-
PDF
- Split View
-
Views
-
Cite
Cite
Juergen Prattes, Daniele R Giacobbe, Cristina Marelli, Alessio Signori, Silvia Dettori, Greta Cattardico, Jonas Frost, Florian Reizine, Matteo Bassetti, Jean-Pierre Gangneux, Martin Hoenigl, P-1032. Posaconazole for Prevention of COVID-19 Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients: the POSACOVID Study, Open Forum Infectious Diseases, Volume 12, Issue Supplement_1, February 2025, ofae631.1222, https://doi.org/10.1093/ofid/ofae631.1222
Close - Share Icon Share
Abstract
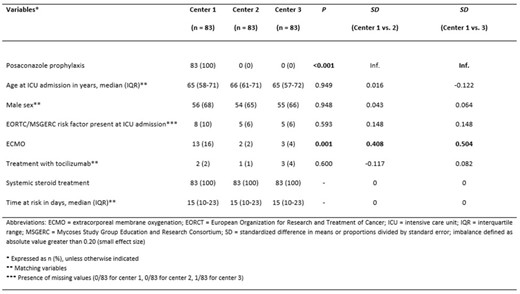
Demographic and clinical characteristics of patients after matching
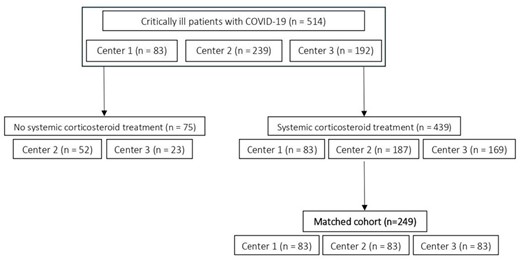
Matched cohorts after 1:1 and 1:1:1 matching. Both composed of 249 patients, although some different controls could have been selected.
Eighty-three consecutive patients receiving POSA were identified at center 1 and matched to 166 controls (Table 1). The pre-matching CAPA incidence rates were 1.69 CAPA/1000 ICU days in center 1, 1.42 CAPA/1000 ICU days in center 2 and 9.53 CAPA/1000 ICU days in center 3. The CAPA incidence rate ratio before matching was 2.38 (95% CI 0.87–9.08; p = 0.072) for those not receiving prophylaxis versus those who did. In post-matching multivariable logistic regression, presence of an EORTC/MSG risk factor at ICU admission (OR 4.35) and Center (Center 3 versus 1: OR 6.07; 95% CI 1.76 – 20.91; p = 0.004; Center 2 versus 1: not significant) were associated with CAPA development.
The impact of POSA prophylaxis depends on the baseline CAPA incidence rate, which varies widely between centers and underlying individual patient risk factors. Future trials should therefore investigate targeted antifungal prophylaxis in COVID-19 patients.
Juergen Prattes, MD, PhD, AbbVie Inc.: Stocks/Bonds (Private Company)|Gilead: Honoraria|MSD: Grant/Research Support|Novo Nordisk: Stocks/Bonds (Private Company)|Pfizer: Grant/Research Support|Pfizer: Honoraria Daniele R. Giacobbe, M.D., bioMerieux: Grant/Research Support|Gilead Italia: Grant/Research Support|Menarini: Honoraria|Pfizer: Grant/Research Support|Pfizer: Honoraria|Shionogi: Grant/Research Support|Tillotts Pharma: Advisor/Consultant Matteo Bassetti, PhD, Angelini: Advisor/Consultant|Angelini: Honoraria|Astellas: Advisor/Consultant|Astellas: Honoraria|bioMerieux: Advisor/Consultant|bioMerieux: Honoraria|Cidara: Advisor/Consultant|Cidara: Honoraria|Gilead: Advisor/Consultant|Gilead: Honoraria|Menarini: Advisor/Consultant|Menarini: Honoraria|MSD: Advisor/Consultant|MSD: Honoraria|Nabriva: Advisor/Consultant|Nabriva: Honoraria|Pfizer: Advisor/Consultant|Pfizer: Honoraria|Tetraphase: Advisor/Consultant|Tetraphase: Honoraria Jean-Pierre Gangneux, Prof., Gilead: Honoraria|MundiPharma: Advisor/Consultant|MundiPharma: Honoraria|Pfizer: Honoraria|Shionogi: Honoraria Martin Hoenigl, MD, Aicuris: Advisor/Consultant|Astra Zeneca: Honoraria|Gilead: Grant/Research Support|Gilead: Honoraria|IMMY: Grant/Research Support|Melinta: Grant/Research Support|Melinta: Honoraria|MSD: Grant/Research Support|Mundipharma: Grant/Research Support|Mundipharma: Honoraria|Pfizer: Grant/Research Support|Pulmocide: Advisor/Consultant|Pulmocide: Grant/Research Support|Scynexis: Advisor/Consultant|Scynexis: Grant/Research Support|Shionogi: Honoraria
Author notes
Study Group:
Session: 137. Medical Mycology
Friday, October 18, 2024: 12:15 PM
- adrenal corticosteroids
- bronchopulmonary aspergillosis
- respiratory failure, acute
- glucocorticoids
- consultants
- demography
- disclosure
- intensive care unit
- vaccination
- mineralocorticoids
- mortality
- posaconazole
- mechanical ventilation
- antifungal prophylaxis
- european organization for research and treatment of cancer
- tocilizumab
- standard of care
- prevention
- covid-19





Comments