-
PDF
- Split View
-
Views
-
Cite
Cite
Mary Nantongo, David C Nguyen, Eunjeong Shin, Christopher Bethel, Magdalena A Taracila, Khalid M Dousa, Sebastian G Kurz, Liem Nguyen, Barry N Kreiswirth, Wilem Boom, Robert A Bonomo, P-788. Exploring β-Lactam Interactions with DacB1: Unraveling Optimal Therapies for Combating Drug-Resistant Mycobacterium tuberculosis, Open Forum Infectious Diseases, Volume 12, Issue Supplement_1, February 2025, ofae631.982, https://doi.org/10.1093/ofid/ofae631.982
Close - Share Icon Share
Abstract
Tab. 1: β-lactam and β-lactamase Inhibitor MICs for Mtb H37Rv, H37Ra and representative Clinical Isolates
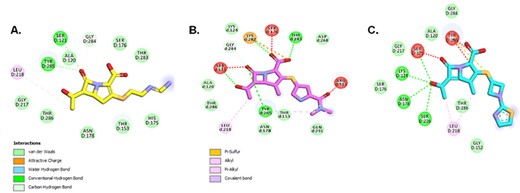
The 2D representation of Michaelis-Menten complexes of DacB1 and IPM (A.), MEM (B.), and TPB (C.) with the formation of initial interactions. The carbonyl group of all the carbapenems is positioned into the oxyanion hole fromed by the catalytic Ser121:N and by Tyr285 amide. the hydroxyethyl group on the β-lactam ring present on all carbapenes produce a hydrophobic interaction with Leu218 and helps the initial positioning od the carbonyl into the oxyanion hole of DacB1. The C1 methyl group in both MEM and TBP, with additional hydrophobic interactions (B. and C.), contribute to the slower acyl-enzyme formation fro those compounds.
MICs for IPM, MEM, and CRO ranged from 0.5 → >64, 1 → 32, and 0.25 → 16 µg/mL respectively (Tab. 1). Combining IPM or MEM with CRO further lowered MICs up to 32-fold (0.13 → 4 µg/mL), all within clinically achievable concentrations. Acyl-enzyme adducts of DacB1 (MW = 34414 Da) with carbapenems were captured; but not with AMX, CRO, or BLIs even after 120 min incubation. DacB1-IPM complexes were detected with 5 min incubation (Δ+299 Da) while at 15 min and 30 min for -MEM (Δ+383 Da) and -TBP (Δ+384 Da) complexes respectively.
IPM preferentially binds to DacB1 compared to MEM and TBP, and BLs, shedding light on crucial SAR for drug development. We postulate that the carbonyl group present in all carbapenems assists with positioning into the oxyanion hole of DacB1. However, the C1 methyl in MEM and TBP with their additional hydrophobic interactions contribute to steric hindrance and slower acyl-enzyme formation (Fig. 1). This study advances our understanding of the molecular mechanisms governing DacB1 interactions with BLs, offering insights into how carbapenems may inhibit another important BL target in Mtb peptidoglycan synthesis.
All Authors: No reported disclosures
Author notes
Study Group: Yes
Session: 67. Tuberculosis and other Mycobacterial Infections
Thursday, October 17, 2024: 12:15 PM
- amoxicillin
- antibiotics
- ceftriaxone
- basic life support
- carbapenem
- amides
- bloom syndrome
- catalysis
- disclosure
- drug delivery systems
- imipenem
- lactams
- mycobacterium tuberculosis
- peptidoglycan
- spectrometry, mass, electrospray ionization
- sulbactam
- world health
- carboxypeptidase
- enzymes
- meropenem
- drug development
- minimum inhibitory concentration measurement
- penicillin-binding proteins
- bare lymphocyte syndrome
- biosynthesis
- clavulanate
- redundancy
- hydrophobic interactions
- binding (molecular function)
- complex
- malnutrition-inflammation-cachexia syndrome






Comments