-
PDF
- Split View
-
Views
-
Cite
Cite
Henry W. Nabeta, Nathan C. Bahr, Joshua Rhein, Nicholas Fossland, Agnes N. Kiragga, David B. Meya, Stephen J. Dunlop, David R. Boulware, Accuracy of Noninvasive Intraocular Pressure or Optic Nerve Sheath Diameter Measurements for Predicting Elevated Intracranial Pressure in Cryptococcal Meningitis, Open Forum Infectious Diseases, Volume 1, Issue 3, Fall 2014, ofu093, https://doi.org/10.1093/ofid/ofu093
Close - Share Icon Share
Abstract
Background. Cryptococcal meningitis is associated with increased intracranial pressure (ICP). Therapeutic lumbar puncture (LP) is recommended when the initial ICP is >250 mm H2O, yet the availability of manometers in Africa is limited and not always used where available. We assessed whether intraocular pressure could be a noninvasive surrogate predictor to determine when additional therapeutic LPs are necessary.
Methods. Ninety-eight human immunodeficiency virus-infected Ugandans with suspected meningitis (81% Cryptococcus) had intraocular pressure measured using a handheld tonometer (n = 78) or optic nerve sheath diameter (ONSD) measured by ultrasound (n = 81). We determined the diagnostic performance of these methods for predicting ICP vs a standard manometer.
Results. The median ICP was 225 mm H2O (interquartile range [IQR], 135–405 mm H2O). The median intraocular pressure was 28 mm Hg (IQR, 22–37 mm Hg), and median ultrasound ONSD was 5.4 mm (IQR, 4.95–6.1 mm). ICP moderately correlated with intraocular pressure (ρ = 0.45, P < .001) and with ultrasound ONSD (ρ = 0.44, P < .001). There were not discrete threshold cutoff values for either tonometry or ultrasound ONSD that provided a suitable cutoff diagnostic value to predict elevated ICP (>200 mm H2O). However, risk of elevated ICP >200 mm H2O was increased with an average intraocular pressure >28 mm Hg (relative risk [RR] = 3.03; 95% confidence interval [CI], 1.55–5.92; P < .001) or an average of ONSD >5 mm (RR = 2.39; 95% CI, 1.42–4.03; P = .003). As either intraocular pressure or ONSD increased, probability of elevated ICP increased (ie, positive predictive value increased).
Conclusions. Noninvasive intraocular pressure measurements by tonometry or ultrasound correlate with cerebrospinal fluid opening pressure, but both are a suboptimal replacement for actual ICP measurement with a manometer.
Cryptococcal meningitis is an AIDS-defining opportunistic infection causing 20%–25% of AIDS-related mortality worldwide, with a disproportionate mortality rate of 30%–70% in sub-Saharan Africa [1–6]. Due to the burden of the human immunodeficiency virus (HIV) epidemic, cryptococcal meningitis is the most common etiology of adult meningitis in sub-Saharan Africa [7]. Elevated intracranial pressure (ICP) is a contributing factor to this high mortality rate, and failure to manage elevated ICP leads to worse outcomes including death and adverse neurologic sequelae [6, 8–10]. Repeated therapeutic lumbar punctures (LPs) as well as temporary external lumbar drainage devices can manage ICP [10, 11]. Despite their clinical importance, the availability of manometers to measure cerebrospinal fluid (CSF) opening pressure is limited throughout Africa. An improvised manometer, using intravenous (IV) tubing, a 3-way stopcock, and a measuring stick, can provide an accurate measurement of CSF opening pressure [12]. However, even when available or improvised, CSF opening pressure is frequently not measured in routine care, as the cryptococcal meningitis diagnosis is not made until after the LP is completed. Thus, physicians are often left guessing as to whether increased ICP is present, basing clinical management decisions on imprecise measures such as headache, decreased Glasgow coma scale score, or fundoscopic papilledema [13]. An objective, noninvasive ICP surrogate would be of substantial clinical utility to guide when additional therapeutic LPs are necessary so as to prevent morbidity and mortality.
Intraocular pressure (IOP) may be one noninvasive surrogate to detect increased ICP, as the optic nerve sheath communicates between the eye and the central nervous system, potentially transmitting increased ICP into increased IOP. Handheld tonometers are common devices used to screen for glaucoma. Tonometers are easy to use, have excellent interoperator reproducibility within ±2.5 mm Hg, and are not dependent on patient position [14, 15]. Yet, prior attempts to correlate IOP with ICP have mixed results among studies of traumatic brain injury, idiopathic intracranial hypertension, and subarachnoid or intracranial hemorrhage; overall, the current evidence does not support IOP measurement as a predictor of ICP in these conditions [16–20]. A 2004 study demonstrated that among traumatic brain injury patients sedated in an intensive care unit, IOPs >20 mm Hg correlated well with increased ICPs >200 mm H2O [16], although this study was not blinded. Although one study of a variety of etiologies of increased ICP reported a strong correlation between IOP and ICP (R2 = 0.91) [16], the direct correlation between the 2 different pressure systems has been imprecise in other studies [18, 19]. Regardless, the potential correlation between IOP and ICP has not been measured.
Second, optic nerve sheath diameter as measured by ultrasound 3 mm behind the globe of the eye may be another noninvasive surrogate to detect increased ICP, as the optic nerve sheath dilates with increased ICP transmitted from the central nervous system [21, 22]. Among 51 pregnant women, when the diameter of the optic nerve sheath was >5.8 mm, this threshold diameter was associated with 95% sensitivity and 100% specificity to detect increased ICP in preeclampsia [22]. In another study of 24 emergency rooms patients, an optic nerve sheath diameter of >5 mm had 100% sensitivity and 75% specificity [23].
Whether handheld tonometry or ultrasound measurement of optic nerve sheath diameter is accurate among conscious HIV-infected patients with meningitis is unknown. The pathophysiology of increased ICP in cryptococcal meningitis is from obstruction of CSF reabsorption in the arachnoid villi due to direct mechanical blockage by cryptococcal organisms and polysaccharide capsule [24]. Given that this represents a diffuse, communicating process of increased CSF pressure, rather than localized trauma or injury manifesting as edema, we hypothesized that the ICP transmitted to the eye may be a noninvasive surrogate to guide when additional follow-up therapeutic LPs are necessary in patients with cryptococcal meningitis.
METHODS
We prospectively screened persons presenting with suspected meningitis on the infectious disease ward of Mulago National Referral Hospital in Kampala, Uganda, from November 2011 to July 2013. Participants were HIV-infected, antiretroviral therapy (ART)–naive adults aged >18 years who had a clinical suspicion of meningitis and were screened as part of the Cryptococcal Optimal ART Timing trial (ClinicalTrials.gov, NCT01075152) and a follow-on observational cohort [25]. Participants or their surrogate provided written informed consent prior to any examination being performed. Study protocols were approved by the institutional review boards of Makerere University, the University of Minnesota, and the Uganda National Council of Science and Technology.
Tonometry measurements were taken by one physician (H.W.N.) after an instructional period of approximately 1 month. After patient counseling, 2 drops of anesthetic (tetracaine 0.5%) were applied to each eye with the participant in the supine position. Intraocular pressure was measured 2 minutes later using a handheld tonometer (Accutome Inc). The tonometry procedure involves placing a clean cover over the tonometer probe, placing the covered tonometer probe on the eye lightly for <5 seconds, and awaiting the measurement from the tonometer via a digital reading. One measurement was performed on each eye. Thereafter, an LP was performed with the patient in the lateral decubitus position by 1 of 2 medical officers (H.W.N. or A.M.) with legs extended upon successful puncture for ICP measurement. CSF opening pressure (ie, ICP) was measured using a 550-mm manometer. CSF was drained until closing pressure was <200 mm H2O. The IOP was then measured again in both eyes at the conclusion of the LP. Normal IOP is <18 mm Hg, and normal ICP is <200 mm H2O [16, 20, 26] (pressure conversion: 1 mm Hg = 13.6 mm H2O).
Handheld ultrasound (Sonosite) was performed beginning in July 2012 with measurements by one physician (H.W.N.) after an intensive 1-week training (S.J.D.). The optic nerve sheath diameter (ONSD) was measured 3 mm behind the globe of the eye. The first 15 measurements during training were discarded for analysis. After handheld ultrasound measurements were performed, LP was performed in the same fashion described above. Among the patients who had both tonometry and ultrasound performed, the IOP measurement was initially performed on both eyes, followed 2–5 minutes later by the ONSD measurement by ultrasound. The LP was then performed as mentioned in the procedure above.
Data analysis focused on the prediction of ICP by the IOP or ultrasound ONSD. In this prospective cohort, diagnoses were unknown at time of initial diagnostic LP. Thus, cases of both cryptococcal meningitis and other causes of meningitis were included in the primary analysis. The noncryptococcal meningitis cases were deemed important to include as the majority had normal ICPs. A secondary analysis was also performed, restricted to cryptococcal meningitis cases only. Descriptive data are presented using median and interquartile range (IQR). The average of the IOP in both eyes was utilized for comparison. We also considered models utilizing the higher/lower measurement of IOP, as a sensitivity analysis. We performed a receiver-operating characteristic (ROC) curve to determine the possible optimal diagnostic threshold for prediction of increased ICP and evaluated the sensitivity and specificity, positive predictive value (PPV), and negative predictive value (NPV). Spearman rank correlation coefficient was used to assess correlation between surrogate measures and ICP. The analysis was performed with SPSS software, version 22.0 (IBM). Study protocols were approved by Makerere University, the University of Minnesota, and the Uganda National Council of Science and Technology.
RESULTS
Study Participants
We enrolled 98 patients with suspected meningitis. Four persons (4%) had tuberculosis meningitis, 15 (15%) had aseptic (viral) meningitis of unknown etiology, and 79 (81%) had cryptococcal meningitis. Forty-one persons had IOP measurements only, 46 had ONSD measurements only, and 11 had both IOP and ONSD measurements. Among the 52 participants who had handheld tonometry readings, some patients had multiple tonometry measurements with subsequent therapeutic LPs (n = 78 total IOP measurements; n = 31 initial diagnostic LP; n = 35 therapeutic LP for cryptococcosis; n = 12 late diagnostic LP to differentiate relapse vs immune reconstitution inflammatory syndrome). Ultrasound measurement of their ONSD was conducted in 57 patients (n = 84 total ONSD measurements; n = 44 initial diagnostic LP; n = 24 therapeutic LP; n = 16 late diagnostic LP). Table 1 displays the demographics for the participants at the time of their first measurement. In general, patients from both groups had very low CD4 counts, with a baseline median CD4 count of 17 cells/µL (IQR, 5–55 cells/µL). The median CSF quantitative cryptococcal culture was 71 500 colony-forming units (CFU)/mL (IQR, 10 100–215 000 CFU/mL). Altered mental status was common in both groups with a Glasgow coma scale score of <15 present in 31% with cryptococcosis and in 42% without cryptococcosis. At initial diagnostic LP, all the participants were ART naive.
| Variable . | Cryptococcal Meningitis (n = 79) . | Other Meningitis (n = 19) . |
|---|---|---|
| Age, y, median (IQR) | 36 (30–42) | 35 (31–43) |
| Male sex, No. (%) | 26 (58%) | 0 (0%) |
| CD4 count, cells/µL, median (IQR) | 18 (6–53) | 7 (5–65) |
| Glasgow coma scale <15, No. (%)a | 10 (33%) | 11 (58%) |
| CSF WBC count at baselinea, cells/µL | 28 (<5–99) | 45 (9–73) |
| CSF opening pressure, mm H2O | 226 (142–398) | 95 (76–330) |
| Intraocular pressure, mm Hg | 28 (23.5–35.6) | 32 (16–42) |
| CSF cryptococcal CFU/mL, median (IQR)a | 71 500 (10 100–215 000) | … |
| Variable . | Cryptococcal Meningitis (n = 79) . | Other Meningitis (n = 19) . |
|---|---|---|
| Age, y, median (IQR) | 36 (30–42) | 35 (31–43) |
| Male sex, No. (%) | 26 (58%) | 0 (0%) |
| CD4 count, cells/µL, median (IQR) | 18 (6–53) | 7 (5–65) |
| Glasgow coma scale <15, No. (%)a | 10 (33%) | 11 (58%) |
| CSF WBC count at baselinea, cells/µL | 28 (<5–99) | 45 (9–73) |
| CSF opening pressure, mm H2O | 226 (142–398) | 95 (76–330) |
| Intraocular pressure, mm Hg | 28 (23.5–35.6) | 32 (16–42) |
| CSF cryptococcal CFU/mL, median (IQR)a | 71 500 (10 100–215 000) | … |
Abbreviations: CFU, colony-forming units; CSF, cerebrospinal fluid; IQR, interquartile range; WBC, white blood cell.
a At initial presentation.
| Variable . | Cryptococcal Meningitis (n = 79) . | Other Meningitis (n = 19) . |
|---|---|---|
| Age, y, median (IQR) | 36 (30–42) | 35 (31–43) |
| Male sex, No. (%) | 26 (58%) | 0 (0%) |
| CD4 count, cells/µL, median (IQR) | 18 (6–53) | 7 (5–65) |
| Glasgow coma scale <15, No. (%)a | 10 (33%) | 11 (58%) |
| CSF WBC count at baselinea, cells/µL | 28 (<5–99) | 45 (9–73) |
| CSF opening pressure, mm H2O | 226 (142–398) | 95 (76–330) |
| Intraocular pressure, mm Hg | 28 (23.5–35.6) | 32 (16–42) |
| CSF cryptococcal CFU/mL, median (IQR)a | 71 500 (10 100–215 000) | … |
| Variable . | Cryptococcal Meningitis (n = 79) . | Other Meningitis (n = 19) . |
|---|---|---|
| Age, y, median (IQR) | 36 (30–42) | 35 (31–43) |
| Male sex, No. (%) | 26 (58%) | 0 (0%) |
| CD4 count, cells/µL, median (IQR) | 18 (6–53) | 7 (5–65) |
| Glasgow coma scale <15, No. (%)a | 10 (33%) | 11 (58%) |
| CSF WBC count at baselinea, cells/µL | 28 (<5–99) | 45 (9–73) |
| CSF opening pressure, mm H2O | 226 (142–398) | 95 (76–330) |
| Intraocular pressure, mm Hg | 28 (23.5–35.6) | 32 (16–42) |
| CSF cryptococcal CFU/mL, median (IQR)a | 71 500 (10 100–215 000) | … |
Abbreviations: CFU, colony-forming units; CSF, cerebrospinal fluid; IQR, interquartile range; WBC, white blood cell.
a At initial presentation.
Tonometry
The distributions of the ICPs and IOPs are presented in Figure 1. The median CSF opening pressure was 225 mm H2O (IQR, 135–405 mm H2O) with an average CSF volume drained of 23 mL (SD, 14 mL). The median IOP was 28 mm Hg (IQR, 22–37 mm Hg) based on the average of the 2 eyes before the LP and with a mean reduction of 6.3 mm Hg (95% confidence interval [CI], 2.9–9.8 mm Hg) after the LP. There was moderate correlation (Spearman correlation coefficient, ρ = 0.45; P < .001) between the ICPs and IOPs (Figure 2). Among persons with increased ICP >200 mm H2O, 94% (44/47) had increased IOP >20 mm Hg. Above the median IOP of 28 mm Hg, the risk was 3-fold higher of having elevated ICP >200 mm H2O (relative risk [RR] = 3.03; 95% CI, 1.55–5.92; P < .001).
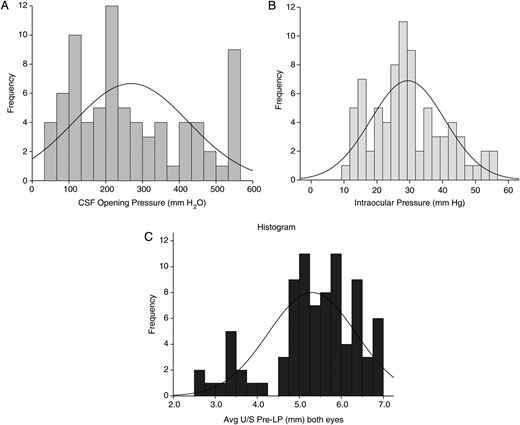
Distribution of intracranial and intraocular pressure in study cohort. A, Frequency of various cerebrospinal fluid opening pressures in mm H2O. B, Frequency of various intraocular pressure measurements as measured by tonometry (mm Hg). C, Frequency of mean optic disk diameter measurement in both eyes as measured by ultrasonography in millimeters. Abbreviations: CSF, cerebrospinal fluid; LP, lumbar puncture; U/S, ultrasound.
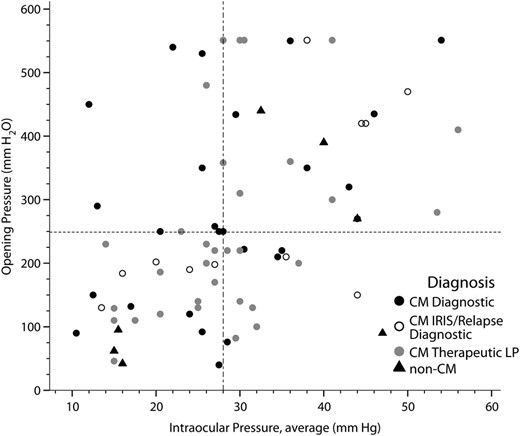
Scatterplot relating mean intraocular pressure and intracranial pressure. The scatterplot displays the distribution of cerebrospinal fluid opening pressure during lumbar puncture vs the intraocular pressure, averaged between both eyes among human immunodeficiency virus-infected persons with suspected meningitis (Spearman ρ = 0.45; P < .001). Abbreviations: CM, cryptococcal meningitis; IRIS, immune reconstitution inflammatory syndrome; LP, lumbar puncture.
Based on the ROC curve, above an IOP threshold of >28 mm Hg (median of cohort) and using the average of both eyes, there was 68% (32/47) sensitivity and 74% (23/31) specificity for predicting a CSF ICP >200 mm H2O, a threshold above which the ICP is considered elevated. The PPV was 80% (32/40) and the NPV was 61% (23/38) (Table 2).
As the Intracranial Pressure Increased, the PPV by Tonometry to Detect Substantially Elevated Pressures Increased
| Intraocular Pressure Measured From Both Eyes . | Performance to Detect Intracranial Pressure (>200 mm H2O) . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| Average >20 mm Hg | 94% (44/47) | 39% (19/31) | 70% (44/63) | 80% (12/15) | 9.3 (2.3–36.7) | .001 |
| Average ≥25 mm Hg | 83% (39/47) | 58% (18/31) | 75% (39/47) | 69% (18/26) | 6.7 (2.4–19.2) | <.001 |
| Average ≥28 mm Hg | 68% (32/47) | 74% (23/31) | 80% (32/40) | 61% (23/38) | 6.1 (2.2–16.9) | <.001 |
| Average ≥35 mm Hg | 40% (19/47) | 90% (28/31) | 87% (19/22) | 50% (28/56) | 6.3 (1.7–23.8) | .004 |
| Minimum ≥35 mm Hg in either eye | 30% (14/47) | 94% (29/31) | 88% (14/16) | 47% (29/62) | 11.9 (2.4–57) | <.001 |
| Intraocular Pressure Measured From Both Eyes . | Performance to Detect Intracranial Pressure (>200 mm H2O) . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| Average >20 mm Hg | 94% (44/47) | 39% (19/31) | 70% (44/63) | 80% (12/15) | 9.3 (2.3–36.7) | .001 |
| Average ≥25 mm Hg | 83% (39/47) | 58% (18/31) | 75% (39/47) | 69% (18/26) | 6.7 (2.4–19.2) | <.001 |
| Average ≥28 mm Hg | 68% (32/47) | 74% (23/31) | 80% (32/40) | 61% (23/38) | 6.1 (2.2–16.9) | <.001 |
| Average ≥35 mm Hg | 40% (19/47) | 90% (28/31) | 87% (19/22) | 50% (28/56) | 6.3 (1.7–23.8) | .004 |
| Minimum ≥35 mm Hg in either eye | 30% (14/47) | 94% (29/31) | 88% (14/16) | 47% (29/62) | 11.9 (2.4–57) | <.001 |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
As the Intracranial Pressure Increased, the PPV by Tonometry to Detect Substantially Elevated Pressures Increased
| Intraocular Pressure Measured From Both Eyes . | Performance to Detect Intracranial Pressure (>200 mm H2O) . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| Average >20 mm Hg | 94% (44/47) | 39% (19/31) | 70% (44/63) | 80% (12/15) | 9.3 (2.3–36.7) | .001 |
| Average ≥25 mm Hg | 83% (39/47) | 58% (18/31) | 75% (39/47) | 69% (18/26) | 6.7 (2.4–19.2) | <.001 |
| Average ≥28 mm Hg | 68% (32/47) | 74% (23/31) | 80% (32/40) | 61% (23/38) | 6.1 (2.2–16.9) | <.001 |
| Average ≥35 mm Hg | 40% (19/47) | 90% (28/31) | 87% (19/22) | 50% (28/56) | 6.3 (1.7–23.8) | .004 |
| Minimum ≥35 mm Hg in either eye | 30% (14/47) | 94% (29/31) | 88% (14/16) | 47% (29/62) | 11.9 (2.4–57) | <.001 |
| Intraocular Pressure Measured From Both Eyes . | Performance to Detect Intracranial Pressure (>200 mm H2O) . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| Average >20 mm Hg | 94% (44/47) | 39% (19/31) | 70% (44/63) | 80% (12/15) | 9.3 (2.3–36.7) | .001 |
| Average ≥25 mm Hg | 83% (39/47) | 58% (18/31) | 75% (39/47) | 69% (18/26) | 6.7 (2.4–19.2) | <.001 |
| Average ≥28 mm Hg | 68% (32/47) | 74% (23/31) | 80% (32/40) | 61% (23/38) | 6.1 (2.2–16.9) | <.001 |
| Average ≥35 mm Hg | 40% (19/47) | 90% (28/31) | 87% (19/22) | 50% (28/56) | 6.3 (1.7–23.8) | .004 |
| Minimum ≥35 mm Hg in either eye | 30% (14/47) | 94% (29/31) | 88% (14/16) | 47% (29/62) | 11.9 (2.4–57) | <.001 |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Because IOP was a continuous measurement positively correlating with ICP, as the IOP increased, the probability of having increased ICP increased and vice versa. At IOPs of 25, 30, and 35 mm Hg, the probability of having substantially elevated ICP of ≥250 mm H2O was 60% (31/52), 67% (22/33), and 77% (17/22), respectively.
An unexpected observation was the occasional difference between the measurements of IOP in both eyes with a mean difference of 2.3 mm Hg (SD, 15 mm Hg) (P = .23), which may imply that pressure is not always being transmitted equally. There was no correlation between the IOP and the fungal burden by quantitative CSF culture. Increased IOP persisted beyond the initial diagnostic LP, but the impact of this was unknown. Within the cohort, formal follow-up detailed visual testing was not performed.
Restricted Analysis—Cryptococcal Meningitis Only
Among only patients with cryptococcal meningitis, the median opening pressure was 238 mm H2O (IQR, 150–366 mm H2O) with an average CSF volume drained of 23 mL (SD, 13 mL). The median IOP was 28 mm Hg (IQR, 22–35 mm Hg) based on the average of the 2 eyes before the LP. There was moderate correlation (Spearman correlation coefficient, ρ = 0.438; P < .001) between the ICPs and IOPs. Among persons with increased ICP >200 mm H2O, 93% (41/44) had increased IOP >20 mm Hg. Above the median IOP of 28 mm Hg, the risk was 3-fold higher of having elevated ICP >200 mm H2O (RR = 2.94; 95% CI, 1.43–6.06; P < .001). Above >28 mm Hg (median of cohort) and using the average of both eye measurements, there was 66% (29/44) sensitivity and 74% (20/27) specificity for predicting a CSF ICP >200 mm H2O (a threshold above which the ICP is considered elevated). The PPV was 81% (29/36) and NPV was 57% (20/35).
As with the more inclusive analysis, as the IOP increased, the probability of having increased ICP increased and vice versa. At IOPs of 25, 30, and 35 mm Hg, the probability of having substantially elevated ICP of ≥250 mm H2O was 58% (28/48), 66% (19/25), and 79% (15/19), respectively. There was moderate positive correlation between the IOP and the fungal burden by quantitative CSF culture (ρ = 0.460; P = .009).
Ultrasound of the ONSD
The median ONSD was 5.5 mm (IQR, 5.0–6.0 mm) based on the average of the 2 eyes before the LP and 5.5 mm (IQR, 4.9–5.9 mm) after the LP. There was a moderate positive correlation (Spearman correlation coefficient, ρ = 0.44; P < .001) between the ICP and ONSD (Figure 3). Based on the ROC curve, above an average ONSD from both eyes of ≥5 mm, there was 85% (33/39) sensitivity and 59% (16/27) specificity for predicting a CSF ICP >200 mm H2O. The PPV was 75% (33/44) and NPV was 73% (16/22) (RR = 3.85; 95% CI, 1.73–8.57; P < .001).
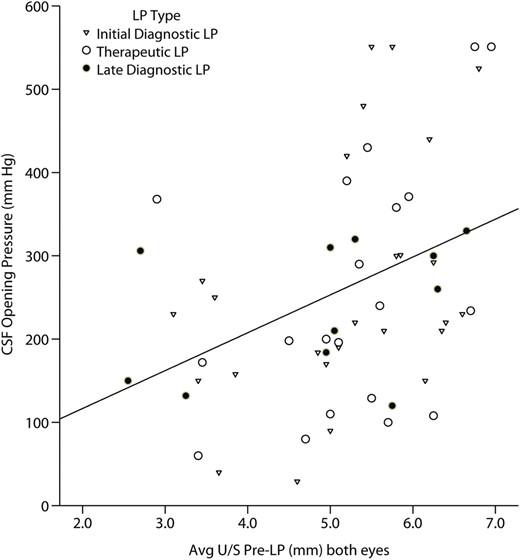
Scatterplot relating mean optic nerve sheath diameter and intracranial pressure. The scatterplot displays the distribution of cerebrospinal fluid opening pressure during lumbar puncture vs the average optic nerve sheath diameter, averaged between both eyes among human immunodeficiency virus-infected persons with suspected meningitis (Spearman ρ = 0.44; P < .001). Abbreviations: CSF, cerebrospinal fluid; LP, lumbar puncture; U/S, ultrasound.
As the ICP became more elevated, the ONSD was more frequently increased. At ICP of >350 mm H2O, the sensitivity was 93% (14/15) at 5 mm ONSD with a 95% (21/22) NPV, whereby if the ONSD was <5 mm, the ICP was <350 mm H2O (Table 3). Conversely, as the ONSD increased, the probability of having elevated ICP increased. When the average ONSD ≥6 mm was used to predict ICP at 250 mm H2O, the sensitivity was 36% (14/39), specificity 93% (25/27), PPV 88% (14/16), and NPV 50% (25/50).
As the Intracranial Pressure Increased, the Sensitivity by Ultrasound to Detect Substantially Elevated Pressures Increased
| Performance to Detect Intracranial Pressure . | Ultrasound Optic Nerve Sheath Diameter >5 mm . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| >200 mm H2O | 85% (33/39) | 59% (16/27) | 75% (33/44) | 73% (16/22) | 8.0 (2.5–25.5) | <.001 |
| >250 mm H2O | 83% (24/29) | 46% (17/37) | 54% (24/44) | 77% (17/22) | 4.0 (1.3–13.0) | .018 |
| >300 mm H2O | 91% (20/22) | 45% (20/44) | 45% (20/44) | 91% (20/22) | 8.3 (1.7–40.1) | .005 |
| >350 mm H2O | 93% (14/15) | 41% (21/51) | 32% (14/44) | 95% (21/22) | 9.8 (1.2–80.3) | .013 |
| Performance to Detect Intracranial Pressure . | Ultrasound Optic Nerve Sheath Diameter >5 mm . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| >200 mm H2O | 85% (33/39) | 59% (16/27) | 75% (33/44) | 73% (16/22) | 8.0 (2.5–25.5) | <.001 |
| >250 mm H2O | 83% (24/29) | 46% (17/37) | 54% (24/44) | 77% (17/22) | 4.0 (1.3–13.0) | .018 |
| >300 mm H2O | 91% (20/22) | 45% (20/44) | 45% (20/44) | 91% (20/22) | 8.3 (1.7–40.1) | .005 |
| >350 mm H2O | 93% (14/15) | 41% (21/51) | 32% (14/44) | 95% (21/22) | 9.8 (1.2–80.3) | .013 |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
As the Intracranial Pressure Increased, the Sensitivity by Ultrasound to Detect Substantially Elevated Pressures Increased
| Performance to Detect Intracranial Pressure . | Ultrasound Optic Nerve Sheath Diameter >5 mm . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| >200 mm H2O | 85% (33/39) | 59% (16/27) | 75% (33/44) | 73% (16/22) | 8.0 (2.5–25.5) | <.001 |
| >250 mm H2O | 83% (24/29) | 46% (17/37) | 54% (24/44) | 77% (17/22) | 4.0 (1.3–13.0) | .018 |
| >300 mm H2O | 91% (20/22) | 45% (20/44) | 45% (20/44) | 91% (20/22) | 8.3 (1.7–40.1) | .005 |
| >350 mm H2O | 93% (14/15) | 41% (21/51) | 32% (14/44) | 95% (21/22) | 9.8 (1.2–80.3) | .013 |
| Performance to Detect Intracranial Pressure . | Ultrasound Optic Nerve Sheath Diameter >5 mm . | |||||
|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | PPV . | NPV . | Odds Ratio (95% CI) . | P Value . | |
| >200 mm H2O | 85% (33/39) | 59% (16/27) | 75% (33/44) | 73% (16/22) | 8.0 (2.5–25.5) | <.001 |
| >250 mm H2O | 83% (24/29) | 46% (17/37) | 54% (24/44) | 77% (17/22) | 4.0 (1.3–13.0) | .018 |
| >300 mm H2O | 91% (20/22) | 45% (20/44) | 45% (20/44) | 91% (20/22) | 8.3 (1.7–40.1) | .005 |
| >350 mm H2O | 93% (14/15) | 41% (21/51) | 32% (14/44) | 95% (21/22) | 9.8 (1.2–80.3) | .013 |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Similar to the tonometry, there were slight differences in ONSD measurements between eyes with a mean difference of 0.6 mm (SD, 0.5 mm). There was no correlation between IOP by tonometry and ONSD by ultrasound (ρ = 0.22; P = .42), although the sample size was small (n = 16) with both tests at the same time point. There was no correlation between the ONSD and the fungal burden by quantitative CSF culture.
Restricted Analysis—Cryptococcal Meningitis Only
When considering only persons with cryptococcal meningitis, the median ONSD was 5.4 mm (IQR, 4.9–6.0 mm) based on the average of the 2 eyes. There was a moderate positive correlation (Spearman correlation coefficient ρ = 0.43; P < .001) between the ICP and ONSD. Above an average ONSD of ≥5 mm from both eyes, there was 86% (30/35) sensitivity and 63% (15/24) specificity for predicting a CSF ICP >200 mm H2O. The PPV was 77% (30/39), and NPV was 75% (15/20) (RR = 3.25; 95% CI, 1.74–6.10; P < .001). As the ICP became more elevated, the ONSD was more frequently increased. At ICP of >350 mm H2O, the sensitivity was 93% (13/14) at 5 mm ONSD with a 95% (19/20) NPV. There was no correlation between ONSD and fungal burden by quantitative CSF culture (ρ = −204; P = .26).
DISCUSSION
Among a prospective cohort of 98 HIV-infected Ugandans presenting with suspected meningitis, there was a moderate sensitivity to detect increased ICP using noninvasive measurement of IOP with a handheld tonometer or ultrasound. Both of these noninvasive measures showed moderated correlation with ICP; however, the correlation was not precise. As a continuous measure, there was not a particular discrete threshold cutoff value that provided diagnostic utility. Neither noninvasive measure would be a replacement for a LP performed with a manometer to measure CSF opening pressure.
Yet, the lack of a threshold cutoff value that provided diagnostic certainty does not mean that these noninvasive measures were completely noninformative. We found positive correlation between ICP and IOP, as have others [16, 27]. Above the median IOP of 28 mm Hg, the risk of elevated ICP increased 3-fold. Above an ONSD of 5 mm measured by ultrasound, the risk of elevated ICP increased 2.4-fold. Furthermore, as the IOP and ONSD increased, the probability of having elevated ICP (ie, PPV) increased with 88% PPV for measurements ≥35 mm Hg for IOP and ≥6 mm for ONSD by ultrasound. These noninvasive methods could provide further objective data to support or diminish the need to perform a therapeutic LP (ideally using a manometer) and help to avoid delays in performing necessary LPs. An alternative approach would be to perform scheduled therapeutic LPs regardless of the prior opening pressure.
In addition, there may be some utility in the NPV of these measures. NPV for an IOP ≥20 mm Hg was 80% for detection of an intracranial pressure >200 mm H2O. Unfortunately, this clearly is far from 100% and the NPV values decrease with increased thresholds of IOP measurement. ONSD measurement resulted in similar performance, with ONSD of ≥5 mm having 73% NPV to detect intracranial pressures >200 mm H2O and 77% to detect intracranial pressures ≥250 mm H2O. Although ONSD performance did improve for detection of the intracranial thresholds of 300 or 350 mm H2O, respectively (91% and 95%), these are less clinically meaningful values as they are all essentially worsening values that require LP just as 200 mm H2O does.
In resource-rich settings, several but not all studies have evaluated and found a correlation between IOP and ICP. The likely mechanism is due to communication between the subarachnoid and the space that surrounds the optic nerve, allowing any increase in the ICP to be transmitted to the eye, resulting in raised IOP [28]. The enlarged retrobulbar optic nerve may exert anteroposterior compression on the globe, evidenced by the posterior scleral flattening associated with idiopathic intracranial hypertension and the difference in axial lengths [29]. Sajjadi et al also demonstrated that the correlation between the IOP and ICP is independent of other factors such as body mass index, age, and disease type [26]. These variables appeared also to have no significant correlation in our study. Fungal burden positively correlated with IOP but not ONSD. This imperfect or lack of correlation may reflect the complex dynamics of the mechanism of increased ICP in cryptococcosis, whereby the cryptococcal polysaccharide capsule size is more important than the fungal burden by either quantitative culture or cryptococcal antigen titer [30].
Among the challenges and limitations of this study were obtaining patient consent to using the handheld tonometer. IOP measurement for glaucoma screening is not commonly performed in Uganda, and so misconceptions about possible eye trauma and/or visual impairment due to the procedure were common. A strength of tonometry is the interoperator variability, which has been studied extensively and is minimal [14, 15]. A limitation is that despite anesthesia with tetracaine, some conscious patients found the procedure uncomfortable. A noncontact tonometer might have better patient acceptance. Ultrasound was well tolerated and acceptable. However, ultrasound has interoperator variability, with training being essential. Although the cost of the tonometer ($630 used) is not negligible, neither is the repeated cost of disposable monometers ($8/manometer) were they available in Uganda.
Ultrasound has been suggested as an alternative approach for surrogate ICP measurement. Studies have been performed among pregnant women with preeclampsia and in emergency room patients to detect increased intracranial pressure by measuring the ONSD [22, 23]. ONSD measurement has also been found to correlate well with actual ICP in patients of severe traumatic brain injury [31] or with severity of acute mountain sickness [32]. We found a moderate positive correlation of ONSD with ICP in our study, but not precision in predicting ICP.
Finally, this study is most generalizable to persons with cryptococcosis, wherein ICP management becomes vitally important. Iatrogenic reticence to conduct repeated therapeutic LPs can be a fatal mistake. In a patient with cryptococcal meningitis with elevated IOP where manometers are not available, removal of approximately 20 mL of CSF may be reasonable based on the volumes removed in this cohort (mean volume removed, 23 mL [SD, 14 mL]). A small volume diagnostic CSF sample (eg, approximately 5 mL) is virtually always insufficient to normalize ICP.
In conclusion, our study showed moderate sensitivity to detect increased intracranial pressure using noninvasive measurement of IOP with a handheld tonometer or ultrasound, although neither technology should be considered as a replacement for LPs with manometers, as the correlations are imprecise. In settings where manometers are unavailable to measure CSF opening pressure, improvised manometers using IV tubing perform suitably well [33] and would be preferred to tonometry or ultrasound. In the absence of an LP, increasing IOP or dilation of the optic nerve sheath increase the risk of elevated ICP being present. Yet, these noninvasive measurements are not a replacement for an LP with measuring ICP. We strongly and unequivocally advocate for the use of manometers to measure CSF opening pressure during lumbar punctures; however, in the absence of a measured ICP, IOP measurement via a handheld tonometer or ultrasound may help inform the probability of elevated ICP being present. The role of increased IOP with visual loss after cryptococcosis requires further exploration. Further research in optimizing IPC management in cryptococcal meningitis is needed.
Notes
Acknowledgments. We thank Drs Paul Bohjanen, Andrew Kambugu, Alex Coutinho, Aaron Friedman, Darlisha Williams, Lois King, and Richard King for institutional support. We thank Sonosite for the loan of a portable ultrasound in 2012–2013 and Dr Abdu Musubire for clinical care.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases and Fogarty International Center at the National Institutes of Health (grant numbers K23AI073192, U01AI089244, R25TW009345, T32AI055433, R21NS065713).
Potential conflicts of interest. None declared.
References
Author notes
H. W. N. and N. C. B. contributed equally to this manuscript.





Comments