-
PDF
- Split View
-
Views
-
Cite
Cite
Anaïs Lesourd, Jérémie Leporrier, Valérie Delbos, Guillemette Unal, Patricia Honoré, Manuel Etienne, Olivier Bouchaud, François Caron, Antiretroviral Therapy as Prevention of … Pneumococcal Infections?, Open Forum Infectious Diseases, Volume 3, Issue 4, Fall 2016, ofw228, https://doi.org/10.1093/ofid/ofw228
Close - Share Icon Share
Abstract
Despite antiretroviral therapy, it is generally believed that the risk for pneumococcal infections (PnIs) is high among patients infected with human immunodeficiency virus (HIV). However, most studies in this field have been conducted before 2010, and the proportion of virologically suppressed patients has drastically increased in these latter years thanks to larger indications and more effective antiretroviral regimens. This study aimed to re-evaluate the current risk of PnI among adult patients infected with HIV.
The incidence of PnI was evaluated between 1996 and 2014 in 2 French regional hospitals. The 80 most recent cases of PnI (2000–2014) were retrospectively compared with 160 controls (HIV patients without PnI) to analyze the residual risk factors of PnI.
Among a mean annual follow-up cohort of 1616 patients, 116 PnIs were observed over 18 years. The risk factors of PnI among patients infected with HIV were an uncontrolled HIV infection or “classic” risk factors of PnI shared by the general population such as addiction, renal or respiratory insufficiency, or hepatitis B or C coinfection. Pneumococcal vaccination coverage was low and poorly targeted, because only 5% of the cases had been previously vaccinated. The incidence of invasive PnIs among HIV patients with a nonvirologically suppressed infection or comorbidities was 12 times higher than that reported in the general population at the country level (107 vs 9/100000 patients), whereas the incidence among virologically suppressed HIV patients without comorbidities was lower (7.6/100000 patients).
Human immunodeficiency virus infection no longer per se seems to be a significant risk factor for PnI, suggesting a step-down from a systematic to an “at-risk patient” targeted pneumococcal vaccination strategy.
During the early years of the human immunodeficiency virus (HIV) outbreak, it was established that patients at an advanced stage of HIV infection were at increased risk for pneumococcal infections [1–4]. A higher rate of bacteremia and recurrent pneumococcal infections were described in this population, and they were included in the Centers for Disease Control and Prevention (CDC) criteria for acquired immune deficiency syndrome [5–7]. As a result, the polysaccharide pneumococcal vaccination was recommended (either optional or mandatory) to patients infected with HIV in numerous countries [5, 8, 9]. More recently, the conjugated pneumococcal vaccine used in a prime-boost sequence of immunization has demonstrated a better immunological response in patients infected with HIV as well as in other recipients [10–12]. Therefore, since 2013, a double vaccination by the 13-valence pneumococcal conjugate vaccine followed 8 weeks later by the 23-valent pneumococcal polysaccharide vaccine is recommended in all patients infected with HIV in France [13]. These recommendations are based on different studies showing a higher risk of pneumococcal infections in patients infected with HIV than in the general population [1, 2, 14, 15], despite the progress in antiretroviral therapy (ART) [16]. Few recent epidemiological studies have assessed the residual risk of pneumococcal infections in patients infected with HIV [16–18], and the proportion of virologically suppressed patients infected with HIV has dramatically increased these last years, thanks to more effective regimens and larger indications.
In daily care, it seems that pneumococcal infections are becoming an uncommon complication of HIV infection: in our ward, patients hospitalized for pneumococcal meningitis, bacteremia, or pneumonia are no longer infected with HIV, which suggests that pneumococcal infections in patients infected with HIV may no longer represent a significant burden. It also seems that pneumococcal vaccination is not applied to all patients infected with HIV despite the recommendations [19], possibly because the perceived benefits are not so obvious. Thus, the current study aimed to re-evaluate the current risk of pneumococcal infections in patients infected with HIV and the coverage for pneumococcal vaccination among a high proportion of virologically suppressed subjects.
METHODS
This retrospective case-control study was conducted in 2 French regional university-hospitals, located in Rouen (Normandy) and Bobigny (Ile-de-France). Both hospitals are reference centers for the management of HIV infection, and they each currently observe approximately 1200 adults/year (2400/years for both centers).
Pneumococcal infections were identified from the computerized database from each center. Pneumococcal infections included the following: (1) noninvasive pneumococcal infections, ie, respiratory infection for which Streptococcus pneumoniae was isolated in a respiratory sample or by urinary antigen (Binax-NOW); and (2) invasive pneumococcal infections, ie, infections such as bacteremia, meningitis, pleural effusion, or arthritis for which S pneumoniae was isolated in a normally sterile site (blood, cerebrospinal fluid, pleural fluid, or joint fluid). A recurrent infection was defined by the subsequent isolation of an S pneumoniae at least 30 days after a previous episode.
First, we evaluated the evolution of pneumococcal infection in HIV patients between 1996 and 2014. Therefore, the annual incidence of pneumococcal infections leading to hospitalization (both invasive and noninvasive infections) in adult patients infected with HIV (expressed for 100000 patients) was estimated for the total 1996–2014 cohort (mean of 1616 patients in annual follow-up among the total study cohort). The denominator was the number of HIV patients in follow-up per year summed for both hospitals; it was obtained via NADIS (French national HIV database). The incidence of invasive pneumococcal infections (restricted to bacteriemia and meningitis) was then compared with the data available at the country level for adults aged >18 years from the French control diseases center (Santé Publique France) that do not include pleural effusion and arthritis [20].
Second, a case-control (1:2) analysis was performed to identify residual risk factors for pneumococcal infections among adult patients infected with HIV. To assess a population with higher access to ART, the period of analysis was truncated to 2000–2014 (the 2000–2014 years correspond with a trend for early treatment in the course of HIV disease). For each case, 2 HIV-infected adult patients free of pneumococcal infections were matched by their date of HIV diagnosis in the same center. In that way, cases and their controls had the same duration of follow-up for their HIV infection.
Clinical and biological data for each subject were collected from the patient’s electronic medical record. A French national computerized database for every patient infected with HIV in follow-up was implemented in 1988 (DIM-1), with multiple new versions since then (DIM-2 in 1992, NADIS in 2007), listing the patients’medical history, HIV-status, biological parameters, treatments, and vaccinal status. In case of a missing data, the patient’s paper file was consulted.
For each subject (case or control), the following data were collected: demographic data (age, sex, geographic origin), comorbidities, HIV-infection characteristics (mode of contamination, CDC classification [21], nadir of CD4, zenith of HIV plasma viral load, length of follow up, ART instauration, and number of lines), and pneumococcal infections characteristics (site of infection, CD4 cell counts, and HIV plasma viral load at the time of the infection). At the same time, the pneumococcal vaccination status and the date of vaccination, both of which were available in the computerized patient’s file, were retrieved for each patient. The Charlson comorbidity index was calculated for each patient, using the modified index excluding the HIV item [22]. A patient was considered to have an undetectable viral load (or virologically suppressed) according to the following definition: HIV viral load <50 copies/mL, which is the highest threshold of detection in the laboratories of the 2 hospitals since 2000.
Finally, the cases and their controls were pooled to analyze the adhesion to pneumococcal vaccination. For the paired case-control study, the McNemar test was used to compare the distribution of categorical variables, and the Student t test was used for continuous variables in a univariate analysis. Variables associated with pneumococcal infections with P < .20 in the univariate analysis were retained in a multivariate analysis. Using unconditional analysis (realized by forcing the matching variables) and forward stepwise regression, variables independently associated with pneumococcal infections at the 5% level were selected. For the pneumococcal vaccination study, the χ2 test (or Fisher’s exact test) was used for categorical variables, and the Student t test was used for continuous variables. Differences were considered significant at P < .05, corresponding to 95% confidence intervals (CIs). Epi Info 3.5.4 statistical software was used to perform the analysis. According to the French legislation, because this observational study did not modify the physicians’ clinical decisions, neither the patient’s consent nor an ethics committee group agreement were required.
RESULTS
Incidence of Pneumococcal Infections
Between 1996 and 2014, 116 cases of pneumococcal infections were identified, occurring among 93 patients. They included 53 (46%) patients presenting with noninvasive pneumococcal infections, 40 (34%) patients with invasive pneumococcal infections (36 bacteremia, 2 meningitis, 1 pleural infection, 1 arthritis), and 23 (20%) recurrent infections affecting 18 of these patients (2–4 episodes of pneumococcal infections per subject). The mortality rate was low, with only 2 attributable deaths (1.7% of the cases, 2.2% of the total cohort).
The mean annual incidence of pneumococcal infections for the total cohort was of 373/100000 patients, decreasing by more than half from 377/100000 in 1996 to 174/100000 in 2014, with the highest incidences reached in 1999 (663/100 000), 2003 (646/100 000), and 2008 (521/100 000). The mean annual incidence of noninvasive pneumococcal infections and invasive pneumococcal infections were of 178/100000 and 132/100000 patients, respectively, and the incidence of each decreased by 77% in 18 years (same incidence for both in 1996 and 2014 decreasing from 188/100000 in 1996 to 43/100000 in 2014).
As shown in Figure 1, during the 2000–2014 period, the pneumococcal infection incidence in patients infected with HIV progressively decreased as the incidence of patients with an undetectable viral load inversely increased. The same observation was made when comparing the evolution of the incidence of pneumococcal infections with the incidence of patients with CD4 T cells >500/mm3.
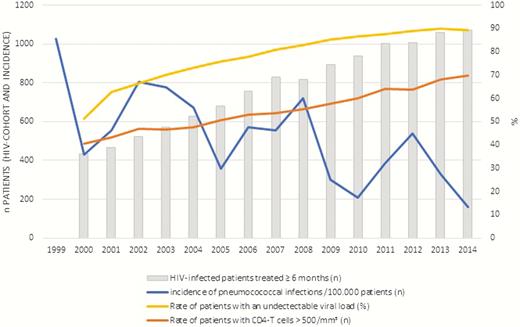
Cross-evolution of the incidence of pneumococcal infections and rate of patients with an undetectable viral load and CD4 T cells >500/mm3 in the Rouen human immunodeficiency virus (HIV) cohort (data unavailable for all years in Avicenne).
Risk Factors for Pneumococcal Infection
Between 2000 and 2014, 98 pneumococcal infections were identified in 80 patients. They represented 57 (58%) noninvasive pneumococcal infections and 41 (42%) invasive pneumococcal infections, 18 (18%) of which were recurrent infections. Of note, the hospitalization for pneumococcal infections led to a diagnosis of HIV infection for 17 (21%) patients. Among the patients previously diagnosed with HIV, 67% (42 of 63) already received ART, 45% (28 of 62) of whom reached an undetectable viral load. Overall, 59% (47 of 80) of the patients had a C3-stage HIV infection (CDC classification) before the diagnosis of a pneumococcal infection. The mean age was 44 years (range, 18–80; standard deviation = 11.4). A comorbidity known to increase the risk of pneumococcal infections in the general population was identified for 65 (85%) patients, the most frequent being malnutrition (29%), chronic respiratory insufficiency (15%), and chronic renal failure (10%).
The 80 cases and 160 controls had a similar mean age (44 years), sex ratio (3 men for 1 female), ethnic origin (half of Caucasians, and half of Africans or Caribbean), average duration of HIV infection at the time of the analysis (8 ± 6.7 years), and HIV transmission mode (58% of cases and 60% of controls registered as heterosexuals).
As shown in Table 1, cases significantly presented with more comorbidities than controls, both for malnutrition, Charlson comorbidity modified index, addiction (either in alcohol, tobacco, or injectable drugs), chronic renal or respiratory insufficiency, or hepatitis B or C coinfection. The mean Charlson comorbidity index was significantly more pejorative for cases than controls (4.6 ± 3.6 vs 2.3 ± 3.1; P < .001). Cases also had a history of more severe HIV infection, with a lower nadir of CD4 cells (mean, 119 ± 151 vs 219 ± 186/mm3; P < .001) and a higher zenith of HIV-1 plasma viral load (mean, 9.2 ± 0.41 × 105 vs 2.7 ± 6.5 × 105 cp/mL; P = .001), as well as a significantly less controlled HIV infection at the time of the pneumococcal infection, with lower CD4 cells (mean, 233 ± 279 vs 476 ± 196/mm3; P < .01) and a higher HIV plasma viral load (1.4 ± 2.2 × 105 vs 9.9 ± 60.8 × 104 cp/mL, P < 10–4). Antiretroviral therapy was a protective factor of pneumococcal infections because 42 of the 63 previously diagnosed HIV patients (66.7%) were receiving ART compared with 105 of 124 (85.7%) of the controls (odds ratio [OR] = .37; 95% CI, .17–.77; P = .03). There was no difference between cases and controls concerning an antibiotic chemoprophylaxis with trimethoprim-sulfamethoxazole because 16 of 63 (25.4%) cases and 25 of 158 (20.2%) controls received such treatment (OR = 1.33; 95% CI, .67–2.64; P = .51).
Risk Factors of Pneumococcal Infections Among HIV-Infected Patients: Characteristics of 80 Pneumococcal Infections Cases and 160 Controls
| Characteristic . | Cases n = 80 (%) . | Controls n = 160 (%) . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Comorbidities | ||||
| BMI <19 kg/m2 | 20/66 (30.3) | 17/129 (13.2) | 3.39 (1.43–8.01) | .005 |
| BMI ≥19 kg/m2 | 46/66 (69.7) | 112/129 (86.8) | 1 | |
| Charlson comorbidity index (CCIb): | ||||
| CCI <2 | 25 (31.2) | 94 (58,7) | 1 | |
| CCI ≥2 | 55 (68.8) | 66 (41,3) | 4.86 (2.45– 9.66) | <.001 |
| Alcohol abuse | 23/75 (30.7) | 17/144 (11.8) | 3.48 (1.75–6.92) | <.001 |
| Current smoking | 42/76 (55.3) | 44/147 (29.9) | 3.64 (1.79–7.41) | <.001 |
| Injectable drugs use | 16/76 (21.1) | 11/147 (7.5) | 3.28 (1.34–8.32) | .005 |
| Chronic renal failure | 8 (10) | 3 (1.9) | 5.33 (1.41–20.1) | .01 |
| Cardiopathy | 4 (5) | 10 (6.3) | .80 (.25–2.55) | .51 |
| Chronic respiratory insufficiency | 12 (15) | 8 (5) | 3.29 (1.28–8.45) | .02 |
| Diabetes | 2 (2.5) | 14 (8.8) | .29 (.07–1.24) | .06 |
| HBV infection | 9 (11.3) | 5 (3.1) | 5.33 (1.38–20.6) | .02 |
| HCV infection | 13 (16.3) | 13 (8.1) | 2.86 (1.07–7.64) | .04 |
| Evolutive neoplasia | 6 (7.5) | 9 (5.6) | 1.43 (.45–4.53) | .77 |
| Steroids therapy | 4 (5) | 1 (0.6) | 8 (.9–71.6) | .08 |
| HIV-infection characteristics | ||||
| CDC stage: | ||||
| A | 14 (17.5) | 78 (48.8) | 1 | |
| B | 11 (13.8) | 34 (21.3) | 1.80 (.74–4.37) | <.001 |
| C | 55 (68.8) | 48 (46.6) | 5.26 (2.64–10.46) | |
| CDC C3 stage versus other stages | 47 (58.8) | 42 (26.3) | 4.0 (2.18–7.36) | <.001 |
| CD4 cell count at the time of the PnI: | ||||
| >500/mm3 | 10 (12.5) | 62 (38.6) | 1 | |
| 200–500/mm3 | 23 (28.8) | 59 (36.9) | 2.60 (1.13–5.83) | <.01 |
| <200/mm3 | 47 (58.8) | 39 (24.4) | 19.7 (8.21–47.15) | |
| HIV plasma viral load at the time of the PnI: | ||||
| ≥50 cp/mL | 52/71 (73.2) | 53/141 (37.6) | 1 | |
| <50 cp/mL | 19/71 (26.8) | 88/141 (62.4) | .22 (.11–0.43) | .003 |
| Characteristic . | Cases n = 80 (%) . | Controls n = 160 (%) . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Comorbidities | ||||
| BMI <19 kg/m2 | 20/66 (30.3) | 17/129 (13.2) | 3.39 (1.43–8.01) | .005 |
| BMI ≥19 kg/m2 | 46/66 (69.7) | 112/129 (86.8) | 1 | |
| Charlson comorbidity index (CCIb): | ||||
| CCI <2 | 25 (31.2) | 94 (58,7) | 1 | |
| CCI ≥2 | 55 (68.8) | 66 (41,3) | 4.86 (2.45– 9.66) | <.001 |
| Alcohol abuse | 23/75 (30.7) | 17/144 (11.8) | 3.48 (1.75–6.92) | <.001 |
| Current smoking | 42/76 (55.3) | 44/147 (29.9) | 3.64 (1.79–7.41) | <.001 |
| Injectable drugs use | 16/76 (21.1) | 11/147 (7.5) | 3.28 (1.34–8.32) | .005 |
| Chronic renal failure | 8 (10) | 3 (1.9) | 5.33 (1.41–20.1) | .01 |
| Cardiopathy | 4 (5) | 10 (6.3) | .80 (.25–2.55) | .51 |
| Chronic respiratory insufficiency | 12 (15) | 8 (5) | 3.29 (1.28–8.45) | .02 |
| Diabetes | 2 (2.5) | 14 (8.8) | .29 (.07–1.24) | .06 |
| HBV infection | 9 (11.3) | 5 (3.1) | 5.33 (1.38–20.6) | .02 |
| HCV infection | 13 (16.3) | 13 (8.1) | 2.86 (1.07–7.64) | .04 |
| Evolutive neoplasia | 6 (7.5) | 9 (5.6) | 1.43 (.45–4.53) | .77 |
| Steroids therapy | 4 (5) | 1 (0.6) | 8 (.9–71.6) | .08 |
| HIV-infection characteristics | ||||
| CDC stage: | ||||
| A | 14 (17.5) | 78 (48.8) | 1 | |
| B | 11 (13.8) | 34 (21.3) | 1.80 (.74–4.37) | <.001 |
| C | 55 (68.8) | 48 (46.6) | 5.26 (2.64–10.46) | |
| CDC C3 stage versus other stages | 47 (58.8) | 42 (26.3) | 4.0 (2.18–7.36) | <.001 |
| CD4 cell count at the time of the PnI: | ||||
| >500/mm3 | 10 (12.5) | 62 (38.6) | 1 | |
| 200–500/mm3 | 23 (28.8) | 59 (36.9) | 2.60 (1.13–5.83) | <.01 |
| <200/mm3 | 47 (58.8) | 39 (24.4) | 19.7 (8.21–47.15) | |
| HIV plasma viral load at the time of the PnI: | ||||
| ≥50 cp/mL | 52/71 (73.2) | 53/141 (37.6) | 1 | |
| <50 cp/mL | 19/71 (26.8) | 88/141 (62.4) | .22 (.11–0.43) | .003 |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio; PnI, pneumococcal infection. Significant risk factors in univariate analysis are in bold text.
Risk Factors of Pneumococcal Infections Among HIV-Infected Patients: Characteristics of 80 Pneumococcal Infections Cases and 160 Controls
| Characteristic . | Cases n = 80 (%) . | Controls n = 160 (%) . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Comorbidities | ||||
| BMI <19 kg/m2 | 20/66 (30.3) | 17/129 (13.2) | 3.39 (1.43–8.01) | .005 |
| BMI ≥19 kg/m2 | 46/66 (69.7) | 112/129 (86.8) | 1 | |
| Charlson comorbidity index (CCIb): | ||||
| CCI <2 | 25 (31.2) | 94 (58,7) | 1 | |
| CCI ≥2 | 55 (68.8) | 66 (41,3) | 4.86 (2.45– 9.66) | <.001 |
| Alcohol abuse | 23/75 (30.7) | 17/144 (11.8) | 3.48 (1.75–6.92) | <.001 |
| Current smoking | 42/76 (55.3) | 44/147 (29.9) | 3.64 (1.79–7.41) | <.001 |
| Injectable drugs use | 16/76 (21.1) | 11/147 (7.5) | 3.28 (1.34–8.32) | .005 |
| Chronic renal failure | 8 (10) | 3 (1.9) | 5.33 (1.41–20.1) | .01 |
| Cardiopathy | 4 (5) | 10 (6.3) | .80 (.25–2.55) | .51 |
| Chronic respiratory insufficiency | 12 (15) | 8 (5) | 3.29 (1.28–8.45) | .02 |
| Diabetes | 2 (2.5) | 14 (8.8) | .29 (.07–1.24) | .06 |
| HBV infection | 9 (11.3) | 5 (3.1) | 5.33 (1.38–20.6) | .02 |
| HCV infection | 13 (16.3) | 13 (8.1) | 2.86 (1.07–7.64) | .04 |
| Evolutive neoplasia | 6 (7.5) | 9 (5.6) | 1.43 (.45–4.53) | .77 |
| Steroids therapy | 4 (5) | 1 (0.6) | 8 (.9–71.6) | .08 |
| HIV-infection characteristics | ||||
| CDC stage: | ||||
| A | 14 (17.5) | 78 (48.8) | 1 | |
| B | 11 (13.8) | 34 (21.3) | 1.80 (.74–4.37) | <.001 |
| C | 55 (68.8) | 48 (46.6) | 5.26 (2.64–10.46) | |
| CDC C3 stage versus other stages | 47 (58.8) | 42 (26.3) | 4.0 (2.18–7.36) | <.001 |
| CD4 cell count at the time of the PnI: | ||||
| >500/mm3 | 10 (12.5) | 62 (38.6) | 1 | |
| 200–500/mm3 | 23 (28.8) | 59 (36.9) | 2.60 (1.13–5.83) | <.01 |
| <200/mm3 | 47 (58.8) | 39 (24.4) | 19.7 (8.21–47.15) | |
| HIV plasma viral load at the time of the PnI: | ||||
| ≥50 cp/mL | 52/71 (73.2) | 53/141 (37.6) | 1 | |
| <50 cp/mL | 19/71 (26.8) | 88/141 (62.4) | .22 (.11–0.43) | .003 |
| Characteristic . | Cases n = 80 (%) . | Controls n = 160 (%) . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Comorbidities | ||||
| BMI <19 kg/m2 | 20/66 (30.3) | 17/129 (13.2) | 3.39 (1.43–8.01) | .005 |
| BMI ≥19 kg/m2 | 46/66 (69.7) | 112/129 (86.8) | 1 | |
| Charlson comorbidity index (CCIb): | ||||
| CCI <2 | 25 (31.2) | 94 (58,7) | 1 | |
| CCI ≥2 | 55 (68.8) | 66 (41,3) | 4.86 (2.45– 9.66) | <.001 |
| Alcohol abuse | 23/75 (30.7) | 17/144 (11.8) | 3.48 (1.75–6.92) | <.001 |
| Current smoking | 42/76 (55.3) | 44/147 (29.9) | 3.64 (1.79–7.41) | <.001 |
| Injectable drugs use | 16/76 (21.1) | 11/147 (7.5) | 3.28 (1.34–8.32) | .005 |
| Chronic renal failure | 8 (10) | 3 (1.9) | 5.33 (1.41–20.1) | .01 |
| Cardiopathy | 4 (5) | 10 (6.3) | .80 (.25–2.55) | .51 |
| Chronic respiratory insufficiency | 12 (15) | 8 (5) | 3.29 (1.28–8.45) | .02 |
| Diabetes | 2 (2.5) | 14 (8.8) | .29 (.07–1.24) | .06 |
| HBV infection | 9 (11.3) | 5 (3.1) | 5.33 (1.38–20.6) | .02 |
| HCV infection | 13 (16.3) | 13 (8.1) | 2.86 (1.07–7.64) | .04 |
| Evolutive neoplasia | 6 (7.5) | 9 (5.6) | 1.43 (.45–4.53) | .77 |
| Steroids therapy | 4 (5) | 1 (0.6) | 8 (.9–71.6) | .08 |
| HIV-infection characteristics | ||||
| CDC stage: | ||||
| A | 14 (17.5) | 78 (48.8) | 1 | |
| B | 11 (13.8) | 34 (21.3) | 1.80 (.74–4.37) | <.001 |
| C | 55 (68.8) | 48 (46.6) | 5.26 (2.64–10.46) | |
| CDC C3 stage versus other stages | 47 (58.8) | 42 (26.3) | 4.0 (2.18–7.36) | <.001 |
| CD4 cell count at the time of the PnI: | ||||
| >500/mm3 | 10 (12.5) | 62 (38.6) | 1 | |
| 200–500/mm3 | 23 (28.8) | 59 (36.9) | 2.60 (1.13–5.83) | <.01 |
| <200/mm3 | 47 (58.8) | 39 (24.4) | 19.7 (8.21–47.15) | |
| HIV plasma viral load at the time of the PnI: | ||||
| ≥50 cp/mL | 52/71 (73.2) | 53/141 (37.6) | 1 | |
| <50 cp/mL | 19/71 (26.8) | 88/141 (62.4) | .22 (.11–0.43) | .003 |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio; PnI, pneumococcal infection. Significant risk factors in univariate analysis are in bold text.
In multivariate analysis, there were only 2 independent risk factors of pneumococcal infections: alcohol abuse (OR = 3.5; 95% CI, 1.3–9.0; P = .01) and CD4 cell counts <500/mm3 (OR = 4.0; 95% CI, 2.29 - 7.0, P < 10–4). The more the CD4 cell count decreased, the more the risk of pneumococcal infections increased: 3-fold increase of risk with CD4 cell count between 200 and 500/mm3 (OR = 2.6; 95% CI, .98–7.1; P = .05); 15-fold increase of risk with CD4 cell count <200/mm3 (OR = 15.3; 95% CI, 5.1–45.8; P < .0001), compared with patients with a CD4 cell count >500/mm3 (OR = 1).
Considering the vaccinal status, only 5 (6%) patients had been vaccinated before the onset of a pneumococcal infection, compared with 91 of 160 (57%) patients in the control group. Therefore, a pneumococcal vaccination appeared to be significantly protective, with an OR = .05, (95% CI, .02–.15; P < .001).
Determinants of Pneumococcal Vaccination
Among the 240 patients infected with HIV (80 cases with a history of pneumococcal infections and 160 controls without a history of pneumococcal infections), 135 (56%) had received at least 1 dose of pneumococcal vaccination. The pneumococcal vaccination coverage was similar between controls (91 of 160; 57%) and cases (44 of 80; 55%; P = .89), with only 5 (6%) of the cases vaccinated before the first pneumococcal infectious episode. The serotypes of the pneumococcal strains in each of these 5 cases were not available. Therefore, it was impossible to reach the conclusion of a true vaccinal failure or of a lack of protection due to a nonvaccine strain. Vaccination coverage markedly varied over time with (1) a low rate (<5%) between 2000 and 2008, (2) an inflation to 25% in 2009, contemporary of the national A/H1N1-flu pandemic preparedness vaccination plan, and (3) progressively increased to 56% in 2014. Eighty percent of the vaccinated patients (108 of 135) only received the 23-valent polysaccharide vaccine. The vaccinated patients had a higher CD4 cell count than nonvaccinated patients (CD4 = 296 ± 343/mm3 vs 152 ± 190/mm3; P = .03). Other determinants of pneumococcal vaccination were a past flu vaccination (P < .001) and a regular follow-up until 2014 for HIV infection (P < .001). In contrast, comorbidities such as addiction, renal or respiratory insufficiency, and hepatitis coinfection were not associated with pneumococcal vaccination.
DISCUSSION
The management of patients infected with HIV has drastically changed in the last decades, with a marked enhancement of the proportion of virogically suppressed patients, thanks to more effective and well tolerated ART and larger indications. These findings extend to universal treatment of all infected individuals regardless of their CD4 cell counts [23]. According to the French national database, 88% of the HIV-adult patients were receiving ART in 2011; among these, 88% had an undetectable viral load and 59% a CD4 cell count >500/mm3 [24]. Antiretroviral therapy is now promoted as a method of prevention of HIV infection, at the individual level as well as at the collective level. This concept derives from the “herd immunity” in vaccinology. The goal is to reduce the HIV infectiousness leading the most optimistics to modelize the end of the HIV outbreak even in the absence of further potent drugs discoveries [25].
Our study was conducted in 2 French regional-university hospitals with a distinct population recruitment. The differential characteristics were the ethnic origin and mode of HIV transmission, with a majority of Caucasian patients and homosexual contamination in Rouen, whereas the population in Bobigny was mainly from sub-Saharan Africa and heterosexual transmission was predominant. Overall, this broad recruitment reflected the current French HIV epidemiology [24].
The risk factors for pneumococcal infections in this current series are in agreement with those classically described in the general population: serious comorbidities reflected by a pejorative Charlson comorbidity index, addiction (to alcohol, tobacco, or injectable drugs), malnutrition, and renal or respiratory insufficiency [26, 27]. Some other classic risk factors such as myeloma or asplenia [28] were not found, due to the low prevalence of such diseases in the HIV cohorts of the 2 participating centers. Of note, pneumococcal infections were more common in patients with hepatits B or C and chronic hepatitis, a fact previously described in HIV-positive as well as in HIV-negative patients [29–31].
A major result was the spectacular decrease of the incidence of pneumococcal infections by 58% of patients over 18 years of age. It was impossible to compare the incidence of pneumococcal infections to that of the general French population in the absence of national data concerning the estimation of the burden of noninvasive pneumococcal infections. However, a comparison was possible for invasive pneumococcal infections (bacteremia and meningitis). The French control disease center was able to estimate an incidence in the general adult population aged over 18 years of 9/100000 for the year 2013 [20]. Of note, the national data include only a minority of patients infected with HIV. The number of adult HIV-infected patients living in France is estimated to be approximately 150000 [24] among a total adult French population of approximately 50 million (less than 0.5%). Therefore, the incidence of invasive pneumococcal infections among patients with comorbidities (addiction, respiratory, or renal failure) and/or uncontrolled HIV infection can be estimated 12 times higher than in the general French population (107/100000 vs 9/100000), whereas the incidence among healthy and virologically suppressed patients with HIV is estimated lower (7.6/100000).
Thus, the incidence of invasive pneumococcal infections in HIV-infected patients virologically suppressed and free of comorbidities seems lower than that of the general French population. Therefore, we can consider that this category of HIV patients may not require pneumococcal vaccination. A similar trend is suspected for noninvasive pneumococcal infections, but it cannot be proved without the estimated incidence of such forms of the disease in the country. These results are mostly attributed to an increase in the use of ART, because the coverage by pneumococcal vaccination was modest in our study.
Other studies have reported a spectacular reduction over time of the incidence of pneumococcal infections among patients infected with HIV [17, 31, 32]. The incidence of invasive pneumococcal infections among patients infected with HIV in Canada decreased from 342/100000 person-year in 2000 to 187/100000 person-year in 2011 [31]. Likewise, in England, Yin et al [16] found an average incidence rate of pneumococcal infections between 2000 and 2009 to be drastically lower than in the pre-highly active antiretroviral therapy era. However, Yin et al [16] reported that the incidence in HIV-infected patients remained higher than in non-HIV-infected persons, exceeding 20 times that of the general population. As a reminder, until 2013, British guidelines have recommended ARV only for patients with CD4 cell counts <350/mm3 [33], suggesting that a high percentage of HIV-infected patients were not virosuppressed at the time of the study.
Finally, the pneumococcal vaccination coverage remained insufficient in this cohort of HIV-infected patients, despite a proven efficacy in the literature as well as in the current study (p<.001). According to the case-control analysis, only half of the cohort had received at least 1 dose of any pneumococcal vaccine. A similar percentage (56.4%) has been described in another French study carried out in Lyon in 2010 [34]. The reasons for nonvaccination where unknown, except for 2 patients who had categorically refused the vaccine. The French population does not have an easy acceptance of vaccination, but doctors also have a responsibility: according to the work by Mohseni et al [19], the most frequent reasons for nonvaccination in HIV patients turned out to be a nonproposal by the physician, a lack of expected effectiveness, and fear of an immunovirological adverse effect. Not only was the vaccinal coverage low, but it was also poorly targeted, because neither comorbidities nor HIV immunovirological parameters were predictive factors of coverage.
CONCLUSIONS
According to the guidelines, a pneumococcal vaccination is recommended to all patients infected with HIV regardless of their CD4 cell count (the response to vaccination does not seem to be impacted by a lower CD4 cell count; the only predictive factor of a better response to conjugate and polysaccharide vaccines in patients infected with HIV seems to be an undetectable HIV plasma viral load [35]). This study suggests that HIV infection is no longer per se a significant risk factor of pneumococcal infections, raising the question of whether to interrupt the “systematic” pneumococcal vaccination not well applied in real life and concentrate the efforts on the most at-risk patients: those sharing the classic risk factors for pneumococcal infections of the general population or failing to achieve control of their HIV infection.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
Correspondence: A. Lesourd, MD, CHU Charles Nicolle - Service de Maladies infectieuses et Tropicales, 1 rue de Germont, 76000 Rouen ([email protected]).





Comments