-
PDF
- Split View
-
Views
-
Cite
Cite
Soazic Grard, Gaud Catho, Florent Valour, Anissa Bouaziz, Thomas Perpoint, Evelyne Braun, François Biron, Patrick Miailhes, Tristan Ferry, Christian Chidiac, Pierre-Jean Souquet, Sébastien Couraud, Gérard Lina, Sylvain Goutelle, Nicolas Veziris, Oana Dumitrescu, Florence Ader, for the Lyon TB Study Group, F. Ader, F. Biron, A. Boibieux, A. Bouaziz, K. Bouledrak, E. Braun, G. Catho, C. Chidiac, W. Chumbi-Flores, S. Couraud, G. Devouassoux, O. Dumitrescu, T. Ferry, N. Freymond, S. Gardes, S. Gerbier-Colomban, Y. Gillet, S. Goutelle, J. Grando, R. Grima, L. Hees, V. Jubin, L. Kiakouama-Maleka, G. Lina, J.M. Maury, P. Miailhes, T. Perpoint, Em. Perrot, Emm Perrot, P. Reix, A.S. Renaud-Baron, V. Ronin, A. Senechal, P.J. Souquet, H. Thai Van, F. Tronc, F. Valour, P. Vanhems, for the Lyon TB Study Group, Linezolid in the Starter Combination for Multidrug-Resistant Tuberculosis: Time to Move on to Group Four?, Open Forum Infectious Diseases, Volume 2, Issue 4, Fall 2015, ofv175, https://doi.org/10.1093/ofid/ofv175
Close - Share Icon Share
Abstract
Linezolid (LNZ), a group 5 antituberculous drug (unclear efficacy), was used in the starter regimens of 23 adults with multidrug-resistant tuberculosis. The LNZ-containing regimens were effective in achieving culture conversions and relapse-free outcomes. The most frequent LNZ-related side effect was neuropathy. We propose that LNZ should be reclassified among bactericidal second-line drugs.
Multidrug-resistant (MDR) tuberculosis (TB) is caused by strains of Mycobacterium tuberculosis that are simultaneously resistant to isoniazid and rifampin. Additional resistance to quinolones (QNL) and a single or more injectable agent(s) (capreomycin, kanamycin, or amikacin) defines extensively drug-resistant (XDR) TB [1]. Multidrug-resistant and XDR TB are growing concerns in high-income public health, low-TB prevalence countries. The World Health Organization (WHO) guidelines recommend the following 5-agent treatment regimen for MDR TB: pyrazinamide; a parenteral aminoglycosides (group 2); a later generation QNL (group 3); ethionamide and either cycloserine or para-aminosalicylic acid (PAS) (group 4). Other agents, not recommended as initial treatment, are linezolid (LNZ), imipenem, amoxicillin/clavulanate, and clarithromycin (group 5) [1]. The difficulty of MDR-TB treatments is to design the most appropriate drug regimen combining efficacy, safety, and tolerability for each patient. In 2013, the estimated global number of MDR and XDR TB cases among newly diagnosed patients with pulmonary TB was 480 000. In some eastern Europe and central Asia countries, 9%–32% of new cases have MDR TB, including more than 50% of previously treated cases [1]. Although France has a low prevalence of MDR TB (1.8%), a recent dramatic increase in the number of cases has been observed, mostly imported from higher prevalence countries [2]. The challenge that many European western countries is facing is to optimally control MDR-TB strains to prevent a shift towards an endemoepidemic status. A dominant microbiological feature of culture-positive strains collected in France is phenotypic resistance to ethionamide (ETO), which is as high as 73% nationwide and 77% in our center. A literature-based approach reveals an extremely wide range of ETO-resistant strains across the world (from 10.8% to 100%) due to the heterogeneity of the disease across various populations and the uncertainties regarding the reproducibility and reliability of ETO-susceptibility testing [3–5]. Thus, WHO guidelines do not recommend performing routine drug-susceptibility testing for group 4 drugs, although ETO is a pivotal drug in the MDR-TB starter regimen [6]. High-ETO resistance has led us to systematically include LNZ in the MDR-TB starter combination. Linezolid has been used primarily for patients considered to be complicated or “intractable” for whom second-line regimen failed or TB strains were phenotypically resistant to second-line drugs [7, 8]. A progressive shift in LNZ use is observed, because systematic reviews found that treatment outcomes with LNZ-containing regimens for complicated cases of MDR TB are noninferior for both MDR and XDR TB [7–14]. Thus, we report our experience with LNZ use as group 4 drug in the starter combination of MDR-TB treatment.
PATIENTS AND METHODS
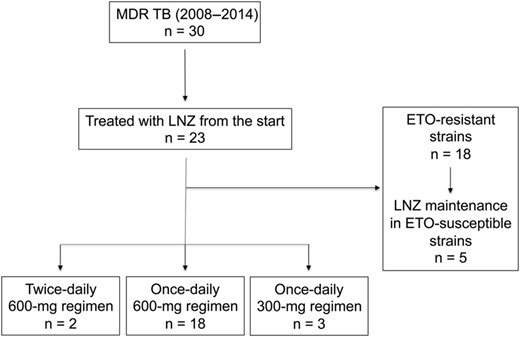
Among the 30 patients who were treated for MDR TB in our center between 2008 and 2014, 23 patients were treated from the start with LNZ. It was used either instead of ETO in patients with ETO-resistant strains (78%, n = 18) or in combination with ETO for the few patients with ETO-susceptible strains (Figure 1). The majority of the patients (78%, n = 18) received a single-dose 600 mg regimen, 2 (8%) patients received a twice-daily 600 mg regimen, and 3 (12.5%) patients received a single-dose 300 mg regimen. Direct-observed therapy was the rule during hospitalization, whereas treatment combination was delivered once monthly to outpatients. The peak and trough LNZ plasma concentrations of 9 patients on the 600 mg daily regimen were measured with a validated high-performance liquid chromatography method [15]. Data related to treatment monitoring and outcomes were collected and assessed as previously described [16, 17].
RESULTS
The patients' general characteristics are summarized in Table 1. The median length of treatment with LNZ was 274 days (interquartile range [IQR], 195–365). The median time to sputum culture conversion was 39 days (IQR, 21–76.5; 6 missing data) (Table 1). The majority of patients (91%) experienced relapse-free outcome with a median follow up after treatment completion of 16.3 months to date (IQR, 12–24.3). Two patients defaulted at 5.5 and 7 months after treatment initiation. The most frequent LNZ-related side effect was neuropathy, which occurred in 10 (43.5%) patients at a median time of 240 days (IQR, 120–265) after LNZ initiation. Among these neuropathies, 8 were peripheral, 1 was optic, and 1 was both, resulting in drug interruption. Hematological disorders accounted for a single drug interruption due to acute anemia that required transfusion support in 1 patient receiving 1200 mg daily. Platelet count below 100 000 platelets/mm3 was observed in 2 patients. The LNZ plasma concentrations were below the lower limit of quantification in 2 patients suspected of nonadherence. In the 7 other monitored patients, the median peak was 9.1 mg/L (IQR, 8.3–14.82) and the median trough was 0.8 mg/L (IQR, 0.4–1.8). The most frequently administered drugs associated with LNZ in the background regimens were QNL (92%, n = 21), injectable aminoglycosides (87%, n = 20), cycloserine (83%, n = 19), PAS (74%, n = 17), and bedaquiline ([BDQ] 35%, n = 8). Ethionamide and imipenem with amoxicillin/clavulanate were sporadically used (22%, n = 5 and 13%, n = 3, respectively; Table 1). Six patients underwent surgical resection of cavitary lesions or destroyed lungs.
| Characteristics . | Linezolid in the Starter Therapy . |
|---|---|
| Subjects, n | 23 |
| Age (years, min-max) | 40 (15–71) |
| Gender (male) | 18 (78) |
| XDR-TB | 2 (8.7) |
| MDR-TB | 21 (91.3) |
| Type of disease | |
| Lung TB with positive sputum culture | 17 (74) |
| Lung TB with negative sputum cultureb | 4 (17.3) |
| Extrapulmonary TB with positive culture samples | 2 (8.7) |
| Course of disease | |
| Time to sputum conversion (days) | 39 (21–76.5) |
| Patients cured | 19 (82.6) |
| Patients in the continuation phase | 2 (8.7) |
| Patients defaulting | 2 (8.7) |
| Follow-up duration (months) | 16.3 (12–24.3) |
| Susceptibility tests result resistance (%) | |
| Ethionamide | 78 |
| Pyrazinamide | 65.2 |
| Fluoroquinolones | 22 |
| Cycloserine | 22 |
| PAS | 22 |
| Amikacin | 13 |
| Background regimen | |
| Fluoroquinolones | 21 (91) |
| Amikacin, streptomycin | 20 (87) |
| Cycloserine | 19 (83) |
| PAS | 17 (74) |
| Bedaquiline | 8 (34.7) |
| Ethionamide | 5 (21.7) |
| Imipenem + clavulanate | 3 (13) |
| Adjuvant thoracic surgery, n (%) | 6 (26) |
| Characteristics . | Linezolid in the Starter Therapy . |
|---|---|
| Subjects, n | 23 |
| Age (years, min-max) | 40 (15–71) |
| Gender (male) | 18 (78) |
| XDR-TB | 2 (8.7) |
| MDR-TB | 21 (91.3) |
| Type of disease | |
| Lung TB with positive sputum culture | 17 (74) |
| Lung TB with negative sputum cultureb | 4 (17.3) |
| Extrapulmonary TB with positive culture samples | 2 (8.7) |
| Course of disease | |
| Time to sputum conversion (days) | 39 (21–76.5) |
| Patients cured | 19 (82.6) |
| Patients in the continuation phase | 2 (8.7) |
| Patients defaulting | 2 (8.7) |
| Follow-up duration (months) | 16.3 (12–24.3) |
| Susceptibility tests result resistance (%) | |
| Ethionamide | 78 |
| Pyrazinamide | 65.2 |
| Fluoroquinolones | 22 |
| Cycloserine | 22 |
| PAS | 22 |
| Amikacin | 13 |
| Background regimen | |
| Fluoroquinolones | 21 (91) |
| Amikacin, streptomycin | 20 (87) |
| Cycloserine | 19 (83) |
| PAS | 17 (74) |
| Bedaquiline | 8 (34.7) |
| Ethionamide | 5 (21.7) |
| Imipenem + clavulanate | 3 (13) |
| Adjuvant thoracic surgery, n (%) | 6 (26) |
Abbreviations: MDR, multidrug-resistant; PAS, para-aminosalicylic acid; TB, tuberculosis; XDR, extensively drug-resistant.
a The data are presented as the numbers (%) or the medians with the interquartile ranges Q1–Q3 unless otherwise stated.
b Clinically diagnosed TB cases (close contacts of bacteriologically confirmed TB case) that received a full course of TB treatment.
| Characteristics . | Linezolid in the Starter Therapy . |
|---|---|
| Subjects, n | 23 |
| Age (years, min-max) | 40 (15–71) |
| Gender (male) | 18 (78) |
| XDR-TB | 2 (8.7) |
| MDR-TB | 21 (91.3) |
| Type of disease | |
| Lung TB with positive sputum culture | 17 (74) |
| Lung TB with negative sputum cultureb | 4 (17.3) |
| Extrapulmonary TB with positive culture samples | 2 (8.7) |
| Course of disease | |
| Time to sputum conversion (days) | 39 (21–76.5) |
| Patients cured | 19 (82.6) |
| Patients in the continuation phase | 2 (8.7) |
| Patients defaulting | 2 (8.7) |
| Follow-up duration (months) | 16.3 (12–24.3) |
| Susceptibility tests result resistance (%) | |
| Ethionamide | 78 |
| Pyrazinamide | 65.2 |
| Fluoroquinolones | 22 |
| Cycloserine | 22 |
| PAS | 22 |
| Amikacin | 13 |
| Background regimen | |
| Fluoroquinolones | 21 (91) |
| Amikacin, streptomycin | 20 (87) |
| Cycloserine | 19 (83) |
| PAS | 17 (74) |
| Bedaquiline | 8 (34.7) |
| Ethionamide | 5 (21.7) |
| Imipenem + clavulanate | 3 (13) |
| Adjuvant thoracic surgery, n (%) | 6 (26) |
| Characteristics . | Linezolid in the Starter Therapy . |
|---|---|
| Subjects, n | 23 |
| Age (years, min-max) | 40 (15–71) |
| Gender (male) | 18 (78) |
| XDR-TB | 2 (8.7) |
| MDR-TB | 21 (91.3) |
| Type of disease | |
| Lung TB with positive sputum culture | 17 (74) |
| Lung TB with negative sputum cultureb | 4 (17.3) |
| Extrapulmonary TB with positive culture samples | 2 (8.7) |
| Course of disease | |
| Time to sputum conversion (days) | 39 (21–76.5) |
| Patients cured | 19 (82.6) |
| Patients in the continuation phase | 2 (8.7) |
| Patients defaulting | 2 (8.7) |
| Follow-up duration (months) | 16.3 (12–24.3) |
| Susceptibility tests result resistance (%) | |
| Ethionamide | 78 |
| Pyrazinamide | 65.2 |
| Fluoroquinolones | 22 |
| Cycloserine | 22 |
| PAS | 22 |
| Amikacin | 13 |
| Background regimen | |
| Fluoroquinolones | 21 (91) |
| Amikacin, streptomycin | 20 (87) |
| Cycloserine | 19 (83) |
| PAS | 17 (74) |
| Bedaquiline | 8 (34.7) |
| Ethionamide | 5 (21.7) |
| Imipenem + clavulanate | 3 (13) |
| Adjuvant thoracic surgery, n (%) | 6 (26) |
Abbreviations: MDR, multidrug-resistant; PAS, para-aminosalicylic acid; TB, tuberculosis; XDR, extensively drug-resistant.
a The data are presented as the numbers (%) or the medians with the interquartile ranges Q1–Q3 unless otherwise stated.
b Clinically diagnosed TB cases (close contacts of bacteriologically confirmed TB case) that received a full course of TB treatment.
DISCUSSION
Linezolid belongs to the group 5 drugs (unclear clinical efficacy) and is not currently recommended by the WHO in the starter regimen of MDR TB [1]. In our experience, the use of LNZ in the starter regimen of 23 MDR-TB patients (including 2 XDR TB) yielded sputum culture conversion rates and relapse-free outcome results that are consistent with those of previous larger studies that evaluated LNZ as a second-line combination in 39 and 45 patients, respectively [8, 13]. In addition, a single-center study that detailed the numerous therapeutic adaptations required for MDR-TB treatment optimization revealed that the tolerability of second-line drugs such as aminoglycosides and PAS did not exceed 3–6 months [18]. This finding emphasizes the need for alternative options.
When using a 1200 mg daily dose regimen, myelosuppression appears first within a delay of 2–8 weeks, in a dose-dependent manner [19]. It has been suggested that lower dosages (eg, 600 mg daily or 300 mg once or twice daily) might limit adverse events in patients on prolonged LNZ therapy for MDR TB [7]. When using lower dosages, the safety and tolerability concerns of prolonged LNZ therapy should focus on neurological disorders, especially peripheral neuropathies [8, 10, 18, 20, 21]. Although the reported incidences of neuropathy are similar (40%–50%), the onset of peripheral symptoms is variable. In our series, the median onset is 8 months, which contrasts with the delay of 2–4 months reported by Tang et al [20]. This difference may be related to the use of a 600 mg daily dose regimen from the start, whereas Tang et al [20] opted for an induction phase with a 1200 mg daily dose regimen over the first 4–6 weeks; this difference suggests a dose-dependent effect of LNZ. Thus, LNZ-induced neuropathy must be cautiously monitored with neurological follow ups, electromyograms, and ophthalmological evaluations upon the onset of symptoms, and alternative regimens without LNZ should be introduced when possible. In addition, the use of LNZ should be carefully evaluated before being implemented in patients with increased risk of toxicity, such as patients with diabetes.
Treatment monitoring via plasma concentration assays allowed the random detection of 2 nonadherent patients. The 7 other patients had median LNZ trough concentrations above 0.5 mg/L, which is considered to be the minimum inhibitory concentration (MIC)90 for MDR-TB strains. A Cmin of at least 1 mg/L should be targeted in order to both achieve anti-TB effect and to prevent resistance mutations occurrence. Our troughs are rather low because previous data reported similar Cmin 2.1 ± 1.3 mg/L values (range, 0.4–4.5 mg/L) in patients receiving 300 mg LNZ daily (half the dose received by our patients) [22]. However, LNZ concentrations achieved in the lung epithelial lining fluid (ELF), the most frequent active infection site, are known to be approximately 3–8 times greater than the serum concentrations [23]. Thus, the LNZ concentrations in the ELF are expected to be substantially greater than the MIC90 and the mutation prevention concentration that has previously been determined in vitro to be 1.2 mg/L [24].
An important issue is the LNZ appropriate daily dose when used as a starter drug. The 1200 mg daily dose regimen provides optimized LNZ tissue concentrations but is not acceptably tolerated for the treatment durations required for MDR TB. The 600 mg daily dose regimen may be a fairly good compromise between drug exposure and long-term tolerability, although, at some point, this dose was limited by the occurrence of neuropathy in nearly half of the patients. As previously reported by Lee et al [13], the 300 mg daily dose regimen provides pharmacokinetic profile sufficient to maintain serum concentrations above the MIC of most isolates. However, data are lacking to support this dose in settings such as high-burden MDR TB (multiple lung excavations) or extrapulmonary MDR TB or XDR TB. Finally, the arrival of promising new drugs, such as BDQ, that exhibit high activity against M tuberculosis bacilli indicates the need for further trials evaluating the most appropriate candidate drugs for the starter regimen of MDR TB.
We believe that in areas with high levels of second-line drug resistance (ie, high proportion of XDR among MDR-TB cases), LNZ should be used as part of the empirical starter regimen. This choice is based on the following: (1) the high efficacy of LNZ-containing regimens even in case of extensive drug resistance; (2) the low prevalence of LNZ resistance among MDR strains (<5% in France) (N.V., personal data); (3) the low in vitro mutation rate, which limits the risk of selection of resistant mutant strains [25]; and (4) the relatively late occurrence of peripheral neuropathy when using the 600 mg daily dose regimen.
CONCLUSIONS
In conclusion, we propose that LNZ should leave the group 5 drugs (antituberculous drugs with unclear clinical efficacy) according to the WHO classification and should be considered for integration with the group 4 drugs (proven bactericidal activity).
Acknowledgments
We acknowledge Christine Bernard, Jérôme Robert, and Vincent Jarlier from the French National Reference Center for Mycobacteria (Assistance Publique des Hôpitaux de Paris) and Alban Le Monnier and Najoua El-Helali from the Hôpital Saint Joseph for performing the linezolid dosages.
The Lyon TB Study Group: Ader F, Biron F, Boibieux A, Bouaziz A, Bouledrak K, Braun E, Catho G, Chidiac C, Chumbi-Flores W, Couraud S, Devouassoux G, Dumitrescu O, Ferry T, Freymond N, Gardes S, Gerbier-Colomban S, Gillet Y, Goutelle S, Grando J, Grima R, Hees L, Jubin V, Kiakouama-Maleka L, Lina G, Maury JM, Miailhes P, Perpoint T, Perrot Em., Perrot Emm., Reix P, Renaud-Baron AS, Ronin V, Senechal A, Souquet PJ, Thai Van H, Tronc F, Valour F, Vanhems P.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
O. D. and F. A. contributed equally to this work.





Comments