-
PDF
- Split View
-
Views
-
Cite
Cite
Charitha Gowda, Craig W. Newcomb, Qing Liu, Dena M. Carbonari, James D. Lewis, Kimberly A. Forde, David S. Goldberg, K. Rajender Reddy, Jason A. Roy, Amy R. Marks, Jennifer L. Schneider, Jay R. Kostman, Janet P. Tate, Joseph K. Lim, Amy C. Justice, Matthew Bidwell Goetz, Douglas A. Corley, Vincent Lo Re, Risk of Acute Liver Injury With Antiretroviral Therapy by Viral Hepatitis Status, Open Forum Infectious Diseases, Volume 4, Issue 2, Spring 2017, ofx012, https://doi.org/10.1093/ofid/ofx012
Close - Share Icon Share
Abstract
The risk of hepatotoxicity with antiretroviral therapy (ART) remains unknown. We determined the comparative risk of acute liver injury (ALI) for antiretroviral drugs, classes, and regimens, by viral hepatitis status.
We followed a cohort of 10 083 human immunodeficiency virus (HIV)-infected persons in Kaiser Permanente Northern California (n = 2099) from 2004 to 2010 and the Veterans Aging Cohort Study (n = 7984) from 2004 to 2012. Within the first year of ART, we determined occurrence of (1) liver aminotransferases >200 U/L and (2) severe ALI (coagulopathy with hyperbilirubinemia). We used Cox regression to determine hazard ratios (HRs) with 95% confidence intervals (CIs) of endpoints among initiators of nucleos(t)ide analogue combinations, antiretroviral classes, and ART regimens, all stratified by viral hepatitis status.
Liver aminotransferases >200 U/L developed in 206 (2%) persons and occurred more frequently among HIV/viral hepatitis-coinfected than HIV-monoinfected persons (116.1 vs 20.7 events/1000 person-years; P < .001). No evidence of differential risk was found between initiators of abacavir/lamivudine versus tenofovir/emtricitabine among coinfected (HR, 0.68; 95% CI, .29–1.57) or HIV-monoinfected (HR, 1.19; 95% CI, .47–2.97) groups. Coinfected patients had a higher risk of aminotransferases >200 U/L after initiation with a protease inhibitor than nonnucleoside reverse-transcriptase inhibitor (HR, 2.01; 95% CI, 1.36–2.96). Severe ALI (30 events; 0.3%) occurred more frequently in coinfected persons (15.9 vs 3.1 events/1000 person-years; P < .001) but was too uncommon to evaluate in adjusted analyses.
Within the year after ART initiation, aminotransferase elevations were infrequently observed and rarely led to severe ALI. Protease inhibitor use was associated with a higher risk of aminotransferase elevations among viral hepatitis-coinfected patients.
The introduction of new antiretroviral drugs over the last decade has transformed the treatment of human immunodeficiency virus (HIV) infection [1, 2]. Current antiretrovirals, including the integrase strand transfer inhibitors (INSTIs) and newer protease inhibitors (PIs) such as atazanavir and darunavir, are highly efficacious [3–5]. However, antiretrovirals have been associated with acute liver injury (ALI) [6–8], manifested by liver aminotransferase elevations or, in more serious cases, hepatic dysfunction (characterized by coagulopathy and hyperbilirubinemia [9]) and acute liver failure (ALF).
Prior studies of HIV-infected patients initiating antiretroviral therapy (ART) reported that the incidence of ALI ranged from 2% to 18%, with higher risk associated with increasing age, viral hepatitis coinfection, and pre-existing liver aminotransferase elevations [6, 10–12]. However, these studies focused on risks associated with individual antiretrovirals used in the early ART era and did not stratify results by viral hepatitis status [6, 7, 11–15]. Analyses that evaluate antiretroviral-associated hepatotoxicity in clinical practice settings are important to ensure the safety of these medications among HIV-infected individuals, particularly those with viral hepatitis coinfection.
We determined the absolute and comparative risks of ALI associated with nucleos(t)ide analogue combinations, antiretroviral classes, and commonly used ART regimens by examining incident development of liver aminotransferase elevations and hepatic dysfunction within the first year after ART initiation among those with and without viral hepatitis coinfection. We also determined factors associated with ALI. To accomplish these objectives, we combined data from 2 integrated health systems, Kaiser Permanente Northern California (KPNC) and the Veterans Health Administration, to create a large, nationally representative cohort of HIV-infected persons initiating ART in whom to evaluate ALI events.
METHODS
Study Design and Data Sources
We conducted a retrospective cohort study of HIV-infected persons who initiated ART within KPNC and the Veterans Aging Cohort Study (VACS), both of which utilize electronic medical records, permitting determination of dispensed antiretrovirals and laboratory-based definitions of ALI [16, 17].
The KPNC is an integrated healthcare organization that provides inpatient and outpatient services to Northern California residents [16]. The KPNC HIV Registry identifies members with a positive HIV antibody test, detectable HIV ribonucleic acid (RNA), antiretroviral prescription, or HIV/acquired immune deficiency syndrome (AIDS)-related diagnosis. Medical chart review is performed to confirm diagnoses. Data collected from KPNC include demographics, inpatient/outpatient International Classification of Diseases, Ninth Revision (ICD-9) diagnoses, procedures, laboratory results, and dispensed medications. Deaths are identified within the KPNC mortality database.
The VACS consists of electronic medical record data from HIV-infected patients receiving care at Veterans Affairs (VA) medical facilities across the United States. Data include demographics, inpatient/outpatient ICD-9 diagnoses, procedures, laboratory results, and pharmacy data. Deaths are identified from the VA Vital Status file [18].This study was approved by the Institutional Review Boards of the University of Pennsylvania, KPNC, and Corporal Michael J. Crescenz Philadelphia VA Medical Center.
Study Patients
Patients were eligible if they were (1) HIV antibody- and/or RNA-positive, (2) ≥18 years old, (3) dispensed ART (defined as use of ≥3 antiretrovirals from 2 different classes [19]) in an outpatient setting within KPNC between January 1, 2004 and December 31, 2010 or within VACS between January 1, 2004 and September 30, 2012, (4) without any antiretroviral fills in the prior 12 months, and (5) continuously enrolled in KPNC or VACS for ≥1 year. Patients were excluded if they had baseline evidence of any ALI outcome (defined below) or received warfarin (preventing identification of coagulopathy due to severe ALI).
The index date was the date the ART regimen was initially dispensed. The 12 months before this date represented the baseline period. Follow-up continued until (1) study endpoint (defined below), (2) death, (3) warfarin dispensation, (4) ART discontinuation (ie, no further antiretroviral medication fills within 30 days after the last prescription’s days’ supply) or change in ART regimen, (5) 1 year after index date, or (6) last contact before December 31, 2010 in KPNC or December 31, 2012 in VACS, whichever occurred first. For patients who discontinued ART, follow-up was censored on the end of the days’ supply of the first antiretroviral discontinued.
Main Study Outcomes
We examined 3 categories of outcomes within 12 months after ART initiation (to increase the likelihood that ALI events were antiretroviral-associated). First, we determined development of liver aminotransferase elevations, defined as an inpatient or outpatient alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >200 U/L (~5 times the upper limit of normal [ULN] of the assays used), a threshold that represents clinically important hepatic injury [20] and ~10 times what has been considered normal liver aminotransferase levels for males (30 U/L) and females (19 U/L) [21]. As a secondary endpoint, we determined incident grade 3 or 4 aminotransferase elevations determined by the toxicity grade scale used by the AIDS Clinical Trials Group [22], an outcome evaluated in prior studies of antiretroviral-induced ALI [10, 11, 14]. As was done in those studies [10, 11, 14], patients with pre-ART aminotransferases below ULN (ALT, 36 IU/L; AST, 40 IU/L) were classified, based on changes relative to ULN, as having incident grade 3 (>5 times ULN) or grade 4 (>10 times ULN) elevations. Patients with pre-ART aminotransferases above ULN were classified, based on changes relative to the baseline value, as having incident grade 3 (>3.5 times baseline) or grade 4 (>5 times baseline) elevations. Patients who did not have ALT/AST measured before ART initiation were excluded from analyses of grade 3/4 aminotransferase elevations.
Second, we evaluated severe ALI, defined by development of both international normalized ratio (INR) ≥1.5 and total bilirubin >2 times ULN in an inpatient or outpatient setting within 30 days of each other [23]. This definition indicates severe hepatic dysfunction and has been used by the US Food and Drug Administration’s Sentinel Initiative to assess serious drug-induced hepatotoxicity in the postmarketing setting [23].
Finally, we evaluated incident ALF among ART initiators without viral hepatitis, because pre-existing liver disease precludes an ALF diagnosis [24]. Acute liver failure was defined by coagulopathy (INR ≥1.5) plus either hepatic encephalopathy or liver transplantation [24].
Data Collection
Baseline clinical data included the following: age, sex, race, obesity (body mass index >30 kg/m2), alcohol dependence/abuse, cancer (excluding nonmelanoma skin cancers), diabetes mellitus, heart failure, and ART regimen. Alcohol dependence/abuse [25] and heart failure [26] were defined by validated ICD-9 diagnoses. Within KPNC, diabetes and cancer were determined by registries. Within VACS, diabetes was defined by random glucose ≥200 mg/dL, ICD-9 diagnosis, and/or antidiabetic medication use [27], and cancer was determined by ICD-9 diagnosis. Baseline laboratory data included ALT, AST, INR, total bilirubin, platelets, pre-ART CD4 count and HIV RNA, hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) antibody, and HCV RNA. To minimize misclassification of viral hepatitis status, patients who ever had a positive HBsAg, HCV antibody, or HCV RNA during the study period were classified as viral hepatitis-coinfected.
Data collected during follow-up included outpatient and inpatient ALT, AST, INR, and total bilirubin. We determined ALF events among patients without viral hepatitis using a method we previously described [9, 28]. Patients were screened for potential ALF if, during the year after ART initiation, they had the following: (1) a hospital ICD-9 diagnosis suggestive of ALF (Supplementary Appendix 1) and (2) both an inpatient INR ≥1.5 and peak total bilirubin ≥5.0 mg/dL. Hospital records of these patients were independently reviewed by 2 hepatologists (K.A.F. and D.S.G.). Acute liver failure was confirmed if a patient was hospitalized and had (1) no chronic liver disease, (2) coagulopathy (INR ≥1.5), and (3) either hepatic encephalopathy or liver transplantation [24]. Disagreements in ALF classification were arbitrated by a third hepatologist (K.R.R.).
Statistical Analysis
One-year cumulative risk and incidence rates (events/1000 person-years) of outcomes with 95% confidence intervals (CIs) of outcomes were calculated for individual antiretrovirals, nucleos(t)ide reverse-transcriptase inhibitor (NRTI) combinations, antiretroviral classes, and commonly prescribed ART regimens. Results were stratified by viral hepatitis status.
Cox regression was used to estimate adjusted hazard ratios (HRs) of endpoints among initiators of NRTI combinations, antiretroviral classes, and ART regimens [29]. We determined whether HRs of outcomes differed by viral hepatitis status through use of statistical tests of interaction within Cox models. Severe ALI and ALF were too rare to evaluate with multivariable Cox regression. Thus, multivariable analyses focused on liver aminotransferase elevations. Three models were developed, stratified by viral hepatitis status, to assess the risk of this endpoint among initiators of the following: (1) NRTI combinations; (2) PI, INSTI, and non-NRTI classes; and (3) common ART regimens. For each analysis, the most frequently prescribed NRTI combination (tenofivir plus either emtricitabine or lamivudine), antiretroviral class (non-NRTI), or ART regimen (efavirenz plus tenofovir/emtricitabine) was the reference. Analyses were adjusted for variables that were significantly (P < .05) associated with incident outcomes in univariable analyses. Proportionality of hazards was assessed by Schoenfeld residuals [30].
Cox regression was also used to identify risk factors for aminotransferases >200 U/L in multivariable analysis and severe ALI in univariable analyses (given the rarity of these events). Age, sex, race, obesity, viral hepatitis, alcohol dependence/abuse, diabetes, cancer, heart failure, pre-ART CD4 count, pre-ART HIV RNA level, and baseline ALT were evaluated as risk factors.
To avoid bias from excluding subjects with missing data for models evaluating aminotransferases >200 U/L, we implemented multiple imputation using chained equations [31]. Ten imputed datasets were created using all variables. Imputed variables included baseline ALT, obesity, and pre-ART CD4 count and HIV RNA. Results across the 10 datasets were combined to arrive at CIs that accounted for within- and across-dataset variances [32]. Data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
Among 24 868 patients dispensed ART within KPNC (n = 8789) and VACS (n = 16 079), 10 083 persons (2099 in KPNC; 7984 in VACS) met eligibility and were followed for 5461 person-years (Figure 1). The VACS patients were older, more commonly male or African American, less commonly Hispanic, and had a higher prevalence of viral hepatitis, alcohol dependence/abuse, and diabetes than KPNC members (Table 1). Median pre-ART CD4, pre-ART HIV RNA, and baseline ALT were similar between the groups.
Baseline Characteristics of HIV-Infected Patients Who Were Dispensed Antiretroviral Therapy in an Outpatient Setting Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012)
| Characteristic . | Overall (n = 10 083) . | Veterans Aging Cohort Study (n = 7984) . | Kaiser Permanente Northern California (n = 2099) . |
|---|---|---|---|
| Median follow-up (months, IQR) | 5.29 (2.14–12.0) | 4.75 (1.94–12.0) | 9.53 (2.99–12.0) |
| Age (n, %) | |||
| Median age (years, IQR) | 48.0 (40.0–55.0) | 49.0 (42.0–56.0) | 43.0 (37.0–50.0) |
| 18–50 years | 5686 (56.4%) | 4151 (52.0%) | 1535 (73.1%) |
| 50–59 years | 4397 (43.6%) | 3833 (48.0%) | 564 (26.9%) |
| Female sex (n, %) | 511 (5.1%) | 248 (3.1%) | 263 (12.5%) |
| Race (n, %) | |||
| White | 3973 (39.4%) | 2942 (36.8%) | 1031 (49.1%) |
| Black or African American | 4972 (49.3%) | 4573 (57.3%) | 399 (19.0%) |
| Asian/Native Hawaiian/Other Pacific Islander | 200 (2.0%) | 81 (1.0%) | 119 (5.7%) |
| American Indian/Alaska Native | 53 (0.5%) | 37 (0.5%) | 16 (0.8%) |
| Unknown/Multiracial | 885 (8.8%) | 351 (4.4%) | 534 (25.4%) |
| Hispanic (n, %) | 939 (9.3%) | 546 (6.8%) | 393 (18.7%) |
| Unknown | 467 (4.6%) | 0 (0%) | 467 (22.2%) |
| Body mass index ≥30 kg/m2 (n, %) | 1659 (17.3%) | 1275 (16.2%) | 384 (22.1%) |
| Unknown | 473 (4.7%) | 108 (1.4%) | 365 (17.4%) |
| Viral hepatitis coinfection (n, %) | |||
| Hepatitis Ba | 326 (3.2%) | 241 (3.0%) | 85 (4.0%) |
| Hepatitis Cb | 1825 (18.1%) | 1636 (20.5%) | 189 (9.0%) |
| History of alcohol dependence/abuse (n, %) | 2368 (23.5%) | 1980 (24.8%) | 388 (18.5%) |
| Diabetes mellitus (n, %) | 929 (9.2%) | 805 (10.1%) | 124 (5.9%) |
| Cancer (n, %) | 643 (6.4%) | 509 (6.4%) | 134 (6.4%) |
| Heart failure (n, %) | 189 (1.9%) | 169 (2.1%) | 20 (1.0%) |
| Median HIV viral load (log10 copies/mL, IQR) | 4.7 (4.2–5.2) | 4.8 (4.2–5.2) | 4.6 (4.1–5.1) |
| Missing (n, %) | 333 (3.3%) | 84 (1.1%) | 249 (11.9%) |
| CD4 cell count (n, %) | |||
| Median (cells/mm3, IQR) | 244 (119–354) | 237 (110–350) | 265 (160–368) |
| <200 cells/mm3 | 3971 (40.0%) | 3307 (41.8%) | 664 (33.0%) |
| 200–500 cells/mm3 | 4999 (50.4%) | 3884 (49.1%) | 1115 (55.4%) |
| ≥500 cells/mm3 | 950 (9.6%) | 717 (9.1%) | 233 (11.6%) |
| Missing | 163 (1.6%) | 76 (1.0%) | 87 (4.1%) |
| Median ALT (IU/mL, IQR) | 29 (20–43) | 29 (20–44) | 28 (20–41) |
| Missing (n, %) | 744 (7.4%) | 353 (4.4%) | 391 (18.6%) |
| Calendar year of ART initiation, n (%) | |||
| 2004–2006 | 3967 (39.3%) | 3139 (39.3%) | 828 (39.4%) |
| 2007–2009 | 3511 (34.8%) | 2549 (31.9%) | 962 (45.8%) |
| 2010–2012 | 2605 (25.8%) | 2296 (28.8%) | 309 (14.7%) |
| Characteristic . | Overall (n = 10 083) . | Veterans Aging Cohort Study (n = 7984) . | Kaiser Permanente Northern California (n = 2099) . |
|---|---|---|---|
| Median follow-up (months, IQR) | 5.29 (2.14–12.0) | 4.75 (1.94–12.0) | 9.53 (2.99–12.0) |
| Age (n, %) | |||
| Median age (years, IQR) | 48.0 (40.0–55.0) | 49.0 (42.0–56.0) | 43.0 (37.0–50.0) |
| 18–50 years | 5686 (56.4%) | 4151 (52.0%) | 1535 (73.1%) |
| 50–59 years | 4397 (43.6%) | 3833 (48.0%) | 564 (26.9%) |
| Female sex (n, %) | 511 (5.1%) | 248 (3.1%) | 263 (12.5%) |
| Race (n, %) | |||
| White | 3973 (39.4%) | 2942 (36.8%) | 1031 (49.1%) |
| Black or African American | 4972 (49.3%) | 4573 (57.3%) | 399 (19.0%) |
| Asian/Native Hawaiian/Other Pacific Islander | 200 (2.0%) | 81 (1.0%) | 119 (5.7%) |
| American Indian/Alaska Native | 53 (0.5%) | 37 (0.5%) | 16 (0.8%) |
| Unknown/Multiracial | 885 (8.8%) | 351 (4.4%) | 534 (25.4%) |
| Hispanic (n, %) | 939 (9.3%) | 546 (6.8%) | 393 (18.7%) |
| Unknown | 467 (4.6%) | 0 (0%) | 467 (22.2%) |
| Body mass index ≥30 kg/m2 (n, %) | 1659 (17.3%) | 1275 (16.2%) | 384 (22.1%) |
| Unknown | 473 (4.7%) | 108 (1.4%) | 365 (17.4%) |
| Viral hepatitis coinfection (n, %) | |||
| Hepatitis Ba | 326 (3.2%) | 241 (3.0%) | 85 (4.0%) |
| Hepatitis Cb | 1825 (18.1%) | 1636 (20.5%) | 189 (9.0%) |
| History of alcohol dependence/abuse (n, %) | 2368 (23.5%) | 1980 (24.8%) | 388 (18.5%) |
| Diabetes mellitus (n, %) | 929 (9.2%) | 805 (10.1%) | 124 (5.9%) |
| Cancer (n, %) | 643 (6.4%) | 509 (6.4%) | 134 (6.4%) |
| Heart failure (n, %) | 189 (1.9%) | 169 (2.1%) | 20 (1.0%) |
| Median HIV viral load (log10 copies/mL, IQR) | 4.7 (4.2–5.2) | 4.8 (4.2–5.2) | 4.6 (4.1–5.1) |
| Missing (n, %) | 333 (3.3%) | 84 (1.1%) | 249 (11.9%) |
| CD4 cell count (n, %) | |||
| Median (cells/mm3, IQR) | 244 (119–354) | 237 (110–350) | 265 (160–368) |
| <200 cells/mm3 | 3971 (40.0%) | 3307 (41.8%) | 664 (33.0%) |
| 200–500 cells/mm3 | 4999 (50.4%) | 3884 (49.1%) | 1115 (55.4%) |
| ≥500 cells/mm3 | 950 (9.6%) | 717 (9.1%) | 233 (11.6%) |
| Missing | 163 (1.6%) | 76 (1.0%) | 87 (4.1%) |
| Median ALT (IU/mL, IQR) | 29 (20–43) | 29 (20–44) | 28 (20–41) |
| Missing (n, %) | 744 (7.4%) | 353 (4.4%) | 391 (18.6%) |
| Calendar year of ART initiation, n (%) | |||
| 2004–2006 | 3967 (39.3%) | 3139 (39.3%) | 828 (39.4%) |
| 2007–2009 | 3511 (34.8%) | 2549 (31.9%) | 962 (45.8%) |
| 2010–2012 | 2605 (25.8%) | 2296 (28.8%) | 309 (14.7%) |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; RNA, ribonucleic acid.
aHepatitis B virus infection defined by ever-positive hepatitis B surface antigen.
bHepatitis C virus infection defined by ever-positive hepatitis C virus antibody or hepatitis C virus RNA.
Baseline Characteristics of HIV-Infected Patients Who Were Dispensed Antiretroviral Therapy in an Outpatient Setting Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012)
| Characteristic . | Overall (n = 10 083) . | Veterans Aging Cohort Study (n = 7984) . | Kaiser Permanente Northern California (n = 2099) . |
|---|---|---|---|
| Median follow-up (months, IQR) | 5.29 (2.14–12.0) | 4.75 (1.94–12.0) | 9.53 (2.99–12.0) |
| Age (n, %) | |||
| Median age (years, IQR) | 48.0 (40.0–55.0) | 49.0 (42.0–56.0) | 43.0 (37.0–50.0) |
| 18–50 years | 5686 (56.4%) | 4151 (52.0%) | 1535 (73.1%) |
| 50–59 years | 4397 (43.6%) | 3833 (48.0%) | 564 (26.9%) |
| Female sex (n, %) | 511 (5.1%) | 248 (3.1%) | 263 (12.5%) |
| Race (n, %) | |||
| White | 3973 (39.4%) | 2942 (36.8%) | 1031 (49.1%) |
| Black or African American | 4972 (49.3%) | 4573 (57.3%) | 399 (19.0%) |
| Asian/Native Hawaiian/Other Pacific Islander | 200 (2.0%) | 81 (1.0%) | 119 (5.7%) |
| American Indian/Alaska Native | 53 (0.5%) | 37 (0.5%) | 16 (0.8%) |
| Unknown/Multiracial | 885 (8.8%) | 351 (4.4%) | 534 (25.4%) |
| Hispanic (n, %) | 939 (9.3%) | 546 (6.8%) | 393 (18.7%) |
| Unknown | 467 (4.6%) | 0 (0%) | 467 (22.2%) |
| Body mass index ≥30 kg/m2 (n, %) | 1659 (17.3%) | 1275 (16.2%) | 384 (22.1%) |
| Unknown | 473 (4.7%) | 108 (1.4%) | 365 (17.4%) |
| Viral hepatitis coinfection (n, %) | |||
| Hepatitis Ba | 326 (3.2%) | 241 (3.0%) | 85 (4.0%) |
| Hepatitis Cb | 1825 (18.1%) | 1636 (20.5%) | 189 (9.0%) |
| History of alcohol dependence/abuse (n, %) | 2368 (23.5%) | 1980 (24.8%) | 388 (18.5%) |
| Diabetes mellitus (n, %) | 929 (9.2%) | 805 (10.1%) | 124 (5.9%) |
| Cancer (n, %) | 643 (6.4%) | 509 (6.4%) | 134 (6.4%) |
| Heart failure (n, %) | 189 (1.9%) | 169 (2.1%) | 20 (1.0%) |
| Median HIV viral load (log10 copies/mL, IQR) | 4.7 (4.2–5.2) | 4.8 (4.2–5.2) | 4.6 (4.1–5.1) |
| Missing (n, %) | 333 (3.3%) | 84 (1.1%) | 249 (11.9%) |
| CD4 cell count (n, %) | |||
| Median (cells/mm3, IQR) | 244 (119–354) | 237 (110–350) | 265 (160–368) |
| <200 cells/mm3 | 3971 (40.0%) | 3307 (41.8%) | 664 (33.0%) |
| 200–500 cells/mm3 | 4999 (50.4%) | 3884 (49.1%) | 1115 (55.4%) |
| ≥500 cells/mm3 | 950 (9.6%) | 717 (9.1%) | 233 (11.6%) |
| Missing | 163 (1.6%) | 76 (1.0%) | 87 (4.1%) |
| Median ALT (IU/mL, IQR) | 29 (20–43) | 29 (20–44) | 28 (20–41) |
| Missing (n, %) | 744 (7.4%) | 353 (4.4%) | 391 (18.6%) |
| Calendar year of ART initiation, n (%) | |||
| 2004–2006 | 3967 (39.3%) | 3139 (39.3%) | 828 (39.4%) |
| 2007–2009 | 3511 (34.8%) | 2549 (31.9%) | 962 (45.8%) |
| 2010–2012 | 2605 (25.8%) | 2296 (28.8%) | 309 (14.7%) |
| Characteristic . | Overall (n = 10 083) . | Veterans Aging Cohort Study (n = 7984) . | Kaiser Permanente Northern California (n = 2099) . |
|---|---|---|---|
| Median follow-up (months, IQR) | 5.29 (2.14–12.0) | 4.75 (1.94–12.0) | 9.53 (2.99–12.0) |
| Age (n, %) | |||
| Median age (years, IQR) | 48.0 (40.0–55.0) | 49.0 (42.0–56.0) | 43.0 (37.0–50.0) |
| 18–50 years | 5686 (56.4%) | 4151 (52.0%) | 1535 (73.1%) |
| 50–59 years | 4397 (43.6%) | 3833 (48.0%) | 564 (26.9%) |
| Female sex (n, %) | 511 (5.1%) | 248 (3.1%) | 263 (12.5%) |
| Race (n, %) | |||
| White | 3973 (39.4%) | 2942 (36.8%) | 1031 (49.1%) |
| Black or African American | 4972 (49.3%) | 4573 (57.3%) | 399 (19.0%) |
| Asian/Native Hawaiian/Other Pacific Islander | 200 (2.0%) | 81 (1.0%) | 119 (5.7%) |
| American Indian/Alaska Native | 53 (0.5%) | 37 (0.5%) | 16 (0.8%) |
| Unknown/Multiracial | 885 (8.8%) | 351 (4.4%) | 534 (25.4%) |
| Hispanic (n, %) | 939 (9.3%) | 546 (6.8%) | 393 (18.7%) |
| Unknown | 467 (4.6%) | 0 (0%) | 467 (22.2%) |
| Body mass index ≥30 kg/m2 (n, %) | 1659 (17.3%) | 1275 (16.2%) | 384 (22.1%) |
| Unknown | 473 (4.7%) | 108 (1.4%) | 365 (17.4%) |
| Viral hepatitis coinfection (n, %) | |||
| Hepatitis Ba | 326 (3.2%) | 241 (3.0%) | 85 (4.0%) |
| Hepatitis Cb | 1825 (18.1%) | 1636 (20.5%) | 189 (9.0%) |
| History of alcohol dependence/abuse (n, %) | 2368 (23.5%) | 1980 (24.8%) | 388 (18.5%) |
| Diabetes mellitus (n, %) | 929 (9.2%) | 805 (10.1%) | 124 (5.9%) |
| Cancer (n, %) | 643 (6.4%) | 509 (6.4%) | 134 (6.4%) |
| Heart failure (n, %) | 189 (1.9%) | 169 (2.1%) | 20 (1.0%) |
| Median HIV viral load (log10 copies/mL, IQR) | 4.7 (4.2–5.2) | 4.8 (4.2–5.2) | 4.6 (4.1–5.1) |
| Missing (n, %) | 333 (3.3%) | 84 (1.1%) | 249 (11.9%) |
| CD4 cell count (n, %) | |||
| Median (cells/mm3, IQR) | 244 (119–354) | 237 (110–350) | 265 (160–368) |
| <200 cells/mm3 | 3971 (40.0%) | 3307 (41.8%) | 664 (33.0%) |
| 200–500 cells/mm3 | 4999 (50.4%) | 3884 (49.1%) | 1115 (55.4%) |
| ≥500 cells/mm3 | 950 (9.6%) | 717 (9.1%) | 233 (11.6%) |
| Missing | 163 (1.6%) | 76 (1.0%) | 87 (4.1%) |
| Median ALT (IU/mL, IQR) | 29 (20–43) | 29 (20–44) | 28 (20–41) |
| Missing (n, %) | 744 (7.4%) | 353 (4.4%) | 391 (18.6%) |
| Calendar year of ART initiation, n (%) | |||
| 2004–2006 | 3967 (39.3%) | 3139 (39.3%) | 828 (39.4%) |
| 2007–2009 | 3511 (34.8%) | 2549 (31.9%) | 962 (45.8%) |
| 2010–2012 | 2605 (25.8%) | 2296 (28.8%) | 309 (14.7%) |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; RNA, ribonucleic acid.
aHepatitis B virus infection defined by ever-positive hepatitis B surface antigen.
bHepatitis C virus infection defined by ever-positive hepatitis C virus antibody or hepatitis C virus RNA.
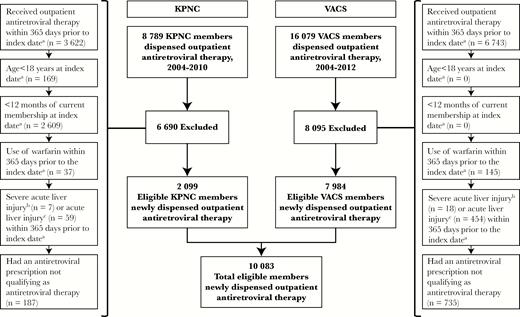
Selection of patients into the study from Kaiser Permanente Northern California and the Veterans Aging Cohort Study. aIndex Date is the earliest qualifying date the antiretroviral therapy was dispensed in an outpatient setting on or after January 1, 2004. bSevere acute liver injury is defined as total bilirubin >2 times the upper limit of normal and an international normalized ratio (INR) ≥1.5 in an inpatient or outpatient setting. cAcute liver injury is defined as (1) inpatient or outpatient alanine aminotransferase or aspartate aminotransferase >200 U/L; (2) a total bilirubin >2 times the upper limit of normal and an INR ≥1.5 in an inpatient or outpatient setting; or (3) inpatient INR ≥1.5 and either hepatic encephalopathy or liver transplantation in the absence of chronic liver disease. KPNC, Kaiser Permanente Northern California; VACS, Veterans Aging Cohort Study.
Risk of Liver Aminotransferase Elevations
During the study period, 206 patients (n = 10 083; 2.0%) developed aminotransferases >200 U/L and 178 (n = 9542; 1.9%) developed grade 3/4 aminotransferase elevations. Viral hepatitis-coinfected patients had higher rates of both aminotransferases >200 U/L (Table 2; Supplementary Appendix 2) and grade 3/4 elevations (Supplementary Appendix 3) than HIV-monoinfected individuals. Because the 2 aminotransferase elevation outcomes yielded comparable results, multivariable analyses were performed using the primary endpoint of aminotransferases >200 U/L.
Cumulative Incidences and Incidence Rates of Liver Aminotransferases >200 U/L Among Initiators of Antiretroviral Drugs and Regimens Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012), Stratified by the Presence of Viral Hepatitis Coinfection
| Regimen/Drug . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person- Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | |
| Overall | 7970 | 93 | 4488 | 1.7 (1.4–2.2) | 20.7 (16.7–25.4) | 2113 | 113 | 973 | 9.0 (7.4–11.0) | 116.1 (95.7–139.6) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 66 | 3464 | 1.6 (1.3–2.1) | 19.1 (14.7–24.2) | 1343 | 73 | 674 | 8.3 (6.6–10.6) | 108.3 (84.9–136.2) |
| ABC/3TC | 392 | 5 | 211 | 2.2 (0.9–5.4) | 23.6 (7.7–55.2) | 162 | 6 | 71 | 5.9 (2.5–13.5) | 84.9 (31.1–184.7) |
| 3TC/ZDV | 1032 | 9 | 481 | 1.3 (0.6–2.7) | 18.7 (8.6–35.5) | 344 | 20 | 129 | 12.9 (7.7–21.0) | 155.0 (94.7–239.3) |
| Othera | 784 | 13 | 332 | 2.8 (1.6–5.1) | 39.2 (20.9–67.0) | 264 | 14 | 100 | 13.3 (7.2–23.9) | 140.7 (76.9–236.0) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 59 | 2948 | 1.7 (1.3–2.3) | 20.0 (15.2–25.8) | 1148 | 47 | 568 | 6.9 (5.1–9.3) | 82.7 (60.7–109.9) |
| PI | 2520 | 25 | 1299 | 1.5 (1.0–2.3) | 19.2 (12.5–28.4) | 833 | 59 | 356 | 12.0 (9.1–15.6) | 165.8 (126.2–213.9) |
| INSTI | 195 | 5 | 112 | 3.4 (1.3–8.6) | 44.7 (14.5–104.4) | 33 | 1 | 14 | 8.3 (1.2–46.1) | 72.8 (1.8–405.8) |
| Otherb | 318 | 4 | 129 | 2.3 (0.8–7.1) | 31.1 (8.5–79.6) | 99 | 6 | 35 | 12.2 (4.5–30.3) | 170.5 (62.6–371.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 42 | 2320 | 1.6 (1.2–2.3) | 18.1 (13.0–24.5) | 761 | 30 | 401 | 6.1 (4.2–8.8) | 74.7 (50.4–106.7) |
| ATV/r + TDF/FTC | 854 | 11 | 511 | 1.8 (0.9–3.3) | 21.5 (10.7–38.5) | 256 | 18 | 124 | 8.6 (5.5–13.5) | 144.7 (85.8–228.8) |
| EFV + 3TC/ZDV | 556 | 2 | 270 | 0.8 (0.2–3.7) | 7.4 (0.9–26.7) | 189 | 8 | 71 | 8.4 (3.9–17.4) | 112.2 (48.4–221.0) |
| LPV/r + TDF/FTC | 276 | 3 | 131 | 1.2 (0.4–3.5) | 22.8 (4.7–66.7) | 90 | 7 | 39 | 14.1 (6.8–28.2) | 179.2 (72.1–369.3) |
| LPV/r + 3TC/ZDV | 221 | 1 | 95 | 1.0 (0.1–6.6) | 10.5 (0.3–58.7) | 70 | 6 | 25 | 20.4 (7.7–47.9) | 238.1 (87.4–518.3) |
| DRV/r + TDF/FTC | 224 | 1 | 119 | 0.5 (0.1–3.2) | 8.4 (0.2–46.6) | 46 | 5 | 22 | 16.1 (6.7–36.0) | 230.1 (74.7–536.9) |
| ATV/r + ABC/3TC | 150 | 2 | 80 | 2.3 (0.6–9.4) | 24.9 (3.0–89.8) | 55 | 3 | 25 | 7.4 (2.4–21.7) | 117.8 (24.3–344.2) |
| RAL + TDF/FTC | 174 | 5 | 102 | 3.7 (1.4–9.3) | 49.1 (16.0–114.6) | 28 | 1 | 13 | 8.3 (1.2–46.1) | 77.0 (2.0–429.2) |
| EFV + TDF/3TC | 126 | 1 | 70 | 1.9 (0.3–12.6) | 14.3 (0.4–79.6) | 30 | 1 | 15 | 4.8 (0.7–29.3) | 68.0 (1.7–379.1) |
| EFV + ABC/3TC | 113 | 2 | 66 | 2.7 (0.6–11.0) | 30.3 (3.7–109.5) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.6) |
| Regimen/Drug . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person- Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | |
| Overall | 7970 | 93 | 4488 | 1.7 (1.4–2.2) | 20.7 (16.7–25.4) | 2113 | 113 | 973 | 9.0 (7.4–11.0) | 116.1 (95.7–139.6) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 66 | 3464 | 1.6 (1.3–2.1) | 19.1 (14.7–24.2) | 1343 | 73 | 674 | 8.3 (6.6–10.6) | 108.3 (84.9–136.2) |
| ABC/3TC | 392 | 5 | 211 | 2.2 (0.9–5.4) | 23.6 (7.7–55.2) | 162 | 6 | 71 | 5.9 (2.5–13.5) | 84.9 (31.1–184.7) |
| 3TC/ZDV | 1032 | 9 | 481 | 1.3 (0.6–2.7) | 18.7 (8.6–35.5) | 344 | 20 | 129 | 12.9 (7.7–21.0) | 155.0 (94.7–239.3) |
| Othera | 784 | 13 | 332 | 2.8 (1.6–5.1) | 39.2 (20.9–67.0) | 264 | 14 | 100 | 13.3 (7.2–23.9) | 140.7 (76.9–236.0) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 59 | 2948 | 1.7 (1.3–2.3) | 20.0 (15.2–25.8) | 1148 | 47 | 568 | 6.9 (5.1–9.3) | 82.7 (60.7–109.9) |
| PI | 2520 | 25 | 1299 | 1.5 (1.0–2.3) | 19.2 (12.5–28.4) | 833 | 59 | 356 | 12.0 (9.1–15.6) | 165.8 (126.2–213.9) |
| INSTI | 195 | 5 | 112 | 3.4 (1.3–8.6) | 44.7 (14.5–104.4) | 33 | 1 | 14 | 8.3 (1.2–46.1) | 72.8 (1.8–405.8) |
| Otherb | 318 | 4 | 129 | 2.3 (0.8–7.1) | 31.1 (8.5–79.6) | 99 | 6 | 35 | 12.2 (4.5–30.3) | 170.5 (62.6–371.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 42 | 2320 | 1.6 (1.2–2.3) | 18.1 (13.0–24.5) | 761 | 30 | 401 | 6.1 (4.2–8.8) | 74.7 (50.4–106.7) |
| ATV/r + TDF/FTC | 854 | 11 | 511 | 1.8 (0.9–3.3) | 21.5 (10.7–38.5) | 256 | 18 | 124 | 8.6 (5.5–13.5) | 144.7 (85.8–228.8) |
| EFV + 3TC/ZDV | 556 | 2 | 270 | 0.8 (0.2–3.7) | 7.4 (0.9–26.7) | 189 | 8 | 71 | 8.4 (3.9–17.4) | 112.2 (48.4–221.0) |
| LPV/r + TDF/FTC | 276 | 3 | 131 | 1.2 (0.4–3.5) | 22.8 (4.7–66.7) | 90 | 7 | 39 | 14.1 (6.8–28.2) | 179.2 (72.1–369.3) |
| LPV/r + 3TC/ZDV | 221 | 1 | 95 | 1.0 (0.1–6.6) | 10.5 (0.3–58.7) | 70 | 6 | 25 | 20.4 (7.7–47.9) | 238.1 (87.4–518.3) |
| DRV/r + TDF/FTC | 224 | 1 | 119 | 0.5 (0.1–3.2) | 8.4 (0.2–46.6) | 46 | 5 | 22 | 16.1 (6.7–36.0) | 230.1 (74.7–536.9) |
| ATV/r + ABC/3TC | 150 | 2 | 80 | 2.3 (0.6–9.4) | 24.9 (3.0–89.8) | 55 | 3 | 25 | 7.4 (2.4–21.7) | 117.8 (24.3–344.2) |
| RAL + TDF/FTC | 174 | 5 | 102 | 3.7 (1.4–9.3) | 49.1 (16.0–114.6) | 28 | 1 | 13 | 8.3 (1.2–46.1) | 77.0 (2.0–429.2) |
| EFV + TDF/3TC | 126 | 1 | 70 | 1.9 (0.3–12.6) | 14.3 (0.4–79.6) | 30 | 1 | 15 | 4.8 (0.7–29.3) | 68.0 (1.7–379.1) |
| EFV + ABC/3TC | 113 | 2 | 66 | 2.7 (0.6–11.0) | 30.3 (3.7–109.5) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.6) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; CI, confidence interval; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV, lopinavir; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; r, ritonavir; RAL, raltegravir; TDF, tenofovir; ZDV, zidovudine.
a“Other” category includes regimens with <2 NRTIs or alternative NRTI combinations than those listed above.
b“Other” includes regimens unable to be classified in the prior categories, such as those with none or >1 antiretroviral class.
Cumulative Incidences and Incidence Rates of Liver Aminotransferases >200 U/L Among Initiators of Antiretroviral Drugs and Regimens Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012), Stratified by the Presence of Viral Hepatitis Coinfection
| Regimen/Drug . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person- Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | |
| Overall | 7970 | 93 | 4488 | 1.7 (1.4–2.2) | 20.7 (16.7–25.4) | 2113 | 113 | 973 | 9.0 (7.4–11.0) | 116.1 (95.7–139.6) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 66 | 3464 | 1.6 (1.3–2.1) | 19.1 (14.7–24.2) | 1343 | 73 | 674 | 8.3 (6.6–10.6) | 108.3 (84.9–136.2) |
| ABC/3TC | 392 | 5 | 211 | 2.2 (0.9–5.4) | 23.6 (7.7–55.2) | 162 | 6 | 71 | 5.9 (2.5–13.5) | 84.9 (31.1–184.7) |
| 3TC/ZDV | 1032 | 9 | 481 | 1.3 (0.6–2.7) | 18.7 (8.6–35.5) | 344 | 20 | 129 | 12.9 (7.7–21.0) | 155.0 (94.7–239.3) |
| Othera | 784 | 13 | 332 | 2.8 (1.6–5.1) | 39.2 (20.9–67.0) | 264 | 14 | 100 | 13.3 (7.2–23.9) | 140.7 (76.9–236.0) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 59 | 2948 | 1.7 (1.3–2.3) | 20.0 (15.2–25.8) | 1148 | 47 | 568 | 6.9 (5.1–9.3) | 82.7 (60.7–109.9) |
| PI | 2520 | 25 | 1299 | 1.5 (1.0–2.3) | 19.2 (12.5–28.4) | 833 | 59 | 356 | 12.0 (9.1–15.6) | 165.8 (126.2–213.9) |
| INSTI | 195 | 5 | 112 | 3.4 (1.3–8.6) | 44.7 (14.5–104.4) | 33 | 1 | 14 | 8.3 (1.2–46.1) | 72.8 (1.8–405.8) |
| Otherb | 318 | 4 | 129 | 2.3 (0.8–7.1) | 31.1 (8.5–79.6) | 99 | 6 | 35 | 12.2 (4.5–30.3) | 170.5 (62.6–371.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 42 | 2320 | 1.6 (1.2–2.3) | 18.1 (13.0–24.5) | 761 | 30 | 401 | 6.1 (4.2–8.8) | 74.7 (50.4–106.7) |
| ATV/r + TDF/FTC | 854 | 11 | 511 | 1.8 (0.9–3.3) | 21.5 (10.7–38.5) | 256 | 18 | 124 | 8.6 (5.5–13.5) | 144.7 (85.8–228.8) |
| EFV + 3TC/ZDV | 556 | 2 | 270 | 0.8 (0.2–3.7) | 7.4 (0.9–26.7) | 189 | 8 | 71 | 8.4 (3.9–17.4) | 112.2 (48.4–221.0) |
| LPV/r + TDF/FTC | 276 | 3 | 131 | 1.2 (0.4–3.5) | 22.8 (4.7–66.7) | 90 | 7 | 39 | 14.1 (6.8–28.2) | 179.2 (72.1–369.3) |
| LPV/r + 3TC/ZDV | 221 | 1 | 95 | 1.0 (0.1–6.6) | 10.5 (0.3–58.7) | 70 | 6 | 25 | 20.4 (7.7–47.9) | 238.1 (87.4–518.3) |
| DRV/r + TDF/FTC | 224 | 1 | 119 | 0.5 (0.1–3.2) | 8.4 (0.2–46.6) | 46 | 5 | 22 | 16.1 (6.7–36.0) | 230.1 (74.7–536.9) |
| ATV/r + ABC/3TC | 150 | 2 | 80 | 2.3 (0.6–9.4) | 24.9 (3.0–89.8) | 55 | 3 | 25 | 7.4 (2.4–21.7) | 117.8 (24.3–344.2) |
| RAL + TDF/FTC | 174 | 5 | 102 | 3.7 (1.4–9.3) | 49.1 (16.0–114.6) | 28 | 1 | 13 | 8.3 (1.2–46.1) | 77.0 (2.0–429.2) |
| EFV + TDF/3TC | 126 | 1 | 70 | 1.9 (0.3–12.6) | 14.3 (0.4–79.6) | 30 | 1 | 15 | 4.8 (0.7–29.3) | 68.0 (1.7–379.1) |
| EFV + ABC/3TC | 113 | 2 | 66 | 2.7 (0.6–11.0) | 30.3 (3.7–109.5) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.6) |
| Regimen/Drug . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person- Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | |
| Overall | 7970 | 93 | 4488 | 1.7 (1.4–2.2) | 20.7 (16.7–25.4) | 2113 | 113 | 973 | 9.0 (7.4–11.0) | 116.1 (95.7–139.6) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 66 | 3464 | 1.6 (1.3–2.1) | 19.1 (14.7–24.2) | 1343 | 73 | 674 | 8.3 (6.6–10.6) | 108.3 (84.9–136.2) |
| ABC/3TC | 392 | 5 | 211 | 2.2 (0.9–5.4) | 23.6 (7.7–55.2) | 162 | 6 | 71 | 5.9 (2.5–13.5) | 84.9 (31.1–184.7) |
| 3TC/ZDV | 1032 | 9 | 481 | 1.3 (0.6–2.7) | 18.7 (8.6–35.5) | 344 | 20 | 129 | 12.9 (7.7–21.0) | 155.0 (94.7–239.3) |
| Othera | 784 | 13 | 332 | 2.8 (1.6–5.1) | 39.2 (20.9–67.0) | 264 | 14 | 100 | 13.3 (7.2–23.9) | 140.7 (76.9–236.0) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 59 | 2948 | 1.7 (1.3–2.3) | 20.0 (15.2–25.8) | 1148 | 47 | 568 | 6.9 (5.1–9.3) | 82.7 (60.7–109.9) |
| PI | 2520 | 25 | 1299 | 1.5 (1.0–2.3) | 19.2 (12.5–28.4) | 833 | 59 | 356 | 12.0 (9.1–15.6) | 165.8 (126.2–213.9) |
| INSTI | 195 | 5 | 112 | 3.4 (1.3–8.6) | 44.7 (14.5–104.4) | 33 | 1 | 14 | 8.3 (1.2–46.1) | 72.8 (1.8–405.8) |
| Otherb | 318 | 4 | 129 | 2.3 (0.8–7.1) | 31.1 (8.5–79.6) | 99 | 6 | 35 | 12.2 (4.5–30.3) | 170.5 (62.6–371.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 42 | 2320 | 1.6 (1.2–2.3) | 18.1 (13.0–24.5) | 761 | 30 | 401 | 6.1 (4.2–8.8) | 74.7 (50.4–106.7) |
| ATV/r + TDF/FTC | 854 | 11 | 511 | 1.8 (0.9–3.3) | 21.5 (10.7–38.5) | 256 | 18 | 124 | 8.6 (5.5–13.5) | 144.7 (85.8–228.8) |
| EFV + 3TC/ZDV | 556 | 2 | 270 | 0.8 (0.2–3.7) | 7.4 (0.9–26.7) | 189 | 8 | 71 | 8.4 (3.9–17.4) | 112.2 (48.4–221.0) |
| LPV/r + TDF/FTC | 276 | 3 | 131 | 1.2 (0.4–3.5) | 22.8 (4.7–66.7) | 90 | 7 | 39 | 14.1 (6.8–28.2) | 179.2 (72.1–369.3) |
| LPV/r + 3TC/ZDV | 221 | 1 | 95 | 1.0 (0.1–6.6) | 10.5 (0.3–58.7) | 70 | 6 | 25 | 20.4 (7.7–47.9) | 238.1 (87.4–518.3) |
| DRV/r + TDF/FTC | 224 | 1 | 119 | 0.5 (0.1–3.2) | 8.4 (0.2–46.6) | 46 | 5 | 22 | 16.1 (6.7–36.0) | 230.1 (74.7–536.9) |
| ATV/r + ABC/3TC | 150 | 2 | 80 | 2.3 (0.6–9.4) | 24.9 (3.0–89.8) | 55 | 3 | 25 | 7.4 (2.4–21.7) | 117.8 (24.3–344.2) |
| RAL + TDF/FTC | 174 | 5 | 102 | 3.7 (1.4–9.3) | 49.1 (16.0–114.6) | 28 | 1 | 13 | 8.3 (1.2–46.1) | 77.0 (2.0–429.2) |
| EFV + TDF/3TC | 126 | 1 | 70 | 1.9 (0.3–12.6) | 14.3 (0.4–79.6) | 30 | 1 | 15 | 4.8 (0.7–29.3) | 68.0 (1.7–379.1) |
| EFV + ABC/3TC | 113 | 2 | 66 | 2.7 (0.6–11.0) | 30.3 (3.7–109.5) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.6) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; CI, confidence interval; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV, lopinavir; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; r, ritonavir; RAL, raltegravir; TDF, tenofovir; ZDV, zidovudine.
a“Other” category includes regimens with <2 NRTIs or alternative NRTI combinations than those listed above.
b“Other” includes regimens unable to be classified in the prior categories, such as those with none or >1 antiretroviral class.
Risk With Nucleos(t)ide Reverse-Transcriptase Inhibitor Combinations
Absolute risks and rates of aminotransferases >200 U/L for initiators of different NRTI combinations were similar among HIV-monoinfected and HIV/viral hepatitis-coinfected patients (Table 2). Relative hazards of this outcome associated with different NRTI combinations did not differ by viral hepatitis status (test of interaction, P > .20). Among HIV-monoinfected and viral hepatitis-coinfected persons, initiators of abacavir/lamivudine and zidovudine/lamivudine did not have a greater risk of aminotransferases >200 U/L versus tenofovir/emtricitabine (or lamivudine) initiators, after adjustment for baseline ALT, year of ART initiation, and data source (Figure 2, Model A).
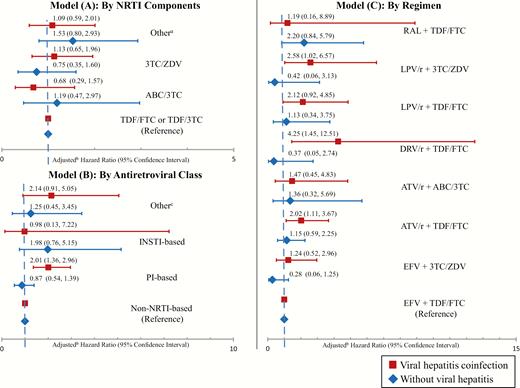
Comparative risk of liver aminotransferase levels >200 U/L among users of different nucleoside reverse-transcriptase inhibitor (NRTI) combinations (Model A), antiretroviral classes (Model B), and antiretroviral regimens (Model C). a“Other” includes regimens with <2 NRTIs or alternative NRTI combinations than those listed. bEach model adjusted for baseline alanine aminotransferase >40 U/L, calendar year of antiretroviral therapy initiation, and data source. cOther refers to regimens with none or >1 of the listed antiretroviral class. ABC, abacavir; ATV/r, boosted atazanvir; DRV/r, boosted darunavir; EFV, efavirenz; FTC, emtricitabine; INSTI, integrase inhibitor; LPV/r, boosted lopinavir; PI, protease inhibitor; TDF, tenofovir; ZDV, zidovudine; 3TC, lamivudine.
Risk With Antiretroviral Classes
Among HIV-monoinfected patients, absolute risks and rates of aminotransferases >200 U/L were similar for initiators of PI, non-NRTI, and INSTI classes (Table 2). Among viral hepatitis-coinfected patients, the rate of aminotransferases >200 U/L was higher for initiators of PI-based ART (165.8 [95% CI, 126.2–213.9] events/1000 person-years) than initiators of non-NRTI-based ART (82.7 [95% CI, 60.7–109.9] events/1000 person-years; P < .01) but not compared with initiators of INSTI-based ART (72.8 [95% CI, 1.8–405.8] events/1000 person-years; P = .40). Hazrd ratios of aminotransferases >200 U/L associated with antiretroviral classes differed by viral hepatitis status (test of interaction, P = .05). Among HIV-monoinfected persons, adjusted HRs did not differ between antiretroviral classes. However, among viral hepatitis-coinfected persons, those who initiated a PI-based regimen had a higher risk of aminotransferases >200 U/L than those initiating non-NRTI-based regimens (HR, 2.01; 95% CI, 1.36–2.96) (Figure 2, Model B). Among coinfected patients, initiation of INSTI-based ART was not associated with a higher risk of aminotransferases >200 U/L versus non-NRTI-based ART (HR, 0.98; 95% CI, 0.13–7.22).
Risk With Antiretroviral Therapy Regimens
Between 2004 and 2012, the most commonly prescribed ART regimens were as follows: efavirenz plus tenofovir/emtricitabine (44.2%) or zidovudine/lamivudine (7.4%); atazanavir/ritonavir plus tenofovir/emtricitabine (11.0%) or abacavir/lamivudine (2.0%); lopinavir/ritonavir plus tenofovir/emtricitabine (3.6%) or zidovudine/lamivudine (2.9%); darunavir/ritonavir plus tenofovir/emtricitabine (2.7%); and raltegravir plus tenofovir/emtricitabine (2.0%). Table 2 presents the absolute risks and rates of aminotransferases >200 U/L for these regimens, stratified by viral hepatitis status. Hazard ratios for different ART regimens did not differ by viral hepatitis status (test of interaction, P > .20). Among HIV-monoinfected persons, there were no significant differences in the risk of aminotransferases >200 U/L associated with initiation of these regimens compared with efavirenz plus tenofovir/emtricitabine, after adjustment for baseline ALT, year of ART initiation, and data source (Figure 2, Model C). Among viral hepatitis-coinfected individuals, there was a higher risk of aminotransferases >200 U/L associated with initiation of darunavir/ritonavir plus tenofovir/emtricitabine (HR, 4.25; 95% CI, 1.45–12.51), atazanavir/ritonavir plus tenofovir/emtricitabine (HR, 2.02; 95% CI, 1.11–3.67), and lopinavir/ritonavir plus zidovudine/lamivudine (HR, 2.58; 95% CI, 1.02–6.57) compared with efavirenz plus tenofovir/emtricitabine.
Severe Acute Liver Injury and Acute Liver Failure
Thirty (0.3%) patients developed severe ALI. Viral hepatitis-coinfected individuals had a higher rate of severe ALI than those without viral hepatitis (Table 3; Supplementary Appendix 4). Severe ALI events were too rare to allow evaluation within multivariable models. Among the 7970 patients without viral hepatitis, none developed ALF within the first year of ART initiation.
Cumulative Incidences and Incidence Rates of Severe Acute Liver Injury Among Initiators of Antiretroviral Drugs and Regimens Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012), Stratified by the Presence of Viral Hepatitis Coinfection
| . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen/Drug . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . |
| Overall | 7970 | 14 | 4515 | 0.2 (0.1–0.4) | 3.1 (1.7–5.2) | 2113 | 16 | 1005 | 1.2 (0.7–2.1) | 15.9 (9.1–25.9) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 12 | 3486 | 0.3 (0.1–0.5) | 3.4 (1.8–6.0) | 1343 | 9 | 695 | 1.1 (0.5–2.2) | 12.9 (5.9–24.6) |
| ABC/3TC | 392 | 0 | 212 | 0.0 (0.0–0.0) | 0.0 (0.0–14.1) | 162 | 2 | 72 | 1.4 (0.4–5.8) | 27.8 (3.4–100.5) |
| 3TC/ZDV | 1032 | 0 | 482 | 0.0 (0.0–0.0) | 0.0 (0.0–6.2) | 344 | 3 | 135 | 1.7 (0.5–6.0) | 22.2 (4.6–65.0) |
| Othera | 5762 | 2 | 334 | 0.3 (0.1–1.0) | 6.0 (0.7–21.6) | 264 | 2 | 103 | 0.8 (0.2–3.1) | 19.4 (2.4–70.2) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 4 | 2968 | 0.1 (0.0–0.3) | 1.3 (0.4–3.5) | 1148 | 10 | 581 | 1.5 (0.7–3.0) | 17.2 (8.3–31.7) |
| PI | 2520 | 10 | 1303 | 0.5 (0.3–0.9) | 7.7 (3.7–14.1) | 833 | 5 | 373 | 0.7 (0.3–1.6) | 13.4 (4.3–31.3) |
| INSTI | 195 | 0 | 114 | 0.0 (0.0–0.0) | 0.0 (0.0–26.3) | 33 | 0 | 14 | 0.0 (0.0–0.0) | 0.0 (0.0–210.8) |
| Otherb | 318 | 0 | 130 | 0.0 (0.0–0.0) | 0.0 (0.0–23.1) | 99 | 1 | 37 | 1.1 (0.2–7.4) | 27.1 (0.7–151.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 4 | 2337 | 0.1 (0.0–0.4) | 1.7 (0.5–4.4) | 761 | 6 | 409 | 1.4 (0.6–3.4) | 14.7 (5.4–31.9) |
| ATV/r + TDF/FTC | 854 | 5 | 513 | 0.8 (0.3–1.9) | 9.7 (3.2–22.7) | 256 | 1 | 132 | 0.4 (0.1–2.8) | 7.6 (0.2–42.3) |
| EFV + 3TC/ZDV | 556 | 0 | 271 | 0.0 (0.0–0.0) | 0.0 (0.0–11.1) | 189 | 3 | 74 | 3.2 (0.9–10.9) | 40.3 (8.3–117.9) |
| LPV/r + TDF/FTC | 276 | 2 | 132 | 0.7 (0.2–2.9) | 15.2 (1.8–54.9) | 90 | 0 | 41 | 0.0 (0.0–0.0) | 0.0 (0.0–72.3) |
| LPV/r + 3TC/ZDV | 221 | 0 | 95 | 0.0 (0.0–0.0) | 0.0 (0.0–31.5) | 70 | 0 | 27 | 0.0 (0.0–0.0) | 0.0 (0.0–109.7) |
| DRV/r + TDF/FTC | 224 | 0 | 120 | 0.0 (0.0–0.0) | 0.0 (0.0–25.0) | 46 | 1 | 23 | 2.3 (0.3–15.4) | 43.8 (1.1–244.0) |
| ATV/r + ABC/3TC | 150 | 0 | 81 | 0.0 (0.0–0.0) | 0.0 (0.0–37.0) | 55 | 0 | 26 | 0.0 (0.0–0.0) | 0.0 (0.0–114.4) |
| RAL + TDF/FTC | 174 | 0 | 104 | 0.0 (0.0–0.0) | 0.0 (0.0–28.9) | 28 | 0 | 13 | 0.0 (0.0–0.0) | 0.0 (0.0–222.5) |
| EFV + TDF/3TC | 126 | 0 | 70 | 0.0 (0.0–0.0) | 0.0 (0.0–42.7) | 30 | 0 | 15 | 0.0 (0.0–0.0) | 0.0 (0.0–201.5) |
| EFV + ABC/3TC | 113 | 0 | 66 | 0.0 (0.0–0.0) | 0.0 (0.0–45.3) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.8) |
| . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen/Drug . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . |
| Overall | 7970 | 14 | 4515 | 0.2 (0.1–0.4) | 3.1 (1.7–5.2) | 2113 | 16 | 1005 | 1.2 (0.7–2.1) | 15.9 (9.1–25.9) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 12 | 3486 | 0.3 (0.1–0.5) | 3.4 (1.8–6.0) | 1343 | 9 | 695 | 1.1 (0.5–2.2) | 12.9 (5.9–24.6) |
| ABC/3TC | 392 | 0 | 212 | 0.0 (0.0–0.0) | 0.0 (0.0–14.1) | 162 | 2 | 72 | 1.4 (0.4–5.8) | 27.8 (3.4–100.5) |
| 3TC/ZDV | 1032 | 0 | 482 | 0.0 (0.0–0.0) | 0.0 (0.0–6.2) | 344 | 3 | 135 | 1.7 (0.5–6.0) | 22.2 (4.6–65.0) |
| Othera | 5762 | 2 | 334 | 0.3 (0.1–1.0) | 6.0 (0.7–21.6) | 264 | 2 | 103 | 0.8 (0.2–3.1) | 19.4 (2.4–70.2) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 4 | 2968 | 0.1 (0.0–0.3) | 1.3 (0.4–3.5) | 1148 | 10 | 581 | 1.5 (0.7–3.0) | 17.2 (8.3–31.7) |
| PI | 2520 | 10 | 1303 | 0.5 (0.3–0.9) | 7.7 (3.7–14.1) | 833 | 5 | 373 | 0.7 (0.3–1.6) | 13.4 (4.3–31.3) |
| INSTI | 195 | 0 | 114 | 0.0 (0.0–0.0) | 0.0 (0.0–26.3) | 33 | 0 | 14 | 0.0 (0.0–0.0) | 0.0 (0.0–210.8) |
| Otherb | 318 | 0 | 130 | 0.0 (0.0–0.0) | 0.0 (0.0–23.1) | 99 | 1 | 37 | 1.1 (0.2–7.4) | 27.1 (0.7–151.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 4 | 2337 | 0.1 (0.0–0.4) | 1.7 (0.5–4.4) | 761 | 6 | 409 | 1.4 (0.6–3.4) | 14.7 (5.4–31.9) |
| ATV/r + TDF/FTC | 854 | 5 | 513 | 0.8 (0.3–1.9) | 9.7 (3.2–22.7) | 256 | 1 | 132 | 0.4 (0.1–2.8) | 7.6 (0.2–42.3) |
| EFV + 3TC/ZDV | 556 | 0 | 271 | 0.0 (0.0–0.0) | 0.0 (0.0–11.1) | 189 | 3 | 74 | 3.2 (0.9–10.9) | 40.3 (8.3–117.9) |
| LPV/r + TDF/FTC | 276 | 2 | 132 | 0.7 (0.2–2.9) | 15.2 (1.8–54.9) | 90 | 0 | 41 | 0.0 (0.0–0.0) | 0.0 (0.0–72.3) |
| LPV/r + 3TC/ZDV | 221 | 0 | 95 | 0.0 (0.0–0.0) | 0.0 (0.0–31.5) | 70 | 0 | 27 | 0.0 (0.0–0.0) | 0.0 (0.0–109.7) |
| DRV/r + TDF/FTC | 224 | 0 | 120 | 0.0 (0.0–0.0) | 0.0 (0.0–25.0) | 46 | 1 | 23 | 2.3 (0.3–15.4) | 43.8 (1.1–244.0) |
| ATV/r + ABC/3TC | 150 | 0 | 81 | 0.0 (0.0–0.0) | 0.0 (0.0–37.0) | 55 | 0 | 26 | 0.0 (0.0–0.0) | 0.0 (0.0–114.4) |
| RAL + TDF/FTC | 174 | 0 | 104 | 0.0 (0.0–0.0) | 0.0 (0.0–28.9) | 28 | 0 | 13 | 0.0 (0.0–0.0) | 0.0 (0.0–222.5) |
| EFV + TDF/3TC | 126 | 0 | 70 | 0.0 (0.0–0.0) | 0.0 (0.0–42.7) | 30 | 0 | 15 | 0.0 (0.0–0.0) | 0.0 (0.0–201.5) |
| EFV + ABC/3TC | 113 | 0 | 66 | 0.0 (0.0–0.0) | 0.0 (0.0–45.3) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.8) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; CI, confidence interval; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV, lopinavir; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; r, ritonavir; RAL, raltegravir; TDF, tenofovir; ZDV, zidovudine.
a“Other” category includes regimens with <2 NRTIs or alternative NRTI combinations than those listed above.
b“Other” includes regimens unable to be classified in the prior categories, such as those with none or >1 antiretroviral class.
Cumulative Incidences and Incidence Rates of Severe Acute Liver Injury Among Initiators of Antiretroviral Drugs and Regimens Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012), Stratified by the Presence of Viral Hepatitis Coinfection
| . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen/Drug . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . |
| Overall | 7970 | 14 | 4515 | 0.2 (0.1–0.4) | 3.1 (1.7–5.2) | 2113 | 16 | 1005 | 1.2 (0.7–2.1) | 15.9 (9.1–25.9) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 12 | 3486 | 0.3 (0.1–0.5) | 3.4 (1.8–6.0) | 1343 | 9 | 695 | 1.1 (0.5–2.2) | 12.9 (5.9–24.6) |
| ABC/3TC | 392 | 0 | 212 | 0.0 (0.0–0.0) | 0.0 (0.0–14.1) | 162 | 2 | 72 | 1.4 (0.4–5.8) | 27.8 (3.4–100.5) |
| 3TC/ZDV | 1032 | 0 | 482 | 0.0 (0.0–0.0) | 0.0 (0.0–6.2) | 344 | 3 | 135 | 1.7 (0.5–6.0) | 22.2 (4.6–65.0) |
| Othera | 5762 | 2 | 334 | 0.3 (0.1–1.0) | 6.0 (0.7–21.6) | 264 | 2 | 103 | 0.8 (0.2–3.1) | 19.4 (2.4–70.2) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 4 | 2968 | 0.1 (0.0–0.3) | 1.3 (0.4–3.5) | 1148 | 10 | 581 | 1.5 (0.7–3.0) | 17.2 (8.3–31.7) |
| PI | 2520 | 10 | 1303 | 0.5 (0.3–0.9) | 7.7 (3.7–14.1) | 833 | 5 | 373 | 0.7 (0.3–1.6) | 13.4 (4.3–31.3) |
| INSTI | 195 | 0 | 114 | 0.0 (0.0–0.0) | 0.0 (0.0–26.3) | 33 | 0 | 14 | 0.0 (0.0–0.0) | 0.0 (0.0–210.8) |
| Otherb | 318 | 0 | 130 | 0.0 (0.0–0.0) | 0.0 (0.0–23.1) | 99 | 1 | 37 | 1.1 (0.2–7.4) | 27.1 (0.7–151.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 4 | 2337 | 0.1 (0.0–0.4) | 1.7 (0.5–4.4) | 761 | 6 | 409 | 1.4 (0.6–3.4) | 14.7 (5.4–31.9) |
| ATV/r + TDF/FTC | 854 | 5 | 513 | 0.8 (0.3–1.9) | 9.7 (3.2–22.7) | 256 | 1 | 132 | 0.4 (0.1–2.8) | 7.6 (0.2–42.3) |
| EFV + 3TC/ZDV | 556 | 0 | 271 | 0.0 (0.0–0.0) | 0.0 (0.0–11.1) | 189 | 3 | 74 | 3.2 (0.9–10.9) | 40.3 (8.3–117.9) |
| LPV/r + TDF/FTC | 276 | 2 | 132 | 0.7 (0.2–2.9) | 15.2 (1.8–54.9) | 90 | 0 | 41 | 0.0 (0.0–0.0) | 0.0 (0.0–72.3) |
| LPV/r + 3TC/ZDV | 221 | 0 | 95 | 0.0 (0.0–0.0) | 0.0 (0.0–31.5) | 70 | 0 | 27 | 0.0 (0.0–0.0) | 0.0 (0.0–109.7) |
| DRV/r + TDF/FTC | 224 | 0 | 120 | 0.0 (0.0–0.0) | 0.0 (0.0–25.0) | 46 | 1 | 23 | 2.3 (0.3–15.4) | 43.8 (1.1–244.0) |
| ATV/r + ABC/3TC | 150 | 0 | 81 | 0.0 (0.0–0.0) | 0.0 (0.0–37.0) | 55 | 0 | 26 | 0.0 (0.0–0.0) | 0.0 (0.0–114.4) |
| RAL + TDF/FTC | 174 | 0 | 104 | 0.0 (0.0–0.0) | 0.0 (0.0–28.9) | 28 | 0 | 13 | 0.0 (0.0–0.0) | 0.0 (0.0–222.5) |
| EFV + TDF/3TC | 126 | 0 | 70 | 0.0 (0.0–0.0) | 0.0 (0.0–42.7) | 30 | 0 | 15 | 0.0 (0.0–0.0) | 0.0 (0.0–201.5) |
| EFV + ABC/3TC | 113 | 0 | 66 | 0.0 (0.0–0.0) | 0.0 (0.0–45.3) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.8) |
| . | Without Viral Hepatitis Coinfection . | With Viral Hepatitis Coinfection . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen/Drug . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . | No. Exposed . | No. Events . | Person-Time . | Cumulative Incidence at 1 Year/100 Persons (95% CI) . | Incidence Rate, Events/1000 Person-Years (95% CI) . |
| Overall | 7970 | 14 | 4515 | 0.2 (0.1–0.4) | 3.1 (1.7–5.2) | 2113 | 16 | 1005 | 1.2 (0.7–2.1) | 15.9 (9.1–25.9) |
| NRTI Components | ||||||||||
| TDF/FTC or TDF/3TC | 5762 | 12 | 3486 | 0.3 (0.1–0.5) | 3.4 (1.8–6.0) | 1343 | 9 | 695 | 1.1 (0.5–2.2) | 12.9 (5.9–24.6) |
| ABC/3TC | 392 | 0 | 212 | 0.0 (0.0–0.0) | 0.0 (0.0–14.1) | 162 | 2 | 72 | 1.4 (0.4–5.8) | 27.8 (3.4–100.5) |
| 3TC/ZDV | 1032 | 0 | 482 | 0.0 (0.0–0.0) | 0.0 (0.0–6.2) | 344 | 3 | 135 | 1.7 (0.5–6.0) | 22.2 (4.6–65.0) |
| Othera | 5762 | 2 | 334 | 0.3 (0.1–1.0) | 6.0 (0.7–21.6) | 264 | 2 | 103 | 0.8 (0.2–3.1) | 19.4 (2.4–70.2) |
| Antiretroviral Class | ||||||||||
| Non-NRTI | 4937 | 4 | 2968 | 0.1 (0.0–0.3) | 1.3 (0.4–3.5) | 1148 | 10 | 581 | 1.5 (0.7–3.0) | 17.2 (8.3–31.7) |
| PI | 2520 | 10 | 1303 | 0.5 (0.3–0.9) | 7.7 (3.7–14.1) | 833 | 5 | 373 | 0.7 (0.3–1.6) | 13.4 (4.3–31.3) |
| INSTI | 195 | 0 | 114 | 0.0 (0.0–0.0) | 0.0 (0.0–26.3) | 33 | 0 | 14 | 0.0 (0.0–0.0) | 0.0 (0.0–210.8) |
| Otherb | 318 | 0 | 130 | 0.0 (0.0–0.0) | 0.0 (0.0–23.1) | 99 | 1 | 37 | 1.1 (0.2–7.4) | 27.1 (0.7–151.1) |
| Commonly Used Regimens | ||||||||||
| EFV/TDF/FTC | 3697 | 4 | 2337 | 0.1 (0.0–0.4) | 1.7 (0.5–4.4) | 761 | 6 | 409 | 1.4 (0.6–3.4) | 14.7 (5.4–31.9) |
| ATV/r + TDF/FTC | 854 | 5 | 513 | 0.8 (0.3–1.9) | 9.7 (3.2–22.7) | 256 | 1 | 132 | 0.4 (0.1–2.8) | 7.6 (0.2–42.3) |
| EFV + 3TC/ZDV | 556 | 0 | 271 | 0.0 (0.0–0.0) | 0.0 (0.0–11.1) | 189 | 3 | 74 | 3.2 (0.9–10.9) | 40.3 (8.3–117.9) |
| LPV/r + TDF/FTC | 276 | 2 | 132 | 0.7 (0.2–2.9) | 15.2 (1.8–54.9) | 90 | 0 | 41 | 0.0 (0.0–0.0) | 0.0 (0.0–72.3) |
| LPV/r + 3TC/ZDV | 221 | 0 | 95 | 0.0 (0.0–0.0) | 0.0 (0.0–31.5) | 70 | 0 | 27 | 0.0 (0.0–0.0) | 0.0 (0.0–109.7) |
| DRV/r + TDF/FTC | 224 | 0 | 120 | 0.0 (0.0–0.0) | 0.0 (0.0–25.0) | 46 | 1 | 23 | 2.3 (0.3–15.4) | 43.8 (1.1–244.0) |
| ATV/r + ABC/3TC | 150 | 0 | 81 | 0.0 (0.0–0.0) | 0.0 (0.0–37.0) | 55 | 0 | 26 | 0.0 (0.0–0.0) | 0.0 (0.0–114.4) |
| RAL + TDF/FTC | 174 | 0 | 104 | 0.0 (0.0–0.0) | 0.0 (0.0–28.9) | 28 | 0 | 13 | 0.0 (0.0–0.0) | 0.0 (0.0–222.5) |
| EFV + TDF/3TC | 126 | 0 | 70 | 0.0 (0.0–0.0) | 0.0 (0.0–42.7) | 30 | 0 | 15 | 0.0 (0.0–0.0) | 0.0 (0.0–201.5) |
| EFV + ABC/3TC | 113 | 0 | 66 | 0.0 (0.0–0.0) | 0.0 (0.0–45.3) | 41 | 1 | 19 | 2.4 (0.3–16.1) | 52.2 (1.3–290.8) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; CI, confidence interval; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV, lopinavir; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; r, ritonavir; RAL, raltegravir; TDF, tenofovir; ZDV, zidovudine.
a“Other” category includes regimens with <2 NRTIs or alternative NRTI combinations than those listed above.
b“Other” includes regimens unable to be classified in the prior categories, such as those with none or >1 antiretroviral class.
Risk Factors for Acute Liver Injury Events
Factors associated with development of aminotransferases >200 U/L included viral hepatitis and baseline ALT >40 U/L (Table 4). Viral hepatitis, heart failure, age >50 years, and higher baseline HIV RNA were associated with severe ALI (Table 4).
Risk Factors for Liver Aminotransferases >200 U/L and Severe Acute Liver Injury Among HIV-Infected Individuals Who Were New Initiators of Antiretroviral Therapy Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012)
| Characteristic . | Unadjusted HR of Liver Aminotransferases >200 U/L (95% CI) . | Adjusted HRa of Liver Aminotransferases >200 U/L (95% CI) . | Unadjusted HRb of Severe ALI (95% CI) . |
|---|---|---|---|
| Age | |||
| 18–50 years | Reference | Reference | Reference |
| ≥50 years | 1.25 (0.95–1.64) | 0.89 (0.66–1.19) | 2.66 (1.25–5.69) |
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 0.87 (0.45–1.70) | 1.11 (0.56–2.21) | 2.10 (0.64–6.93) |
| Race | |||
| Non-Black | Reference | Reference | Reference |
| Black | 1.17 (0.89–1.53) | 0.88 (0.66–1.19) | 1.44 (0.70–2.96) |
| Body Mass Index | |||
| <30 kg/m2 | Reference | Reference | Reference |
| ≥30 kg/m2 | 0.92 (0.64–1.32) | 0.93 (0.64–1.35) | 0.90 (0.34–2.35) |
| Viral Hepatitis Coinfection | |||
| Uninfected | Reference | Reference | Reference |
| HCV or HBV-infected | 5.26 (4.00–6.93) | 4.21 (3.10–5.72) | 4.64 (2.26–9.51) |
| History of Alcohol Dependence/Abuse | |||
| No | Reference | Reference | Reference |
| Yes | 1.63 (1.22–2.19) | 1.10 (0.81–1.50) | 1.47 (0.67–3.20) |
| Diabetes Mellitus | |||
| No | Reference | Reference | Reference |
| Yes | 1.46 (0.97–2.21) | 1.22 (0.80–1.87) | 2.00 (0.77–5.23) |
| Heart Failure | |||
| No | Reference | Reference | Reference |
| Yes | 0.86 (0.27–2.68) | 0.76 (0.24–2.42) | 6.26 (1.90–20.66) |
| HIV RNA (per log10 copies/mL) | 0.95 (0.80–1.12) | 0.90 (0.75–1.08) | 1.82 (1.10–3.01) |
| CD4 cell count | |||
| ≥500 cells/mm3 | Reference | Reference | Reference |
| 200–500 cells/mm3 | 1.39 (0.78–2.48) | 1.38 (0.76–2.48) | 0.65 (0.14–3.14) |
| <200 cells/mm3 | 1.73 (0.96–3.10) | 1.68 (0.91–3.10) | 2.62 (0.61–11.16) |
| Baseline ALT | |||
| ALT <40 IU/mL | Reference | Reference | Reference |
| ALT ≥40 IU/mL | 3.38 (2.55–4.49) | 2.49 (1.85–3.34) | 1.92 (0.93–3.96) |
| Characteristic . | Unadjusted HR of Liver Aminotransferases >200 U/L (95% CI) . | Adjusted HRa of Liver Aminotransferases >200 U/L (95% CI) . | Unadjusted HRb of Severe ALI (95% CI) . |
|---|---|---|---|
| Age | |||
| 18–50 years | Reference | Reference | Reference |
| ≥50 years | 1.25 (0.95–1.64) | 0.89 (0.66–1.19) | 2.66 (1.25–5.69) |
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 0.87 (0.45–1.70) | 1.11 (0.56–2.21) | 2.10 (0.64–6.93) |
| Race | |||
| Non-Black | Reference | Reference | Reference |
| Black | 1.17 (0.89–1.53) | 0.88 (0.66–1.19) | 1.44 (0.70–2.96) |
| Body Mass Index | |||
| <30 kg/m2 | Reference | Reference | Reference |
| ≥30 kg/m2 | 0.92 (0.64–1.32) | 0.93 (0.64–1.35) | 0.90 (0.34–2.35) |
| Viral Hepatitis Coinfection | |||
| Uninfected | Reference | Reference | Reference |
| HCV or HBV-infected | 5.26 (4.00–6.93) | 4.21 (3.10–5.72) | 4.64 (2.26–9.51) |
| History of Alcohol Dependence/Abuse | |||
| No | Reference | Reference | Reference |
| Yes | 1.63 (1.22–2.19) | 1.10 (0.81–1.50) | 1.47 (0.67–3.20) |
| Diabetes Mellitus | |||
| No | Reference | Reference | Reference |
| Yes | 1.46 (0.97–2.21) | 1.22 (0.80–1.87) | 2.00 (0.77–5.23) |
| Heart Failure | |||
| No | Reference | Reference | Reference |
| Yes | 0.86 (0.27–2.68) | 0.76 (0.24–2.42) | 6.26 (1.90–20.66) |
| HIV RNA (per log10 copies/mL) | 0.95 (0.80–1.12) | 0.90 (0.75–1.08) | 1.82 (1.10–3.01) |
| CD4 cell count | |||
| ≥500 cells/mm3 | Reference | Reference | Reference |
| 200–500 cells/mm3 | 1.39 (0.78–2.48) | 1.38 (0.76–2.48) | 0.65 (0.14–3.14) |
| <200 cells/mm3 | 1.73 (0.96–3.10) | 1.68 (0.91–3.10) | 2.62 (0.61–11.16) |
| Baseline ALT | |||
| ALT <40 IU/mL | Reference | Reference | Reference |
| ALT ≥40 IU/mL | 3.38 (2.55–4.49) | 2.49 (1.85–3.34) | 1.92 (0.93–3.96) |
Abbreviations: ALI, acute liver injury; ALT, alanine aminotransferase; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; RNA, ribonucleic acid.
aModel adjusted for all covariates shown as well as calendar year of antiretroviral therapy initiation and data source.
bDue to the low number of severe ALI events, adjusted analyses could not be performed.
Risk Factors for Liver Aminotransferases >200 U/L and Severe Acute Liver Injury Among HIV-Infected Individuals Who Were New Initiators of Antiretroviral Therapy Within Kaiser Permanente Northern California (2004–2010) and the Veterans Aging Cohort Study (2004–2012)
| Characteristic . | Unadjusted HR of Liver Aminotransferases >200 U/L (95% CI) . | Adjusted HRa of Liver Aminotransferases >200 U/L (95% CI) . | Unadjusted HRb of Severe ALI (95% CI) . |
|---|---|---|---|
| Age | |||
| 18–50 years | Reference | Reference | Reference |
| ≥50 years | 1.25 (0.95–1.64) | 0.89 (0.66–1.19) | 2.66 (1.25–5.69) |
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 0.87 (0.45–1.70) | 1.11 (0.56–2.21) | 2.10 (0.64–6.93) |
| Race | |||
| Non-Black | Reference | Reference | Reference |
| Black | 1.17 (0.89–1.53) | 0.88 (0.66–1.19) | 1.44 (0.70–2.96) |
| Body Mass Index | |||
| <30 kg/m2 | Reference | Reference | Reference |
| ≥30 kg/m2 | 0.92 (0.64–1.32) | 0.93 (0.64–1.35) | 0.90 (0.34–2.35) |
| Viral Hepatitis Coinfection | |||
| Uninfected | Reference | Reference | Reference |
| HCV or HBV-infected | 5.26 (4.00–6.93) | 4.21 (3.10–5.72) | 4.64 (2.26–9.51) |
| History of Alcohol Dependence/Abuse | |||
| No | Reference | Reference | Reference |
| Yes | 1.63 (1.22–2.19) | 1.10 (0.81–1.50) | 1.47 (0.67–3.20) |
| Diabetes Mellitus | |||
| No | Reference | Reference | Reference |
| Yes | 1.46 (0.97–2.21) | 1.22 (0.80–1.87) | 2.00 (0.77–5.23) |
| Heart Failure | |||
| No | Reference | Reference | Reference |
| Yes | 0.86 (0.27–2.68) | 0.76 (0.24–2.42) | 6.26 (1.90–20.66) |
| HIV RNA (per log10 copies/mL) | 0.95 (0.80–1.12) | 0.90 (0.75–1.08) | 1.82 (1.10–3.01) |
| CD4 cell count | |||
| ≥500 cells/mm3 | Reference | Reference | Reference |
| 200–500 cells/mm3 | 1.39 (0.78–2.48) | 1.38 (0.76–2.48) | 0.65 (0.14–3.14) |
| <200 cells/mm3 | 1.73 (0.96–3.10) | 1.68 (0.91–3.10) | 2.62 (0.61–11.16) |
| Baseline ALT | |||
| ALT <40 IU/mL | Reference | Reference | Reference |
| ALT ≥40 IU/mL | 3.38 (2.55–4.49) | 2.49 (1.85–3.34) | 1.92 (0.93–3.96) |
| Characteristic . | Unadjusted HR of Liver Aminotransferases >200 U/L (95% CI) . | Adjusted HRa of Liver Aminotransferases >200 U/L (95% CI) . | Unadjusted HRb of Severe ALI (95% CI) . |
|---|---|---|---|
| Age | |||
| 18–50 years | Reference | Reference | Reference |
| ≥50 years | 1.25 (0.95–1.64) | 0.89 (0.66–1.19) | 2.66 (1.25–5.69) |
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 0.87 (0.45–1.70) | 1.11 (0.56–2.21) | 2.10 (0.64–6.93) |
| Race | |||
| Non-Black | Reference | Reference | Reference |
| Black | 1.17 (0.89–1.53) | 0.88 (0.66–1.19) | 1.44 (0.70–2.96) |
| Body Mass Index | |||
| <30 kg/m2 | Reference | Reference | Reference |
| ≥30 kg/m2 | 0.92 (0.64–1.32) | 0.93 (0.64–1.35) | 0.90 (0.34–2.35) |
| Viral Hepatitis Coinfection | |||
| Uninfected | Reference | Reference | Reference |
| HCV or HBV-infected | 5.26 (4.00–6.93) | 4.21 (3.10–5.72) | 4.64 (2.26–9.51) |
| History of Alcohol Dependence/Abuse | |||
| No | Reference | Reference | Reference |
| Yes | 1.63 (1.22–2.19) | 1.10 (0.81–1.50) | 1.47 (0.67–3.20) |
| Diabetes Mellitus | |||
| No | Reference | Reference | Reference |
| Yes | 1.46 (0.97–2.21) | 1.22 (0.80–1.87) | 2.00 (0.77–5.23) |
| Heart Failure | |||
| No | Reference | Reference | Reference |
| Yes | 0.86 (0.27–2.68) | 0.76 (0.24–2.42) | 6.26 (1.90–20.66) |
| HIV RNA (per log10 copies/mL) | 0.95 (0.80–1.12) | 0.90 (0.75–1.08) | 1.82 (1.10–3.01) |
| CD4 cell count | |||
| ≥500 cells/mm3 | Reference | Reference | Reference |
| 200–500 cells/mm3 | 1.39 (0.78–2.48) | 1.38 (0.76–2.48) | 0.65 (0.14–3.14) |
| <200 cells/mm3 | 1.73 (0.96–3.10) | 1.68 (0.91–3.10) | 2.62 (0.61–11.16) |
| Baseline ALT | |||
| ALT <40 IU/mL | Reference | Reference | Reference |
| ALT ≥40 IU/mL | 3.38 (2.55–4.49) | 2.49 (1.85–3.34) | 1.92 (0.93–3.96) |
Abbreviations: ALI, acute liver injury; ALT, alanine aminotransferase; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; RNA, ribonucleic acid.
aModel adjusted for all covariates shown as well as calendar year of antiretroviral therapy initiation and data source.
bDue to the low number of severe ALI events, adjusted analyses could not be performed.
DISCUSSION
In this study of HIV-infected patients initiating ART within 2 of the largest integrated healthcare systems in the United States, we found low absolute risks and rates of ALI within the first year of ART. Although liver aminotransferases >200 U/L and grade 3/4 elevations occurred in 2% of the cohort, severe ALI, manifested by coagulopathy and hyperbilirubinemia, developed in <1%, and no ALF events were identified among patients without viral hepatitis coinfection. Rates of ALI were higher for viral hepatitis-coinfected patients. Among these individuals, initiation of PI-based ART was associated with a higher risk of aminotransferases >200 U/L compared with initiation of non-NRTI-based ART. The risk was significantly higher for coinfected initiators of darunavir/ritonavir plus tenofovir/emtricitabine, atazanavir/ritonavir plus tenofovir/emtricitabine, and lopinavir/ritonavir plus zidovudine/lamivudine compared with those initiating efavirenz plus tenofovir/emtricitabine. Among HIV-monoinfected patients, no differences in the risk of this outcome were found among different NRTI combinations; PI, INSTI, or non-NRTI classes; or ART regimens. Taken together, these results highlight the rarity of antiretroviral-associated ALI and provide evidence for the hepatic safety of these regimens among HIV-infected patients with and without viral hepatitis.
As in prior studies of antiretroviral-associated hepatotoxicity in the early ART era [6, 11, 13, 33], viral hepatitis coinfection was associated with higher rates of ALI. Our observation that use of PI-based ART increased the risk of aminotransferase elevations compared with non-NRTI-based ART among viral hepatitis-coinfected patients is consistent with a study of 155 HIV/HCV-coinfected patients from 5 sites across Italy. Protease inhibitor-based ART initiators had a higher incidence of aminotransferase elevations >5 times ULN than non-NRTI-based ART initiators [15]. A study of 568 HIV-infected patients initiating ART at Johns Hopkins Hospital HIV Clinic found that concurrent PI and non-NRTI use was associated with increased risk of hepatotoxicity among viral hepatitis-coinfected patients [14]. Finally, an analysis of 1982 HIV-infected patients initiating ART at Hospital Carlos III in Spain found that aminotransferase elevations >5 times baseline occurred more commonly in HIV/HCV-coinfected patients treated with PIs, particularly darunavir and atazanavir, than with raltegravir or etravirine [34]. The reasons why PIs might increase the risk of aminotransferase elevations among viral hepatitis-coinfected patients remain unclear. Protease inhibitors are associated with up-regulation of proinflammatory cytokines and alterations in lipid metabolism that could promote liver injury [35, 36], and these effects might be exacerbated by viral hepatitis. Viral hepatitis-mediated liver disease may also impair drug metabolism and clearance, further contributing to drug-related hepatotoxicity [37]. More importantly, although the risk of aminotransferase elevations after PI-based ART initiation was increased in coinfected patients, there were very few severe ALI events among these individuals. Thus, providers should not be reluctant to initiate PIs in viral hepatitis-coinfected patients.
This study identified several factors, including viral hepatitis coinfection, heart failure, older age, and higher pre-ART HIV RNA levels, that might increase ALI risk. Viral hepatitis and heart failure can each contribute to hepatic fibrosis, which could impair the liver’s ability to tolerate acute insults from drug-induced ALI [37, 38]. Older HIV-infected patients are more likely to have other comorbidities and polypharmacy [39], which could increase exposure to hepatotoxic medications or drug-drug interactions. Finally, patients with high pre-ART HIV RNA levels might have more vigorous immune reconstitution after ART initiation. Patients with these characteristics might benefit from closer monitoring of liver aminotransferases after ART initiation.
Our study has several potential limitations. First, severe ALI and ALF events were rare, and analyses of these outcomes could not adjust for potential confounders. Moreover, absolute risks and rates for some antiretrovirals, such as integrase inhibitors, were based on small sample sizes. Second, the ALI outcomes in this study could have been due to other conditions, such as patient comorbidities or concurrent medications for which we did not collect complete data. To minimize this possibility, we focused on ALI events that developed within the first year of ART initiation. Third, because pre-existing liver disease precludes a diagnosis of ALF [24], we did not determine this outcome among viral hepatitis-coinfected patients. For patients with pre-existing liver disease, definitions for the acute deterioration of liver function due to drug-induced or other etiologies (“acute-on-chronic liver failure”) have only recently been proposed [40]. Validated methods to ascertain these events within electronic health data have not been developed, preventing us from ascertaining them among hepatitis-coinfected patients.
CONCLUSIONS
In summary, severe ALI events were rare among HIV-infected persons initiating ART. Patients with viral hepatitis had higher rates of aminotransferase elevations and hepatic dysfunction than those with HIV alone. Among viral hepatitis-coinfected patients, initiation of PI-based ART, particularly with darunavir/ritonavir, atazanavir/ritonavir, and lopinavir/ritonavir, was associated with a higher risk of aminotransferase elevations than with non-NRTI-based ART, but acute hepatic dysfunction was uncommon among these individuals.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. All authors had access to the data and a role in writing this manuscript. C. G. and V. L. R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analyses. C. G., J. R. K., D. A. C., and V. L. R contributed to the study concept and design. D. M. C., K. A. F., D. S. G., K. R. R., A. R. M., J. L. S., J. P. T., A. C. J., D. A. C., and V. L. R. acquired the data. C. G., C. W. N., Q. L., D. M. C., J. A. R., A. R. M., J. L. S., and D. A. C. analyzed and interpreted the data. C. G., C. W. N., D. M. C., J. A. R., J. R. K., D. A. C., and V. L. R. drafted the manuscript. C. G., C. W. N., Q. L., D. M. C., J. D. L., K. A. F., D. S. G., K. R. R., J. A. R., A. R. M., J. L. S., J. R. K., J. P. T., J. K. L., A. C. J., M. B. G., D. A. C., and V. L. R. critically reviewed the manuscript. V. L. R. obtained funding. D. M. C., J. P. T., J. L. S., A. C. J., D. A. C., and V. L. R. supervised the study.
Disclaimer. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Financial support. This study was funded by research grants from the Agency for Healthcare Research and Quality (Grant R01 HS018372; to V. L. R.), the National Institutes of Health (Grants F32 AI120363 [to C. G.] and K24 DK078228 [to J. D. L.]), and the National Institute on Alcohol Abuse and Alcoholism (Grants U01 AA13566, U24 AA20794, and U01 AA20790; to A. C. J.).
Potential conflicts of interest. V. L. R., D. M. C., and J. A. R. have received research grant support (to the University of Pennsylvania) from AstraZeneca. J. D. L. has received research grant support (to the University of Pennsylvania) from Bayer, Nestle Health Science, and Takeda and has served as a consultant to AstraZeneca, Amgen, MedImmune, Merck, Nestle Health Science, Gilead, Pfizer, Rebiotix, and Takeda. D. S. G. has received research grant support (to the University of Pennsylvania) from Bayer HealthCare, Intercept Pharmaceuticals, and Merck. K. R. R. has received research grant support (to the University of Pennsylvania) from Abbvie, Bristol-Myers Squibb, Gilead, Janssen, and Merck and has served as an advisor to Abbvie, Bristol-Myers Squibb, Gilead, Janssen, and Merck. D. A. C. has received research grant support (to Kaiser Permanente) from Pfizer. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Correspondence: V. Lo Re III, MD, MSCE, Center for Clinical Epidemiology and Biostatistics, 836 Blockley Hall, 423 Guardian Drive, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104-6021 ([email protected]).





Comments