whereas the intervention period varied from 2 to 30 months. Importantly, substantial improvements in the rate of inappropriate ASB treatment were reported after the implementation of these relatively simple measures (medianARR, 33%; rangeARR, 16–36%; medianRRR, 53%; rangeRRR, 25–80%). One study reported data on a 1-year maintenance period after the completion of the intervention; a sustained reduction was observed (29% maintenance vs 38% baseline treatment rate).
DISCUSSION
In the “Choosing Wisely Campaign,” the American Geriatrics Society and the American Board of Internal Medicine Foundation listed the unnecessary use of antimicrobials for ASB as one of the top 5 overused services [46]. We found that almost half of the ASB cases were managed inappropriately, a finding that justifies this level of concern, and the rate remained alarmingly high (40%), even after the introduction of the IDSA evidence-based recommendations. Women and patients with gram-negative bacteriuria had 2 and 4 times greater odds of being treated inappropriately, respectively. Also, overinterpretation of urinalysis that places undue emphasis on the presence of pyuria, nitrite positivity, and higher bacterial counts appears to drive inappropriate antimicrobial prescription for ASB. Interestingly, the challenge of reducing ASB overtreatment may be surmountable, as relatively simple educational and/or organizational interventions appear to reduce the incidence of inappropriate prescribing practices.
Challenges in the clinical differentiation between ASB and UTI may offer a partial explanation for the excessive overtreatment of ASB [47]. Of note, the presence of nonspecific signs and symptoms or comorbid conditions in such patients can be misleading [26, 47]. No association between these nonurinary indications and the presence or absence of bacteriuria has been suggested, and there is no evidence to justify treating bacteriuria in this context [48]. Another key aspect in understanding overtreatment is the undue reliance on the results of laboratory studies. For example, in a survey of physicians at a Swiss hospital, decision-making based on laboratory findings alone was the most commonly reported reason for overprescribing (reported by 17 out of 21 participants) [49]. This suggests that practitioners may decide to commence empiric treatment for UTIs based solely on the results of laboratory tests, without considering clinical data. Conversely, this overreliance on laboratory information may also explain why no association was found between the patient’s underlying condition as represented by the site of hospitalization (surgical floor, ICU) or the presence of diabetes and the rate of ASB overtreatment. It is possible that certain “danger” results in urinalysis (bacterial counts ≥100000, positive nitrites, pyuria, and the presence of gram-negative bacteria) are interpreted as strengthening the likelihood of a UTI diagnosis, while the patient’s history and clinical signs/symptoms may be given less consideration. However, as noted above, none of these laboratory findings has been shown to be specific for symptomatic UTI, and evidence-based recommendations discourage the use of antimicrobial agents for asymptomatic ASB in most cases, regardless of laboratory results [8].
Interestingly, among the 30 included studies, 8 implemented interventions aimed at reducing the rate of inappropriate treatment. Most of these initiatives were apparently successful and resulted in up to an 80% reduction in the inappropriate management of ASB. Our findings support that such successful interventions should aim at educating providers regarding the existing clinical guidelines [50], the natural history of ASB, and the diagnostic/therapeutic pitfalls associated with differentiating ASB cases from UTI [12]. Nonetheless, educational efforts are only part of the answer. For example, in a recent survey from a tertiary care hospital, almost 50% of the participants demonstrated a willingness to treat ASB despite knowing that this was not indicated by the IDSA guidelines [12]. Fortunately, relatively simple measures, such as didactic sessions and audit and feedback, are not only effective at reducing overtreatment, which is highlighted by the 25–80% reduction noted among the included studies, but also sustainable [41]. In 2 studies [33, 42], the introduction of the requirement that the physician should contact the microbiology lab by phone in order to acquire the results of urine cultures yielded a 37% and 75% reduction in prescribing, which underlines the role of strategies to promote appropriate ordering of urinary cultures. Specifically, it has been demonstrated that a considerable percentage of urine cultures ordered in everyday practice are not clinically indicated [34]. Importantly, unnecessary ordering of urine cultures not only contributes to inefficient utilization of laboratory resources and excess health care costs, but is also thought to drive inappropriate treatment of ASB [33, 34, 41, 51]. In fact, as demonstrated in a recent major study by Trautner et al. [41], initiatives aiming to restrict the inappropriate ordering of urine cultures significantly reduced the frequency of ASB overtreatment. As such, “urine culture stewardship” clearly has an important role to play in efforts to reduce inappropriate antibiotic prescription in the setting of ASB.
Regarding limitations of our analysis, most of the included studies were performed in inpatient populations in North America and Europe, and thus may not be applicable to other patient populations. Moreover, we were not able to estimate the effect of age on the treatment rates due to lack of uniformity in the reporting of relevant information. In addition, we were unable to perform a separate analysis of patients with spinal cord injury due to the paucity of relevant data. Future studies should investigate this question as it has been suggested that the IDSA guidelines [8] may not be widely implemented among patients with spinal cord injury [52] due to limited relevant evidence and the inherent diagnostic challenges presented by this patient group [52]. Furthermore, given the small number of studies reporting on factors associated with overtreatment, we were unable to perform a multivariate analysis to assess for possible confounders. It should also be noted that the reported lack of an association between the site of patient hospitalization (surgical floor, ICU) or the presence of diabetes and the rate of ASB overtreatment was based on a limited patient sample and may merely stem from reduced statistical power. Future studies are needed to confirm these findings. Regarding the estimation of the effect of interventions, we performed a descriptive analysis instead of pooling the effects due to the disparity between strategies applied by different institutions.
In conclusion, the present study highlights the considerable gap between evidence-based guidelines and contemporary clinical practice in the management of ASB. Undue emphasis on laboratory results, rather than on the patient’s clinical condition, appears to underlie most cases of ASB overtreatment. Evidence suggests that the challenges of differentiating symptomatic from asymptomatic bacteriuria and improving adherence to evidence-based guidelines can be overcome through a combination of educational and organizational interventions and that ASB should be a priority for antimicrobial stewardship programs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online (ofid.oxfordjournals.org). Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgements
Author contributions. Guarantor of the article: Drs. Flokas and Mylonakis accept full responsibility for the conduct of the study, have access to the data, and have control of the decision to publish. Myrto Eleni Flokas: Dr. Flokas conceptualized and designed the study, participated in data collection, extraction, and interpretation, performed the statistical analysis, prepared tables and figures, wrote and drafted the initial manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Nikolaos Andreatos: Dr. Andreatos conceptualized and designed the study, participated in data interpretation, drafted the initial manuscript, revised the manuscript according to the reviewers’ comments, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Michail Alevizakos: Dr. Alevizakos conceptualized and designed the study, participated in data interpretation, drafted the initial manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Alireza Kalbasi: Dr. Kalbasi participated in data collection, extraction, drafted the initial manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Pelin Onur: Dr. Onur participated in data collection, drafted the initial manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Eleftherios Mylonakis: Dr. Mylonakis conceptualized and designed the study, participated in data interpretation, reviewed and revised the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Potential conflicts of interest. All authors: no reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Authors contributed equally to this work.
- urinary tract infections
- drug resistance, microbial
- gram-negative bacteria
- health care costs
- nitrites
- pyuria
- knowledge acquisition
- guidelines
- pathogenic organism
- antimicrobials
- urine culture
- bacteriuria, asymptomatic
- appropriateness
- antimicrobial stewardship
- absolute risk reduction
- adverse event
- overtreatment
- infectious diseases society of america





Comments