-
PDF
- Split View
-
Views
-
Cite
Cite
Claire Janoir, Agnès Lepoutre, Laurent Gutmann, Emmanuelle Varon, on behalf of the Observatoires Régionaux du Pneumocoque Network, Insight Into Resistance Phenotypes of Emergent Non 13-valent Pneumococcal Conjugate Vaccine Type Pneumococci Isolated From Invasive Disease After 13-valent Pneumococcal Conjugate Vaccine Implementation in France, Open Forum Infectious Diseases, Volume 3, Issue 1, Winter 2016, ofw020, https://doi.org/10.1093/ofid/ofw020
Close - Share Icon Share
Abstract
Background. In 2010, the pneumococcal 13-valent conjugate vaccine (PCV13), containing 6 additional serotypes including the multidrug-resistant 19A, replaced the PCV7 in France. This study aimed at analyzing trends in antibiotic resistance in invasive pneumococcal disease (IPD) isolates in France after PCV13 introduction.
Methods. A total of 5243 pneumococci isolated from IPD in 2008–2009 (late PCV7 era) and 2011–2012 (PCV13 era) were studied according to their serotype and antibiotic resistance profile. Multilocus sequence typing analysis was performed on strains of the predominant serotypes (12F and 24F) isolated from young children.
Results. Overall, the prevalence of antibiotic resistance decreased in France (−21.5% for penicillin from 2008–2009 to 2011–2012), mainly driven by the decline of the 19A serotype. Among non-PCV13 serotypes that concomitantly emerged, serotypes 12F, 24F, 15A, and 35B were consistently associated with resistance to 1 or more antibiotics. In children under 2 years, serotypes 15A, 35B, and 24F accounted together for 37.8% and 31.9% of penicillin-nonsusceptible and erythromycin-resistant isolates, respectively. Chloramphenicol and cotrimoxazole resistance were mainly associated with serotypes 12F and 24F, respectively. Genetic analysis showed that although emergence of serotype 12F pneumococci resulted from the expansion of various pre-existing lineages, increase in serotype 24F was related to the clonal expansion of the ST162 penicillin-susceptible cotrimoxazole-resistant lineage.
Conclusions. We showed that decline of PCV13-related IPD was associated with a decline in antibiotic resistance in France, but that it likely favored the spread of several resistant nonvaccine serotypes. However, antibiotic resistance does not seem to be the only element that may drive this phenomenon.
Streptococcus pneumoniae is an important human bacterial pathogen that can cause both mild and severe invasive life-threatening diseases such as bacteremia and meningitis. Its major virulence factor for invasive disease is the antiphagocytic polysaccharide capsule. The populations at risk for invasive pneumococcal disease (IPD) mainly include the elderly, immunocompromised patients, and children <2 years of age. In addition, these latter groups are often carriers of pneumococci in their nasopharynx and thus represent the main reservoir of S pneumoniae. Since introduction of antibiotics, their effectiveness has gradually been offset by the emergence of resistance, especially to β-lactam antibiotics, the most common treatment used for IPD [1]. In France, the rate of penicillin nonsusceptible pneumococci ([PNSP], penicillin minimum inhibitory concentration [MIC] >0.06 mg/L) reached more than 50% of the invasive isolates in 2001–2002 [2]. The decrease in PNSP prevalence observed thereafter relates to the combination of public health measures: (1) in 2001, the national plan to preserve the effectiveness of antibiotics and (2) in 2003, the introduction of the 7-valent conjugate antipneumococcal vaccine (PCV7) in the immunization schedule for children under 2 years at risk for IPD. This vaccine included the world's 7 most prevalent serotypes in IPD, which are also associated with the highest rates of antibiotic resistance [3]. After generalization of PCV7 recommendation in May 2006 to all children under 2 years, its coverage increased gradually to more than 90% after 2008. In contrast to other countries, the decrease of PCV7-related IPD in France was rapidly counterbalanced by an increase in non-PCV7 serotypes IPD, in particular, serotypes 19A, 7F, 1 and 3, and the overall IPD incidence reached 11.2 cases/100 000 in 2008–2009 [4, 5]. In contrast, the decrease in the prevalence of PNSP PCV7-serotypes contributed to an overall gradual decrease of penicillin resistance rates, nonetheless partially counterbalanced by the increased prevalence of PNSP 19A serotype, a serotype commonly associated with penicillin and multidrug resistance (MDR), which ranked first at the end of the PCV7 era [6].
Since June 2010, the 13-valent conjugate vaccine (PCV13) containing 6 additional serotypes (1, 3, 5, 6A, 7F, 19A) has replaced the PCV7 in France, with a high coverage of at least 90% [4]. The introduction of the PCV13 in France in 2010 led to a dramatic modification of pneumococcal serotype distribution, with the fall of 4 of the 6 additional serotypes included in PCV13, among which is serotype 19A [5]. Subsequently, an overall significant decrease in IPD incidence was observed in 2012 relative to 2008–2009 (late PCV7 era) both in children and adults [4]. The aim of this report was to analyze the antibiotic resistance trends in IPD in France after the introduction of the PCV13, taking in account characteristics of the main replacement serotypes.
METHODS
The strains studied are part of the national French ongoing annual survey on IPD and were collected from 400 laboratories through the surveillance network of the Observatoires Régionaux du Pneumocoque (ORP), which covered approximately 70% of the admissions in medical wards. The proportion of isolates studied each year was as follows: 100% of meningitis isolates, 100%, and one sixth of all blood isolates from children (0–15 years) and adults (>15 years), respectively. Duplicate isolates were eliminated; therefore, all samples were independent.
Among 5307 IPD isolates collected during the years 2008, 2009, 2011, and 2012, 5243 isolates (3630 from bacteremia and 1613 from meningitis) were further studied for their serotype and their antibiotic resistance phenotype. Invasive pneumococcal disease isolates collected in 2008 and 2009, the 2 last years of PCV7 exclusive use (2832 isolates), were compared with those collected in 2011 and 2012, the 2 first years of PCV13 exclusive use (2411 isolates). The distribution of strains according to age groups and IPD samples is outlined in Table 1.
Distribution of Invasive Pneumococcal Disease Isolates According to Serotype Group, Specimen, and Age Group
| Age Group . | Serotype Group . | Number of Isolates . | ||||
|---|---|---|---|---|---|---|
| Meningitis . | Bacteremia . | Total . | ||||
| 2008–2009 . | 2011–2012 . | 2008–2009 . | 2011–2012 . | |||
| 0–23 mo | PCV7a | 11 | 8 | 27 | 8 | 54 |
| 6 + PCV13b | 95 | 27 | 203 | 40 | 365 | |
| Non-PCVc | 72 | 99 | 74 | 126 | 371 | |
| Total | 178 | 134 | 304 | 174 | 790 | |
| 2–15 y | PCV7 | 16 | 6 | 18 | 17 | 57 |
| 6 + PCV13 | 32 | 18 | 320 | 181 | 551 | |
| Non-PCV | 57 | 48 | 57 | 103 | 265 | |
| Total | 105 | 72 | 395 | 301 | 873 | |
| 16–64 y | PCV7 | 50 | 34 | 86 | 32 | 202 |
| 6 + PCV13 | 110 | 91 | 339 | 233 | 773 | |
| Non-PCV | 205 | 233 | 179 | 277 | 894 | |
| Total | 365 | 358 | 604 | 542 | 1869 | |
| >64 y | PCV7 | 47 | 24 | 126 | 59 | 256 |
| 6 + PCV13 | 73 | 47 | 289 | 245 | 654 | |
| Non-PCV | 91 | 119 | 255 | 336 | 801 | |
| Total | 211 | 190 | 670 | 640 | 1711 | |
| Total | 859 | 754 | 1973 | 1657 | 5243 | |
| Age Group . | Serotype Group . | Number of Isolates . | ||||
|---|---|---|---|---|---|---|
| Meningitis . | Bacteremia . | Total . | ||||
| 2008–2009 . | 2011–2012 . | 2008–2009 . | 2011–2012 . | |||
| 0–23 mo | PCV7a | 11 | 8 | 27 | 8 | 54 |
| 6 + PCV13b | 95 | 27 | 203 | 40 | 365 | |
| Non-PCVc | 72 | 99 | 74 | 126 | 371 | |
| Total | 178 | 134 | 304 | 174 | 790 | |
| 2–15 y | PCV7 | 16 | 6 | 18 | 17 | 57 |
| 6 + PCV13 | 32 | 18 | 320 | 181 | 551 | |
| Non-PCV | 57 | 48 | 57 | 103 | 265 | |
| Total | 105 | 72 | 395 | 301 | 873 | |
| 16–64 y | PCV7 | 50 | 34 | 86 | 32 | 202 |
| 6 + PCV13 | 110 | 91 | 339 | 233 | 773 | |
| Non-PCV | 205 | 233 | 179 | 277 | 894 | |
| Total | 365 | 358 | 604 | 542 | 1869 | |
| >64 y | PCV7 | 47 | 24 | 126 | 59 | 256 |
| 6 + PCV13 | 73 | 47 | 289 | 245 | 654 | |
| Non-PCV | 91 | 119 | 255 | 336 | 801 | |
| Total | 211 | 190 | 670 | 640 | 1711 | |
| Total | 859 | 754 | 1973 | 1657 | 5243 | |
Abbreviations: PCV, pneumococcal conjugate vaccine; PCV7, 7-valent pneumococcal conjugate vaccine.
a PCV7, serotypes included in PCV7 (4, 6B, 9V, 14, 18C, 19F, and 23F).
b 6 + PCV13, six additional serotypes included in PCV13 (1, 3, 5, 6A, 7F, and 19A).
c Non-PCV, serotypes not included in PCV13.
Distribution of Invasive Pneumococcal Disease Isolates According to Serotype Group, Specimen, and Age Group
| Age Group . | Serotype Group . | Number of Isolates . | ||||
|---|---|---|---|---|---|---|
| Meningitis . | Bacteremia . | Total . | ||||
| 2008–2009 . | 2011–2012 . | 2008–2009 . | 2011–2012 . | |||
| 0–23 mo | PCV7a | 11 | 8 | 27 | 8 | 54 |
| 6 + PCV13b | 95 | 27 | 203 | 40 | 365 | |
| Non-PCVc | 72 | 99 | 74 | 126 | 371 | |
| Total | 178 | 134 | 304 | 174 | 790 | |
| 2–15 y | PCV7 | 16 | 6 | 18 | 17 | 57 |
| 6 + PCV13 | 32 | 18 | 320 | 181 | 551 | |
| Non-PCV | 57 | 48 | 57 | 103 | 265 | |
| Total | 105 | 72 | 395 | 301 | 873 | |
| 16–64 y | PCV7 | 50 | 34 | 86 | 32 | 202 |
| 6 + PCV13 | 110 | 91 | 339 | 233 | 773 | |
| Non-PCV | 205 | 233 | 179 | 277 | 894 | |
| Total | 365 | 358 | 604 | 542 | 1869 | |
| >64 y | PCV7 | 47 | 24 | 126 | 59 | 256 |
| 6 + PCV13 | 73 | 47 | 289 | 245 | 654 | |
| Non-PCV | 91 | 119 | 255 | 336 | 801 | |
| Total | 211 | 190 | 670 | 640 | 1711 | |
| Total | 859 | 754 | 1973 | 1657 | 5243 | |
| Age Group . | Serotype Group . | Number of Isolates . | ||||
|---|---|---|---|---|---|---|
| Meningitis . | Bacteremia . | Total . | ||||
| 2008–2009 . | 2011–2012 . | 2008–2009 . | 2011–2012 . | |||
| 0–23 mo | PCV7a | 11 | 8 | 27 | 8 | 54 |
| 6 + PCV13b | 95 | 27 | 203 | 40 | 365 | |
| Non-PCVc | 72 | 99 | 74 | 126 | 371 | |
| Total | 178 | 134 | 304 | 174 | 790 | |
| 2–15 y | PCV7 | 16 | 6 | 18 | 17 | 57 |
| 6 + PCV13 | 32 | 18 | 320 | 181 | 551 | |
| Non-PCV | 57 | 48 | 57 | 103 | 265 | |
| Total | 105 | 72 | 395 | 301 | 873 | |
| 16–64 y | PCV7 | 50 | 34 | 86 | 32 | 202 |
| 6 + PCV13 | 110 | 91 | 339 | 233 | 773 | |
| Non-PCV | 205 | 233 | 179 | 277 | 894 | |
| Total | 365 | 358 | 604 | 542 | 1869 | |
| >64 y | PCV7 | 47 | 24 | 126 | 59 | 256 |
| 6 + PCV13 | 73 | 47 | 289 | 245 | 654 | |
| Non-PCV | 91 | 119 | 255 | 336 | 801 | |
| Total | 211 | 190 | 670 | 640 | 1711 | |
| Total | 859 | 754 | 1973 | 1657 | 5243 | |
Abbreviations: PCV, pneumococcal conjugate vaccine; PCV7, 7-valent pneumococcal conjugate vaccine.
a PCV7, serotypes included in PCV7 (4, 6B, 9V, 14, 18C, 19F, and 23F).
b 6 + PCV13, six additional serotypes included in PCV13 (1, 3, 5, 6A, 7F, and 19A).
c Non-PCV, serotypes not included in PCV13.
All isolates were serotyped by the conventional agglutination method using latex particles sensitized with antisera from the Statens Serum Institute (Copenhagen, Denmark). Susceptibility to penicillin G and cefotaxime was determined by the agar dilution method. Susceptibility to erythromycin, cotrimoxazole, tetracycline, and chloramphenicol was studied by the disk diffusion method. Results were interpreted according to the 2012 European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [7]. The MDR phenotype was defined as nonsusceptibility to penicillin associated with resistance to at least 2 other non-β-lactam antibiotics [8].
Analyses were done on the whole set of isolates studied, with a special focus on those recovered from children under 2 years of age (the primary target for PCV13 vaccination), defined hereinafter as “young children”. Trends between the 2 study periods were analyzed using the 2-sided χ2 test or the Fisher exact test according to the number of isolates, with level of significance P < .05. For children under 2 years of age, we calculated antibiotic resistant- and serotype-specific incidence rates by multiplying the incidence of IPD in this population by the proportion of resistant isolates and of each serotype, respectively.
Multilocus sequence typing (MLST) analysis was carried out on isolates belonging to the 2 predominant serotypes in young children (12F and 24F). Sequence types (STs) were grouped in clonal complexes (CCs) according to eBURST-v3 analysis as previously described [9].
RESULTS
A Global Decrease in Antibiotic Resistance in Invasive Pneumococcal Disease Isolates in the 13-valent Pneumococcal Conjugate Vaccine Era
After the introduction of PCV13 in 2010, an overall significant decrease in the proportion of PNSP was observed, from 28.9% in 2008–2009 to 22.7% in 2011–2012 (P < 10−7), and the decrease was observed both in children and adults. The same significant decline was observed for cefotaxime resistance that decreased from 8.9% in 2008–2009 to 3.7% in 2011–2012 (P < 10−7). During the same period, a remarkable drop in the proportion of resistant IPD isolates was also observed for erythromycin (from 40.8% in 2008–2009 to 27.0% in 2011–2012; P < 10−7) and for cotrimoxazole (from 22.7% in 2008–2009 to 10.4% in 2011–2012; P < 10−7). Among young children isolates, the decrease in the proportion of resistance to penicillin, erythromycin, and cotrimoxazole was even more pronounced, from 35.5%, 48.6%, and 31.5% in 2008–2009 to 23.9%, 30.4%, and 13.8% in 2011–2012, respectively. By contrast, the overall proportion of isolates resistant to tetracycline remained stable in the whole population (25.5% in 2008–2009 vs 24.3% in 2011–2012; P = .32, not significant [NS]), but it decreased significantly in young children (from 34.2% in 2008–2009 to 26.3% in 2011–2012; P = .018). Finally, a moderate but significant increase in the proportion of isolates resistant to chloramphenicol from 3.7% in 2008–2009 to 5.4% in 2011–2012 (P = .004) was observed in the whole population but not in young children where it remained stable (3.1% in 2008–2009 vs 2.6% in 2011–2012; P = .67, NS). To summarize, in terms of incidence of antibiotic resistance, a decrease was observed for all molecules tested both in the whole population, and more specifically in children under 2 years of age, except for the chloramphenicol (Figure 1).
![Incidence rates (number of cases/100 000 population [pop]) of antibiotic nonsusceptible invasive pneumococcal disease cases in the whole population (A) and in children under 2 years of age; Abbreviations: Pen, penicillin; Ery, erythromycin; Tet, tetracycline; Sxt, cotrimoxazole; Chl, chloramphenicol.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/3/1/10.1093_ofid_ofw020/4/m_ofw02001.jpeg?Expires=1747897430&Signature=jYbHyev4GHG2Y~-XCUQx3mgrikNz0m9b4N1VCaRYp-5qWvkKiBt~1EjzIco-tWufKmbBodd1VmBTPnxU1RE3mf93WSaJ~li6PJob~I8IpEgeS1ufLWbkZuP2HFh6T3U2gNB6UbKidOC~YVl7ySt-O9vHufrFL~tZNrIii-K3VY554kRJQtxPUMh3sJQN5hwbKHBUHRMrHDme5foxBf1AkQPFDSK~YnsbruoDjnyrW1fZMPyMjYRgZi7wPOFVenXgRDsoKhg6eiwbAIv6dkBa-3xVp7Hi1YLY8gPDWXrAzXyQJNqUWwOALoteUb8-dVnQBzT0ybk1PrBLyB3arcMzZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Incidence rates (number of cases/100 000 population [pop]) of antibiotic nonsusceptible invasive pneumococcal disease cases in the whole population (A) and in children under 2 years of age; Abbreviations: Pen, penicillin; Ery, erythromycin; Tet, tetracycline; Sxt, cotrimoxazole; Chl, chloramphenicol.
Decrease in Antibiotic Resistance After 13-valent Pneumococcal Conjugate Vaccine Introduction Could Be Primarily Related to Decrease in Prevalence of 19A Isolates
The contribution of serotype 19A isolates to resistance for various antibiotics, in the whole population and in young children, is described for the 2 periods in Table 2. In 2008–2009 at the end of the PCV7 era, serotype 19A accounted for 15.3% of IPD isolates and was commonly associated with antibiotic resistance. In 2011–2012, the decline of penicillin, erythromycin, and cotrimoxazole resistance rates in all age groups appeared to be mostly related to the decrease in prevalence of the serotype 19A, which, nonetheless, remained the most prevalent serotype associated with antibiotic resistance (Figure 2). Over the 2 periods, the proportion of PNSP among 19A IPD isolates remained stable (87% 2008–2009 vs 84% 2011–2012; P = .24; Table 2). By contrast, the proportion of erythromycin resistance among the 19A isolates moderately decreased over the 2 periods (88% in 2008–2009 vs 82% in 2011–2012; P = .036), whereas that of cotrimoxazole resistance dropped from 80% in 2008–2009 to 23% in 2011–2012; P < 10−7). Accordingly, in 2008–2009, 71% (307 of 432) of 19A isolates showed a predominant MDR phenotype including penicillin, erythromycin, tetracycline, and cotrimoxazole, whereas in 2011–2012, the dominant MDR phenotype (147 of 273, 54%) combined resistance to only 3 of these antibiotics: penicillin, erythromycin, and tetracycline.
Contribution of 19A Invasive Isolates to Antibiotic Nonsusceptibility in 2008–2009 and 2011–2012 in France, in the Whole Population and in Children <2 Years of Age
| IPD Isolates According to Study Period . | 2008–2009 . | 2011–2012 . | ||
|---|---|---|---|---|
| All Serotypes . | 19A . | All Serotypes . | 19A . | |
| All Age Groups | 2832 | 432 (15.3) | 2411 | 273 (11.3) |
| Number of IPD nonsusceptible isolates to | N | n (%a) | N | n (%a) |
| Penicillin | 818 | 376 (46.0) | 548 | 229 (41.8) |
| Cefotaxime | 252 | 166 (65.9) | 89 | 60 (67.4) |
| Erythromycin | 1154 | 379 (32.8) | 651 | 224 (34.4) |
| Tetracycline | 722 | 365 (50.6) | 586 | 212 (36.2) |
| Cotrimoxazole | 643 | 344 (53.5) | 251 | 64 (25.5) |
| Chloramphenicol | 105 | 6 (5.7) | 129 | 1 (0.8) |
| <2 y | 482 | 139 (28.8) | 308 | 37 (12.0) |
| Number of IPD nonsusceptible isolates to | N | n (%) | N | n (%) |
| Penicillin | 171 | 121 (70.8) | 74 | 30 (40.5) |
| Cefotaxime | 61 | 59 (96.7) | 14 | 9 (64.3) |
| Erythromycin | 234 | 121 (51.7) | 94 | 30 (31.9) |
| Tetracycline | 165 | 122 (73.9) | 81 | 29 (35.8) |
| Cotrimoxazole | 152 | 113 (74.3) | 43 | 11 (25.6) |
| Chloramphenicol | 15 | 2 (13.3) | 8 | 0 (0) |
| IPD Isolates According to Study Period . | 2008–2009 . | 2011–2012 . | ||
|---|---|---|---|---|
| All Serotypes . | 19A . | All Serotypes . | 19A . | |
| All Age Groups | 2832 | 432 (15.3) | 2411 | 273 (11.3) |
| Number of IPD nonsusceptible isolates to | N | n (%a) | N | n (%a) |
| Penicillin | 818 | 376 (46.0) | 548 | 229 (41.8) |
| Cefotaxime | 252 | 166 (65.9) | 89 | 60 (67.4) |
| Erythromycin | 1154 | 379 (32.8) | 651 | 224 (34.4) |
| Tetracycline | 722 | 365 (50.6) | 586 | 212 (36.2) |
| Cotrimoxazole | 643 | 344 (53.5) | 251 | 64 (25.5) |
| Chloramphenicol | 105 | 6 (5.7) | 129 | 1 (0.8) |
| <2 y | 482 | 139 (28.8) | 308 | 37 (12.0) |
| Number of IPD nonsusceptible isolates to | N | n (%) | N | n (%) |
| Penicillin | 171 | 121 (70.8) | 74 | 30 (40.5) |
| Cefotaxime | 61 | 59 (96.7) | 14 | 9 (64.3) |
| Erythromycin | 234 | 121 (51.7) | 94 | 30 (31.9) |
| Tetracycline | 165 | 122 (73.9) | 81 | 29 (35.8) |
| Cotrimoxazole | 152 | 113 (74.3) | 43 | 11 (25.6) |
| Chloramphenicol | 15 | 2 (13.3) | 8 | 0 (0) |
Abbreviations: IPD, invasive pneumococcal disease.
a %, proportion of nonsusceptible 19A isolates relative to all isolates (n/N).
Contribution of 19A Invasive Isolates to Antibiotic Nonsusceptibility in 2008–2009 and 2011–2012 in France, in the Whole Population and in Children <2 Years of Age
| IPD Isolates According to Study Period . | 2008–2009 . | 2011–2012 . | ||
|---|---|---|---|---|
| All Serotypes . | 19A . | All Serotypes . | 19A . | |
| All Age Groups | 2832 | 432 (15.3) | 2411 | 273 (11.3) |
| Number of IPD nonsusceptible isolates to | N | n (%a) | N | n (%a) |
| Penicillin | 818 | 376 (46.0) | 548 | 229 (41.8) |
| Cefotaxime | 252 | 166 (65.9) | 89 | 60 (67.4) |
| Erythromycin | 1154 | 379 (32.8) | 651 | 224 (34.4) |
| Tetracycline | 722 | 365 (50.6) | 586 | 212 (36.2) |
| Cotrimoxazole | 643 | 344 (53.5) | 251 | 64 (25.5) |
| Chloramphenicol | 105 | 6 (5.7) | 129 | 1 (0.8) |
| <2 y | 482 | 139 (28.8) | 308 | 37 (12.0) |
| Number of IPD nonsusceptible isolates to | N | n (%) | N | n (%) |
| Penicillin | 171 | 121 (70.8) | 74 | 30 (40.5) |
| Cefotaxime | 61 | 59 (96.7) | 14 | 9 (64.3) |
| Erythromycin | 234 | 121 (51.7) | 94 | 30 (31.9) |
| Tetracycline | 165 | 122 (73.9) | 81 | 29 (35.8) |
| Cotrimoxazole | 152 | 113 (74.3) | 43 | 11 (25.6) |
| Chloramphenicol | 15 | 2 (13.3) | 8 | 0 (0) |
| IPD Isolates According to Study Period . | 2008–2009 . | 2011–2012 . | ||
|---|---|---|---|---|
| All Serotypes . | 19A . | All Serotypes . | 19A . | |
| All Age Groups | 2832 | 432 (15.3) | 2411 | 273 (11.3) |
| Number of IPD nonsusceptible isolates to | N | n (%a) | N | n (%a) |
| Penicillin | 818 | 376 (46.0) | 548 | 229 (41.8) |
| Cefotaxime | 252 | 166 (65.9) | 89 | 60 (67.4) |
| Erythromycin | 1154 | 379 (32.8) | 651 | 224 (34.4) |
| Tetracycline | 722 | 365 (50.6) | 586 | 212 (36.2) |
| Cotrimoxazole | 643 | 344 (53.5) | 251 | 64 (25.5) |
| Chloramphenicol | 105 | 6 (5.7) | 129 | 1 (0.8) |
| <2 y | 482 | 139 (28.8) | 308 | 37 (12.0) |
| Number of IPD nonsusceptible isolates to | N | n (%) | N | n (%) |
| Penicillin | 171 | 121 (70.8) | 74 | 30 (40.5) |
| Cefotaxime | 61 | 59 (96.7) | 14 | 9 (64.3) |
| Erythromycin | 234 | 121 (51.7) | 94 | 30 (31.9) |
| Tetracycline | 165 | 122 (73.9) | 81 | 29 (35.8) |
| Cotrimoxazole | 152 | 113 (74.3) | 43 | 11 (25.6) |
| Chloramphenicol | 15 | 2 (13.3) | 8 | 0 (0) |
Abbreviations: IPD, invasive pneumococcal disease.
a %, proportion of nonsusceptible 19A isolates relative to all isolates (n/N).
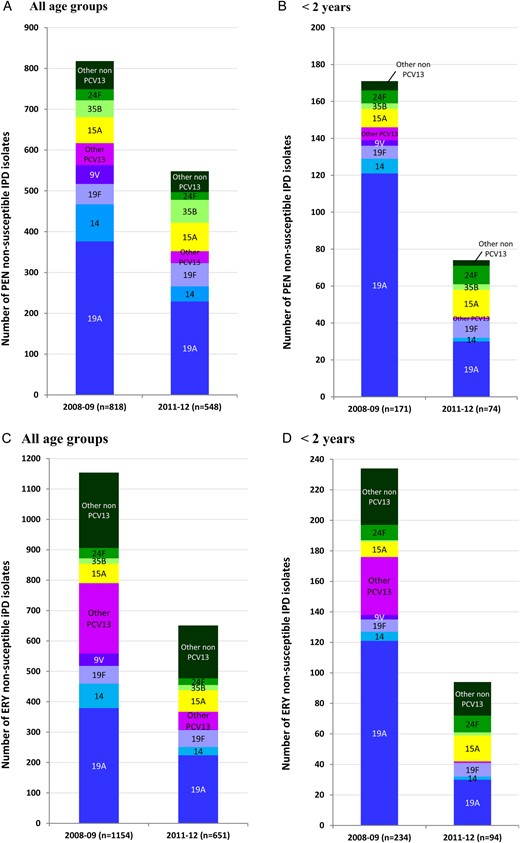
Distribution of penicillin (A and B) and erythromycin (C and D) resistant isolates according to serotype, in 2008–2009 and 2011–2012, in the whole population (A and C) and in children <2 years of age (B and D); n, total number of resistant isolates. Abbreviations: Ery, erythromycin; PCV, pneumococcal conjugate vaccine.
Antibiotic Resistance in Emergent Non 13-valent Pneumococcal Conjugate Vaccine Serotypes After Pneumococcal Virus 13 Introduction
Due to the introduction of the PCV13 in the children immunization schedule, non-PCV13 serotypes are now becoming the most prevalent serotype group in IPD in France, accounting for 55.6% of IPD in the whole population and 73.1% in young children (incidence 13.7/100 000) (Table 1 and Figure 3). Among non-PCV13 serotypes emerging in 2011–2012 [4], we further analyzed those consistently associated with resistance to 1 or more antibiotics: 12F, 24F, 15A, and 35B that represented 12.3%, 4.0%, 3.5%, and 2.6% of all isolates and ranked 1, 7, 9, and 11, respectively, in 2011–2012. Their contribution to antibiotic resistance varied according to age groups. The dramatic rise of serotype 12F was observed both in young children (2.1% in 2008–2009 vs 14.1% in 2011–2012) and in the remaining population (>2 years) (2.9% in 2008–2009 vs 12.1% in 2011–2012). A moderate increase in serotype 35B was also noted in all age groups (1.6% in 2008–2009 vs 2.6% in 2011–2012). By contrast, serotypes 24F and 15A increased significantly in young children from 5.2% and 2.1% in 2008–2009 to 14.0% and 6.2% in 2011–2012, respectively, but were stable in the remaining population. Consequently, the role of these serotypes in antibiotic resistance in IPD varied according to each age group considered (Table 3, Figures 2 and 3).
Antibiotic Resistance Phenotypes According to the Main Emerging non-PCV13 Serotypes From IPD in 2011–2012, in France, in the Whole Population and in Children <2 Years of Age
| IPD Isolates According to Indicated Serotype and Study Period . | 12F . | 24F . | 15A . | 35B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008–2009 . | 2011–2012 . | P Valuea . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | |
| All Age Groups | ||||||||||||
| No. of isolates (N) | 77 | 297 | 88 | 96 | 70 | 84 | 46 | 63 | ||||
| No. of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 3 (4%) | 0 | –c | 27 (31%) | 19 (20%) | .04 | 64 (91%) | 70 (83%) | NS | 41 (89%) | 56 (89%) | NS |
| Erythromycin | 16 (21%) | 10 (3%) | 10−6 | 34 (39%) | 22 (23%) | .01 | 65 (93%) | 71 (85%) | NS | 17 (37%) | 17 (27%) | NS |
| Tetracycline | 29 (38%) | 76 (26%) | .02 | 23 (26%) | 19 (20%) | NS | 54 (77%) | 60 (71%) | NS | 3 (7%) | 9 (14%) | NS |
| Cotrimoxazole | 32 (42%) | 31 (10%) | <10−7 | 26 (30%) | 49 (51%) | .001 | 9 (13%) | 10 (12%) | NS | 3 (7%) | 1 (2%) | NS |
| Chloramphenicol | 31 (40%) | 82 (28%) | .02 | 4 (5%) | 0 (0%) | – | 4 (6%) | 0 (0%) | – | 6 (13%) | 12 (19%) | NS |
| Children <2 y | ||||||||||||
| No of isolates (N) | 10 | 43 | 25 | 43 | 10 | 19 | 3 | 6 | ||||
| No of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 0 | 0 (0%) | – | 7 (28%) | 10 (23%) | NS | 10 (100%) | 15 (79%) | NS | 3 (100%) | 3 (50%) | – |
| Erythromycin | 4 (40%) | 3 (7%) | .01 | 10 (40%) | 11 (26%) | NS | 10 (100%) | 17 (89%) | NS | 1 (33%) | 2 (33%) | – |
| Tetracycline | 4 (40%) | 7 (16%) | NS | 6 (24%) | 10 (23%) | NS | 7 (70%) | 16 (84%) | NS | 0 | 1 (17%) | – |
| Cotrimoxazole | 4 (40%) | 2 (5%) | – | 7 (28%) | 22 (51%) | .03 | 2 (20%) | 2 (11%) | – | 0 | 0 | – |
| Chloramphenicol | 4 (40%) | 6 (14%) | .04 | 2 (8%) | 0 | – | 1 (10%) | 0 | – | 0 | 1 (17%) | – |
| IPD Isolates According to Indicated Serotype and Study Period . | 12F . | 24F . | 15A . | 35B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008–2009 . | 2011–2012 . | P Valuea . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | |
| All Age Groups | ||||||||||||
| No. of isolates (N) | 77 | 297 | 88 | 96 | 70 | 84 | 46 | 63 | ||||
| No. of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 3 (4%) | 0 | –c | 27 (31%) | 19 (20%) | .04 | 64 (91%) | 70 (83%) | NS | 41 (89%) | 56 (89%) | NS |
| Erythromycin | 16 (21%) | 10 (3%) | 10−6 | 34 (39%) | 22 (23%) | .01 | 65 (93%) | 71 (85%) | NS | 17 (37%) | 17 (27%) | NS |
| Tetracycline | 29 (38%) | 76 (26%) | .02 | 23 (26%) | 19 (20%) | NS | 54 (77%) | 60 (71%) | NS | 3 (7%) | 9 (14%) | NS |
| Cotrimoxazole | 32 (42%) | 31 (10%) | <10−7 | 26 (30%) | 49 (51%) | .001 | 9 (13%) | 10 (12%) | NS | 3 (7%) | 1 (2%) | NS |
| Chloramphenicol | 31 (40%) | 82 (28%) | .02 | 4 (5%) | 0 (0%) | – | 4 (6%) | 0 (0%) | – | 6 (13%) | 12 (19%) | NS |
| Children <2 y | ||||||||||||
| No of isolates (N) | 10 | 43 | 25 | 43 | 10 | 19 | 3 | 6 | ||||
| No of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 0 | 0 (0%) | – | 7 (28%) | 10 (23%) | NS | 10 (100%) | 15 (79%) | NS | 3 (100%) | 3 (50%) | – |
| Erythromycin | 4 (40%) | 3 (7%) | .01 | 10 (40%) | 11 (26%) | NS | 10 (100%) | 17 (89%) | NS | 1 (33%) | 2 (33%) | – |
| Tetracycline | 4 (40%) | 7 (16%) | NS | 6 (24%) | 10 (23%) | NS | 7 (70%) | 16 (84%) | NS | 0 | 1 (17%) | – |
| Cotrimoxazole | 4 (40%) | 2 (5%) | – | 7 (28%) | 22 (51%) | .03 | 2 (20%) | 2 (11%) | – | 0 | 0 | – |
| Chloramphenicol | 4 (40%) | 6 (14%) | .04 | 2 (8%) | 0 | – | 1 (10%) | 0 | – | 0 | 1 (17%) | – |
Abbreviations: IPD, invasive pneumococcal disease; NS, not significant.
a Trends between 2008–2009 and 2011–2012, calculated using the 2-sided χ2 test, with level of significance P < .05.
b Proportion of antibiotic nonsusceptible isolates among each serotype (n/N).
c Statistical calculation not possible, because of the low number of isolates.
Antibiotic Resistance Phenotypes According to the Main Emerging non-PCV13 Serotypes From IPD in 2011–2012, in France, in the Whole Population and in Children <2 Years of Age
| IPD Isolates According to Indicated Serotype and Study Period . | 12F . | 24F . | 15A . | 35B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008–2009 . | 2011–2012 . | P Valuea . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | |
| All Age Groups | ||||||||||||
| No. of isolates (N) | 77 | 297 | 88 | 96 | 70 | 84 | 46 | 63 | ||||
| No. of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 3 (4%) | 0 | –c | 27 (31%) | 19 (20%) | .04 | 64 (91%) | 70 (83%) | NS | 41 (89%) | 56 (89%) | NS |
| Erythromycin | 16 (21%) | 10 (3%) | 10−6 | 34 (39%) | 22 (23%) | .01 | 65 (93%) | 71 (85%) | NS | 17 (37%) | 17 (27%) | NS |
| Tetracycline | 29 (38%) | 76 (26%) | .02 | 23 (26%) | 19 (20%) | NS | 54 (77%) | 60 (71%) | NS | 3 (7%) | 9 (14%) | NS |
| Cotrimoxazole | 32 (42%) | 31 (10%) | <10−7 | 26 (30%) | 49 (51%) | .001 | 9 (13%) | 10 (12%) | NS | 3 (7%) | 1 (2%) | NS |
| Chloramphenicol | 31 (40%) | 82 (28%) | .02 | 4 (5%) | 0 (0%) | – | 4 (6%) | 0 (0%) | – | 6 (13%) | 12 (19%) | NS |
| Children <2 y | ||||||||||||
| No of isolates (N) | 10 | 43 | 25 | 43 | 10 | 19 | 3 | 6 | ||||
| No of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 0 | 0 (0%) | – | 7 (28%) | 10 (23%) | NS | 10 (100%) | 15 (79%) | NS | 3 (100%) | 3 (50%) | – |
| Erythromycin | 4 (40%) | 3 (7%) | .01 | 10 (40%) | 11 (26%) | NS | 10 (100%) | 17 (89%) | NS | 1 (33%) | 2 (33%) | – |
| Tetracycline | 4 (40%) | 7 (16%) | NS | 6 (24%) | 10 (23%) | NS | 7 (70%) | 16 (84%) | NS | 0 | 1 (17%) | – |
| Cotrimoxazole | 4 (40%) | 2 (5%) | – | 7 (28%) | 22 (51%) | .03 | 2 (20%) | 2 (11%) | – | 0 | 0 | – |
| Chloramphenicol | 4 (40%) | 6 (14%) | .04 | 2 (8%) | 0 | – | 1 (10%) | 0 | – | 0 | 1 (17%) | – |
| IPD Isolates According to Indicated Serotype and Study Period . | 12F . | 24F . | 15A . | 35B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008–2009 . | 2011–2012 . | P Valuea . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | 2008–2009 . | 2011–2012 . | P Value . | |
| All Age Groups | ||||||||||||
| No. of isolates (N) | 77 | 297 | 88 | 96 | 70 | 84 | 46 | 63 | ||||
| No. of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 3 (4%) | 0 | –c | 27 (31%) | 19 (20%) | .04 | 64 (91%) | 70 (83%) | NS | 41 (89%) | 56 (89%) | NS |
| Erythromycin | 16 (21%) | 10 (3%) | 10−6 | 34 (39%) | 22 (23%) | .01 | 65 (93%) | 71 (85%) | NS | 17 (37%) | 17 (27%) | NS |
| Tetracycline | 29 (38%) | 76 (26%) | .02 | 23 (26%) | 19 (20%) | NS | 54 (77%) | 60 (71%) | NS | 3 (7%) | 9 (14%) | NS |
| Cotrimoxazole | 32 (42%) | 31 (10%) | <10−7 | 26 (30%) | 49 (51%) | .001 | 9 (13%) | 10 (12%) | NS | 3 (7%) | 1 (2%) | NS |
| Chloramphenicol | 31 (40%) | 82 (28%) | .02 | 4 (5%) | 0 (0%) | – | 4 (6%) | 0 (0%) | – | 6 (13%) | 12 (19%) | NS |
| Children <2 y | ||||||||||||
| No of isolates (N) | 10 | 43 | 25 | 43 | 10 | 19 | 3 | 6 | ||||
| No of nonsusceptible isolates to (n , %b) | ||||||||||||
| Penicillin | 0 | 0 (0%) | – | 7 (28%) | 10 (23%) | NS | 10 (100%) | 15 (79%) | NS | 3 (100%) | 3 (50%) | – |
| Erythromycin | 4 (40%) | 3 (7%) | .01 | 10 (40%) | 11 (26%) | NS | 10 (100%) | 17 (89%) | NS | 1 (33%) | 2 (33%) | – |
| Tetracycline | 4 (40%) | 7 (16%) | NS | 6 (24%) | 10 (23%) | NS | 7 (70%) | 16 (84%) | NS | 0 | 1 (17%) | – |
| Cotrimoxazole | 4 (40%) | 2 (5%) | – | 7 (28%) | 22 (51%) | .03 | 2 (20%) | 2 (11%) | – | 0 | 0 | – |
| Chloramphenicol | 4 (40%) | 6 (14%) | .04 | 2 (8%) | 0 | – | 1 (10%) | 0 | – | 0 | 1 (17%) | – |
Abbreviations: IPD, invasive pneumococcal disease; NS, not significant.
a Trends between 2008–2009 and 2011–2012, calculated using the 2-sided χ2 test, with level of significance P < .05.
b Proportion of antibiotic nonsusceptible isolates among each serotype (n/N).
c Statistical calculation not possible, because of the low number of isolates.
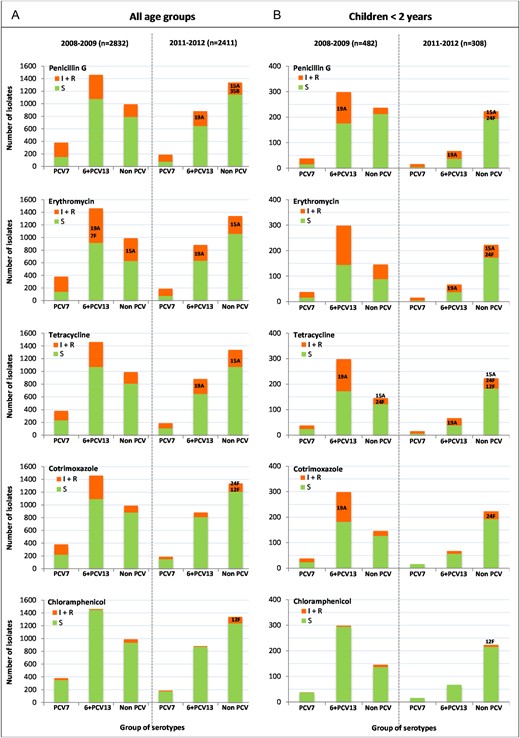
Distribution of susceptible (S) and nonsusceptible (I + R) isolates to penicillin, erythromycin, tetracycline, cotrimoxazole, and chloramphenicol, in the 2 periods of the study, according to 3 groups of serotype defined as follows: PCV7, serotypes included in the 7-valent conjugate vaccine; 6 + PCV13, 6 additional serotypes included in the 13-valent conjugate vaccine; non-PCV: serotypes not included in any conjugate vaccine. The main 6 + PCV13 or non-PCV serotypes associated with antibiotic resistance in 2011–2012 and contributing to more than 10% of all resistant isolates are indicated.
Decreased susceptibility to penicillin (Table 3, Figure 2) was associated with 89%, 83%, and 20% of 35B, 15A, and 24F isolates, respectively, accounting for 26.5% of all PNSP in the whole population and 37.8% of all PNSP in young children. None of these PNSP was strictly resistant to penicillin (MIC > 2 mg/L). Only 4 strains of serotype 15A and 3 strains of serotype 35B displayed a decreased susceptibility to cefotaxime (MIC = 1 or 2 mg/L). Among those, two 15A and one 35B isolates were isolated from meningitis.
Erythromycin resistance was observed in 85% of serotype 15A isolates and in 23% of serotype 24F isolates, contributing together to 14.3% of erythromycin resistance in the whole opulation, and 29.8% in young children (Table 3, Figure 2).
Tetracycline resistance was observed in 71% and 20% of all 15A and 24F isolates, respectively, which contributed together to 13.5% of tetracycline-resistant isolates among all age groups and to 32.1% in young children. Tetracycline resistance was linked to erythromycin resistance in more than 85% of 15A and 24F isolates, a situation frequently found in pneumococci carrying Tn916/Tn1545-related conjugative transposons [10]. It is interesting to note that 26% of 12F isolates, mostly erythromycin susceptible, were tetracycline-resistant and contributed to 13.0% of tetracycline resistance in the whole population and to 8.6% in young children (Table 3, Figure 3).
Among 12F isolates, 28% were resistant to chloramphenicol and contribute to 63.6% of chloramphenicol resistance in the whole population and 75.0% in young children. Chloramphenicol resistance was associated with tetracycline resistance in 77% of the 12F isolates, suggesting the presence of composite Tn5253-related transposons [11]. Chloramphenicol resistance was also expressed by 19% of 35B isolates, which contributed to 9.3% of chloramphenicol resistance in the whole population and 12.5% in young children (Table 3, Figure 3). Of note, none of the 15A and 24F isolates resistant to both erythromycin and tetracycline were resistant to chloramphenicol.
Finally, cotrimoxazole resistance was mainly associated with serotypes 24F and 12F that contributed together to 31.9% of cotrimoxazole resistance in the whole population and 55.8% in young children (Table 3, Figure 3).
Genetic Structure Population of 12F and 24F Strains Isolated in Children <2 Years
In 2011–2012, the 2 main serotypes recovered from IPD in young children were serotype 12F and 24F (incidence of 2.51 cases/100 000 for both). To decipher the genetic structure of these 2 nonvaccine serotypes that emerged in the vaccine-targeted population, we investigated the genetic structure of serotype 12F and 24F isolates responsible for IPD in young children using MLST (Table 4).
Population Structure (Sequence Types) of 12F (n = 53) and 24F (n = 67) Invasive Isolates Recovered From Children <2 Years of Age.
| Serotype . | Clonal Complex . | Sequence Type . | Number of Nonsusceptible Isolates to . | Main Related Serotypesa . | Distribution per Period . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pen . | Ery . | Tet . | Sxt . | Chl . | 2008–2009 . | 2011–2012 . | ||||
| 12F | n = 0 | n = 7 | n = 11 | n = 6 | n = 10 | n = 10 | n = 43 | |||
| 218 | 218 | 0 | 0 | 0 | 0 | 0 | 12F | 1 | 4 | |
| 220 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |||
| 989 | 989 | 0 | 2 | 10 | 5 | 10 | 12F | 5 | 7 | |
| 3774 | 3524 | 0 | 1 | 0 | 0 | 0 | 12F | 2 | 10 | |
| 11 368 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Singleton | 8060 | 0 | 3 | 1 | 1 | 0 | 12F | 2 | 17 | |
| 433 | 433 | 0 | 0 | 0 | 0 | 0 | 22F | 0 | 1 | |
| 460 | 1551 | 0 | 1 | 0 | 0 | 0 | 10A | 0 | 1 | |
| 24F | n = 16 | n = 20 | n = 15 | n = 29 | n = 2 | n = 25 | n = 42b | |||
| 72 | 72 | 0 | 4 | 1 | 0 | 2 | 24F | 16 | 11 | |
| 11 369 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| 156 | 162 | 0 | 1 | 0 | 20 | 0 | 9V | 0 | 20 | |
| 392 | 392 | 0 | 0 | 0 | 1 | 0 | 17F | 0 | 1 | |
| 230 | 230 | 8 | 6 | 5 | 4 | 0 | 14, 19A, 24F | 4 | 5 | |
| 4253 | 7 | 7 | 7 | 3 | 0 | 3 | 4 | |||
| 11 349 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||
| 63 | 5172 | 0 | 1 | 1 | 0 | 0 | 15A | 0 | 1 | |
| Serotype . | Clonal Complex . | Sequence Type . | Number of Nonsusceptible Isolates to . | Main Related Serotypesa . | Distribution per Period . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pen . | Ery . | Tet . | Sxt . | Chl . | 2008–2009 . | 2011–2012 . | ||||
| 12F | n = 0 | n = 7 | n = 11 | n = 6 | n = 10 | n = 10 | n = 43 | |||
| 218 | 218 | 0 | 0 | 0 | 0 | 0 | 12F | 1 | 4 | |
| 220 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |||
| 989 | 989 | 0 | 2 | 10 | 5 | 10 | 12F | 5 | 7 | |
| 3774 | 3524 | 0 | 1 | 0 | 0 | 0 | 12F | 2 | 10 | |
| 11 368 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Singleton | 8060 | 0 | 3 | 1 | 1 | 0 | 12F | 2 | 17 | |
| 433 | 433 | 0 | 0 | 0 | 0 | 0 | 22F | 0 | 1 | |
| 460 | 1551 | 0 | 1 | 0 | 0 | 0 | 10A | 0 | 1 | |
| 24F | n = 16 | n = 20 | n = 15 | n = 29 | n = 2 | n = 25 | n = 42b | |||
| 72 | 72 | 0 | 4 | 1 | 0 | 2 | 24F | 16 | 11 | |
| 11 369 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| 156 | 162 | 0 | 1 | 0 | 20 | 0 | 9V | 0 | 20 | |
| 392 | 392 | 0 | 0 | 0 | 1 | 0 | 17F | 0 | 1 | |
| 230 | 230 | 8 | 6 | 5 | 4 | 0 | 14, 19A, 24F | 4 | 5 | |
| 4253 | 7 | 7 | 7 | 3 | 0 | 3 | 4 | |||
| 11 349 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||
| 63 | 5172 | 0 | 1 | 1 | 0 | 0 | 15A | 0 | 1 | |
Abbreviations: Chl, chloramphenicol; Ery, erythromycin; MLST, multilocus sequence typing; Pen, penicillin; Sxt, cotrimoxazole; Tet, tetracycline.
a From Streptococcus pneumoniae MLST website (http://pubmlst.org/spneumoniae/).
b One 24F bacteremia isolate with decreased susceptibility to Pen, Ery, and Tet was not available for MLST.
Population Structure (Sequence Types) of 12F (n = 53) and 24F (n = 67) Invasive Isolates Recovered From Children <2 Years of Age.
| Serotype . | Clonal Complex . | Sequence Type . | Number of Nonsusceptible Isolates to . | Main Related Serotypesa . | Distribution per Period . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pen . | Ery . | Tet . | Sxt . | Chl . | 2008–2009 . | 2011–2012 . | ||||
| 12F | n = 0 | n = 7 | n = 11 | n = 6 | n = 10 | n = 10 | n = 43 | |||
| 218 | 218 | 0 | 0 | 0 | 0 | 0 | 12F | 1 | 4 | |
| 220 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |||
| 989 | 989 | 0 | 2 | 10 | 5 | 10 | 12F | 5 | 7 | |
| 3774 | 3524 | 0 | 1 | 0 | 0 | 0 | 12F | 2 | 10 | |
| 11 368 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Singleton | 8060 | 0 | 3 | 1 | 1 | 0 | 12F | 2 | 17 | |
| 433 | 433 | 0 | 0 | 0 | 0 | 0 | 22F | 0 | 1 | |
| 460 | 1551 | 0 | 1 | 0 | 0 | 0 | 10A | 0 | 1 | |
| 24F | n = 16 | n = 20 | n = 15 | n = 29 | n = 2 | n = 25 | n = 42b | |||
| 72 | 72 | 0 | 4 | 1 | 0 | 2 | 24F | 16 | 11 | |
| 11 369 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| 156 | 162 | 0 | 1 | 0 | 20 | 0 | 9V | 0 | 20 | |
| 392 | 392 | 0 | 0 | 0 | 1 | 0 | 17F | 0 | 1 | |
| 230 | 230 | 8 | 6 | 5 | 4 | 0 | 14, 19A, 24F | 4 | 5 | |
| 4253 | 7 | 7 | 7 | 3 | 0 | 3 | 4 | |||
| 11 349 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||
| 63 | 5172 | 0 | 1 | 1 | 0 | 0 | 15A | 0 | 1 | |
| Serotype . | Clonal Complex . | Sequence Type . | Number of Nonsusceptible Isolates to . | Main Related Serotypesa . | Distribution per Period . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pen . | Ery . | Tet . | Sxt . | Chl . | 2008–2009 . | 2011–2012 . | ||||
| 12F | n = 0 | n = 7 | n = 11 | n = 6 | n = 10 | n = 10 | n = 43 | |||
| 218 | 218 | 0 | 0 | 0 | 0 | 0 | 12F | 1 | 4 | |
| 220 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |||
| 989 | 989 | 0 | 2 | 10 | 5 | 10 | 12F | 5 | 7 | |
| 3774 | 3524 | 0 | 1 | 0 | 0 | 0 | 12F | 2 | 10 | |
| 11 368 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Singleton | 8060 | 0 | 3 | 1 | 1 | 0 | 12F | 2 | 17 | |
| 433 | 433 | 0 | 0 | 0 | 0 | 0 | 22F | 0 | 1 | |
| 460 | 1551 | 0 | 1 | 0 | 0 | 0 | 10A | 0 | 1 | |
| 24F | n = 16 | n = 20 | n = 15 | n = 29 | n = 2 | n = 25 | n = 42b | |||
| 72 | 72 | 0 | 4 | 1 | 0 | 2 | 24F | 16 | 11 | |
| 11 369 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| 156 | 162 | 0 | 1 | 0 | 20 | 0 | 9V | 0 | 20 | |
| 392 | 392 | 0 | 0 | 0 | 1 | 0 | 17F | 0 | 1 | |
| 230 | 230 | 8 | 6 | 5 | 4 | 0 | 14, 19A, 24F | 4 | 5 | |
| 4253 | 7 | 7 | 7 | 3 | 0 | 3 | 4 | |||
| 11 349 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||
| 63 | 5172 | 0 | 1 | 1 | 0 | 0 | 15A | 0 | 1 | |
Abbreviations: Chl, chloramphenicol; Ery, erythromycin; MLST, multilocus sequence typing; Pen, penicillin; Sxt, cotrimoxazole; Tet, tetracycline.
a From Streptococcus pneumoniae MLST website (http://pubmlst.org/spneumoniae/).
b One 24F bacteremia isolate with decreased susceptibility to Pen, Ery, and Tet was not available for MLST.
Among the 53 serotype 12F strains genotyped (10 strains isolated in 2008–2009, 43 strains isolated in 2011–2012), we found 8 distinct STs that were mainly assigned to 3 CCs (CC218, 7 isolates; CC989, 12 isolates; CC3774, 13 isolates) and 1 singleton (ST8060, 19 isolates). Most of them were related to serotype 12F in the MLST database. The 8 isolates coresistant to chloramphenicol and tetracycline belonged to CC989 and were similarly distributed over the 2 periods. Other CCs seemed to emerge in 2011–2012, although one should be cautious in this interpretation because of the low number of strains studied (Table 4).
Among the 67 24F isolates genotyped (25 from 2008–2009, 42 from 2011–2012), 8 STs were identified that were mainly assigned to 3 CCs: CC72 (28 isolates), CC156 (20 isolates), and CC230 (17 isolates). Although 23 of 28 strains belonging to CC72 were fully susceptible to antibiotics, 13 of 17 strains from CC230 were associated with penicillin, erythromycin, and tetracycline resistance, and all those from CC156 were associated with cotrimoxazole resistance only (Table 4). Of importance, the evolutionary trends of the 24F isolates belonging to the 3 CCs were different; although CC72 and CC230 were present over the 2 periods, CC156 emerged in the PCV13 era. This is shown by the 20 penicillin-susceptible but cotrimoxazole-resistant 24F strains belonging to this CC isolated in 2011–2012 (Table 4).
DISCUSSION
This study is the first to describe the antibiotic resistance trends in IPD isolates following PCV13 introduction in France. The decline in IPD incidence was due to the decreased prevalence of additional serotypes present in PCV13 and was correlated to an overall decrease in antibiotic resistance, as recently reported in the United States, South Africa, and the United Kingdom [8, 12–14].
In 2011–2012, serotype 19A was still the main serotype responsible for decreased susceptibility to both penicillin and erythromycin, although its overall prevalence decreased significantly (Figure 2). Of note, whereas the proportion of PNSP among 19A isolates remained stable among invasive isolates in France, in the United States, a significant decline was observed among 19A isolates recovered from invasive and noninvasive disease between 2008–2009 and 2012–2013 [8]. This could be explained, at least partly, by an increased use of broad-spectrum penicillins in outpatients between 2008 and 2013 in France [15]. By contrast, the decreased proportion of erythromycin and cotrimoxazole resistance among the 19A isolates could be related to the reduced prescription of these antibiotics during the same period in France [15, 16].
The decline of PCV13 serotypes favored the spread of residual resistant strains associated to nonvaccine serotypes, which are gradually increasing, particularly in young children. Serotype 6C that displayed a high proportion of erythromycin resistance was reported previously [9]. Altogether, the replacement serotypes 15A, 35B, and 24F are becoming major contributors to penicillin nonsusceptibility in pneumococci in France, accounting for 26.5% of PNSP in the whole population in 2011–2012, and, more alarming, for 37.8% of PNSP in young children. Moreover, serotypes 15A and 24F contributed to approximately one third of erythromycin-resistant isolates in young children. By contrast, 12F isolates were less frequently resistant to erythromycin, tetracycline, cotrimoxazole, and chloramphenicol in 2011–2012 relative to 2008–2009. Serotype 24F was the single serotype to show a steady increase in cotrimoxazole resistance with up to 50% of resistant isolates in 2011–2012. It is interesting to note that the resistance rates did not change for 15A and 35B isolates between the 2 periods (Table 3), suggesting that their increased prevalence might not be driven by the antibiotic selection pressure. In other countries these serotypes have also increased. In the United States, serotypes 35B and 15A are ranking now at the third and the seventh position, respectively, but their rates of resistance are considerably lower [8]; in the United Kingdom, serotypes 24F and 15A are also increasing [17].
If serotypes 12F and 24F were predominantly found in invasive disease in children in 2011–2012, they appear quite rare in children nasopharyngeal carriage in the early PCV13 era [18]. This suggests a poor potential of colonization beside a high invasive disease potential, as already reported for serotype 12F [19]. We found several lineages among the 12F and 24F strains isolated in young children, reflecting a diverse population structure for both serotypes. The several distinct lineages of 12F serotype were all related to serotype 12F in the MLST database. In contrast, 24F lineages were related to more diverse serotypes according to the MLST database. Clonal complex CC230 included serotypes 14, 19A, and 24F isolates, which are related to clone Denmark14-ST230, a lineage largely distributed in southern European countries [20]. Likewise, all 24F isolates belonging to CC156 displayed a ST162, which is, in the database, mainly associated with serotype 9V strains and related to clone Spain9V-ST156, a major French clone found elsewhere in Europe [21, 22]. These data suggest that capsular switches took place between vaccine serotypes 14, 19A, and 9V, and the nonvaccine serotype 24F.
The main difference observed between these 2 emerging serotypes was their evolution between the late PCV7 and the PCV13 era. Serotype 12F spread could likely be related to vaccine pressure, but a natural “secular wave” could not be ruled out to explain this evolution, as already reported for other outbreaks [23, 24]. Indeed, main 12F lineages were similarly represented during the 2 periods of our study. By contrast, the recent emergence of serotype 24F seems to be related to the clonal expansion of a single lineage, the cotrimoxazole-resistant ST162, which seems to be more successful than multiresistant lineages from CC230, and antibiotic susceptible lineages from CC72. This is unexpected because use of broad-spectrum penicillins significantly increased in outpatients between 2008 and 2013 in France, accompanied by a decline of cotrimoxazole, macrolides, and tetracycline [15, 16]. Thus, the clonal expansion of ST162 might be explained by a higher invasiveness, although the related clone Spain9V-ST156 was shown to have a moderate invasive disease potential [21].
Despite some limitations, this study has several strengths, in particular the large number of isolates studied, and the performance of testing at a central reference laboratory using reference methods. It should be mentioned that 12F and 24F related CCs were distributed all over the country, indicating no local cluster.
CONCLUSIONS
This study emphasizes that continuous survey is needed to confirm whether antibiotic-resistant non-PCV13 serotypes 12F, 24F, 15A, and 35B will continue to expand, according to their resistance or invasive potential, or be overflowed by other non-PCV13 serotypes.
Acknowledgments
We thank all the microbiologists of the Observatoires Régionaux du Pneumocoque network for their active contribution to the French surveillance of Streptococcus pneumoniae (list of the coordinators available at: http://www.sante-limousin.fr/public/observatoires/observatoire-des-pneumocoques/presentation/16e5496f74fa6e0319d340496390862b#bas).
Disclaimer. The sponsor had no role in data collection, data analysis, data interpretation, or writing of the report.
Author contributions. E. V. designed the study and drafted the analysis plan. Strains and clinical data were managed by E. V. and C. J. through the French Observatoires Régionaux du Pneumocoque network. E. V. and C. J. did serotyping, antimicrobial resistance testing, and multilocus sequence typing of case isolates. C. J. wrote the main manuscript. L. G. provided input into the analysis plan and the interpretation of the data. All authors contributed to the interpretation of the findings and the writing of the final manuscript draft. C. J. and E. V. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was funded by the French Institute for Public Health Surveillance.
Potential conflicts of interest. E. V. received fees from Pfizer and GlaxoSmithKline for participation in working groups on pneumococcal vaccines. E. V. also received grant support through her institution from Pfizer. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References





Comments