-
PDF
- Split View
-
Views
-
Cite
Cite
Rebecca Mathews, Claudia Shen, Michael W Traeger, Helen M O’Brien, Christine Roder, Margaret E Hellard, Joseph S Doyle, Enhancing Hepatitis C Virus Testing, Linkage to Care, and Treatment Commencement in Hospitals: A Systematic Review and Meta-analysis, Open Forum Infectious Diseases, Volume 12, Issue 2, February 2025, ofaf056, https://doi.org/10.1093/ofid/ofaf056
Close - Share Icon Share
Abstract
The hospital-led interventions yielding the best hepatitis C virus (HCV) testing and treatment uptake are poorly understood.
We searched Medline, Embase, and Cochrane databases for studies assessing outcomes of hospital-led interventions for HCV antibody or RNA testing uptake, linkage to care, or direct-acting antiviral commencement compared with usual care, a historical comparator, or control group. We systematically reviewed hospital-led interventions delivered in inpatient units, outpatient clinics, or emergency departments. Random-effects meta-analysis estimated pooled odds ratios [pORs] measuring associations between interventions and outcomes. Subgroup analyses explored outcomes by intervention type.
A total of 7872 abstracts were screened with 23 studies included. Twelve studies (222 868 participants) reported antibody testing uptake, 5 (n = 4987) reported RNA testing uptake, 7 (n = 3185) reported linkage to care, and 4 (n = 1344) reported treatment commencement. Hospital-led interventions were associated with increased antibody testing uptake (pOR, 5.83 [95% confidence interval {CI}, 2.49–13.61]; I2 = 99.9%), RNA testing uptake (pOR, 10.65 [95% CI, 1.70–66.50]; I2 = 97.9%), and linkage to care (pOR, 1.75 [95% CI, 1.10–2.79]; I2 = 79.9%) when data were pooled and assessed against comparators. Automated opt-out testing (5 studies: pOR, 16.13 [95% CI, 3.35–77.66]), reflex RNA testing (4 studies: pOR, 25.04 [95% CI, 3.63–172.7]), and care coordination and financial incentives (4 studies: pOR, 2.73 [95% CI, 1.85–4.03]) showed the greatest increases in antibody and RNA testing uptake and linkage to care, respectively. No intervention increased uptake at all care cascade steps.
Automated antibody and reflex RNA testing increase HCV testing uptake in hospitals but have limited impact on linkage to treatment. Other interventions promoting linkage must be explored.
Global hepatitis C virus (HCV) elimination requires more people living with hepatitis C to be diagnosed, progress through the care cascade, and complete curative direct-acting antiviral (DAA) treatment [1, 2]. Hospitals have opportunities to engage patients at all steps of the hepatitis C care cascade. In many countries, people living with or at increased risk of HCV infection are overrepresented in emergency department (ED) presentations [3, 4] and all-cause hospitalizations [5]. Systematic reviews show higher HCV seroprevalence among ED attendees than in the general population [6–8]. Frequent, prolonged hospitalizations are common among people who inject drugs, who are at increased risk of HCV infection and therefore present opportunities for diagnosis, linkage to care, and treatment commencement [9].
Despite the burden of infection, hospitals are missing opportunities to diagnose and treat HCV in patients. Studies exploring this suggest that many people engaged in hospital care do not receive HCV antibody testing despite having identified risk factors [10, 11]. Among those testing antibody positive, many fail to receive RNA testing to confirm the diagnosis [10]. Patients diagnosed with HCV in hospital are seldom successfully referred to attend appointments with health professionals who can treat it, partly because hepatitis is rarely the primary cause for their presentation [12]. DAA commencement while hospitalized is also uncommon [10].
Simple, effective, and efficient interventions are needed to maximize HCV testing, diagnosis, and treatment in hospitals. Currently, the hospital-led interventions yielding the best testing and treatment uptake are poorly understood. Despite reviews of interventions in community settings [13], no systematic reviews have exclusively assessed the effects of hospital-led interventions on uptake at different steps of the hepatitis C care cascade. This review explores hospital-led interventions to compare the impact of different intervention types on HCV testing, linkage to care, and treatment commencement.
METHODS
Search Strategy and Study Selection
The systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. The review protocol was registered prospectively (PROSPERO registration number CRD42019146569).
Scientific literature was searched using Medline, Embase, and the Cochrane Database of Systematic Reviews. Embase included conference abstracts. Multiple search terms related to HCV, interventions, testing, linkage or treatment outcomes, and hospital settings were used (Supplementary Figure 1). Results were limited to studies in English that were published and conducted between 1 January 2014 and 21 June 2023 to align with the period when DAAs were available outside clinical trials. Reference lists of retrieved studies were hand-searched to identify additional studies.
Studies were eligible for inclusion in the systematic review and meta-analysis if they:
Recruited adults engaged in hospital care at risk of or diagnosed with HCV. Studies with populations not receiving hospital care at the time of the intervention, or only comprised of pregnant women, blood donors, transplant, transfusion, or dialysis recipients, were excluded.
Assessed the association between a hospital-led intervention and 1 or more of the following outcomes compared with standard care, a historical comparator, or control group:
HCV antibody testing uptake;
HCV RNA testing uptake;
Linkage to care for RNA-confirmed HCV cases; or
HCV DAA treatment commencement.
Reported randomized controlled trials (RCTs) or nonrandomized studies except for dissertations and study protocols.
Hospital-led interventions included those delivered to patients in inpatient units and outpatient clinics encompassing all medical specialties including psychiatry and in EDs. In outpatient clinics, specialist medical practitioners provide care to patients in the hospital without the patient being admitted. Studies in primary care, sexual health, drug treatment, or community health services were excluded.
Using COVIDence [15], 2 reviewers (R. M. and C. S.) independently screened abstracts and full text articles for eligibility, then extracted data from eligible studies. Authors were contacted for further information required for analysis and studies were excluded if this information could not be obtained. Conflicts in eligibility screening or data extraction were resolved by consensus or a third reviewer (J. S. D.).
Data Collection
Extracted data included patient population (eg, all patients, birth cohort 1945–1965, injecting drug use history, human immunodeficiency virus (HIV) coinfected or multiple HCV risk factors), hospital setting (inpatient unit, ED or outpatient clinic), outcomes, and intervention and comparator arm descriptions. In studies with multiple component interventions, a primary intervention component was assigned to each outcome reported and then categorized into intervention types as defined in Table 1.
| Intervention Type . | Definition . |
|---|---|
| System-level interventions | |
| Electronic health record interventions | |
| Automated opt-out screening | Electronic health record modifications that identify patients eligible for HCV testing and automate the addition of HCV antibody testing to the patients' blood pathology order forms. The clinician (and patient) must opt out of testing. |
| Medical chart reminders | Alerts or reminders within electronic or physical medical records of patient's eligibility for HCV testing and recommendation to offer testing. The clinician (and patient) must opt in to testing. |
| Testing-level interventions | |
| Universal screening | Testing all patients for HCV regardless of risk factors. |
| Bundled testing | Requiring patients to be tested for HCV at the same time as being screened for other blood-borne viruses (eg, HIV). |
| Reflex RNA testing | Laboratory-based testing strategy where blood samples positive for HCV antibodies are automatically tested for HCV RNA. |
| Health professional–level interventions | |
| Clinician education | Educational sessions and or resources provided to hospital health professionals on subjects including HCV screening criteria and processes, referral pathways, and treatment options. |
| Patient-level interventions | |
| Care coordination | An intervention delivered by hospital staff (eg, nursing, allied health, or clerical staff) to help newly diagnosed patients with 1 or more aspects of navigating linkage to care and treatment commencement for HCV. Includes at least 1 of the following: referring patients to treatment, helping patients schedule and or attend appointments for treatment, counseling and educating patients regarding HCV testing results. |
| Financial incentives for patients | Hospital patients receive cash, fuel voucher, or gift voucher during their attendance at hospital appointments including for HCV care or commencement of treatment. |
| Peer support | Structured peer mentor support program where mentors previously successfully treated for HCV regularly meet the “mentees” who are newly diagnosed with HCV to discuss and understand potential barriers to treatment success. Program comprised an initial face-to-face meeting with regular ongoing phone communication including check-in points before, during, and after treatment. |
| Motivational interviewing | A nurse-administered intervention focused on identifying barriers to commencing treatment among HCV-diagnosed patients and building behavioral and motivational skills to address these barriers. |
| Intervention Type . | Definition . |
|---|---|
| System-level interventions | |
| Electronic health record interventions | |
| Automated opt-out screening | Electronic health record modifications that identify patients eligible for HCV testing and automate the addition of HCV antibody testing to the patients' blood pathology order forms. The clinician (and patient) must opt out of testing. |
| Medical chart reminders | Alerts or reminders within electronic or physical medical records of patient's eligibility for HCV testing and recommendation to offer testing. The clinician (and patient) must opt in to testing. |
| Testing-level interventions | |
| Universal screening | Testing all patients for HCV regardless of risk factors. |
| Bundled testing | Requiring patients to be tested for HCV at the same time as being screened for other blood-borne viruses (eg, HIV). |
| Reflex RNA testing | Laboratory-based testing strategy where blood samples positive for HCV antibodies are automatically tested for HCV RNA. |
| Health professional–level interventions | |
| Clinician education | Educational sessions and or resources provided to hospital health professionals on subjects including HCV screening criteria and processes, referral pathways, and treatment options. |
| Patient-level interventions | |
| Care coordination | An intervention delivered by hospital staff (eg, nursing, allied health, or clerical staff) to help newly diagnosed patients with 1 or more aspects of navigating linkage to care and treatment commencement for HCV. Includes at least 1 of the following: referring patients to treatment, helping patients schedule and or attend appointments for treatment, counseling and educating patients regarding HCV testing results. |
| Financial incentives for patients | Hospital patients receive cash, fuel voucher, or gift voucher during their attendance at hospital appointments including for HCV care or commencement of treatment. |
| Peer support | Structured peer mentor support program where mentors previously successfully treated for HCV regularly meet the “mentees” who are newly diagnosed with HCV to discuss and understand potential barriers to treatment success. Program comprised an initial face-to-face meeting with regular ongoing phone communication including check-in points before, during, and after treatment. |
| Motivational interviewing | A nurse-administered intervention focused on identifying barriers to commencing treatment among HCV-diagnosed patients and building behavioral and motivational skills to address these barriers. |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus.
| Intervention Type . | Definition . |
|---|---|
| System-level interventions | |
| Electronic health record interventions | |
| Automated opt-out screening | Electronic health record modifications that identify patients eligible for HCV testing and automate the addition of HCV antibody testing to the patients' blood pathology order forms. The clinician (and patient) must opt out of testing. |
| Medical chart reminders | Alerts or reminders within electronic or physical medical records of patient's eligibility for HCV testing and recommendation to offer testing. The clinician (and patient) must opt in to testing. |
| Testing-level interventions | |
| Universal screening | Testing all patients for HCV regardless of risk factors. |
| Bundled testing | Requiring patients to be tested for HCV at the same time as being screened for other blood-borne viruses (eg, HIV). |
| Reflex RNA testing | Laboratory-based testing strategy where blood samples positive for HCV antibodies are automatically tested for HCV RNA. |
| Health professional–level interventions | |
| Clinician education | Educational sessions and or resources provided to hospital health professionals on subjects including HCV screening criteria and processes, referral pathways, and treatment options. |
| Patient-level interventions | |
| Care coordination | An intervention delivered by hospital staff (eg, nursing, allied health, or clerical staff) to help newly diagnosed patients with 1 or more aspects of navigating linkage to care and treatment commencement for HCV. Includes at least 1 of the following: referring patients to treatment, helping patients schedule and or attend appointments for treatment, counseling and educating patients regarding HCV testing results. |
| Financial incentives for patients | Hospital patients receive cash, fuel voucher, or gift voucher during their attendance at hospital appointments including for HCV care or commencement of treatment. |
| Peer support | Structured peer mentor support program where mentors previously successfully treated for HCV regularly meet the “mentees” who are newly diagnosed with HCV to discuss and understand potential barriers to treatment success. Program comprised an initial face-to-face meeting with regular ongoing phone communication including check-in points before, during, and after treatment. |
| Motivational interviewing | A nurse-administered intervention focused on identifying barriers to commencing treatment among HCV-diagnosed patients and building behavioral and motivational skills to address these barriers. |
| Intervention Type . | Definition . |
|---|---|
| System-level interventions | |
| Electronic health record interventions | |
| Automated opt-out screening | Electronic health record modifications that identify patients eligible for HCV testing and automate the addition of HCV antibody testing to the patients' blood pathology order forms. The clinician (and patient) must opt out of testing. |
| Medical chart reminders | Alerts or reminders within electronic or physical medical records of patient's eligibility for HCV testing and recommendation to offer testing. The clinician (and patient) must opt in to testing. |
| Testing-level interventions | |
| Universal screening | Testing all patients for HCV regardless of risk factors. |
| Bundled testing | Requiring patients to be tested for HCV at the same time as being screened for other blood-borne viruses (eg, HIV). |
| Reflex RNA testing | Laboratory-based testing strategy where blood samples positive for HCV antibodies are automatically tested for HCV RNA. |
| Health professional–level interventions | |
| Clinician education | Educational sessions and or resources provided to hospital health professionals on subjects including HCV screening criteria and processes, referral pathways, and treatment options. |
| Patient-level interventions | |
| Care coordination | An intervention delivered by hospital staff (eg, nursing, allied health, or clerical staff) to help newly diagnosed patients with 1 or more aspects of navigating linkage to care and treatment commencement for HCV. Includes at least 1 of the following: referring patients to treatment, helping patients schedule and or attend appointments for treatment, counseling and educating patients regarding HCV testing results. |
| Financial incentives for patients | Hospital patients receive cash, fuel voucher, or gift voucher during their attendance at hospital appointments including for HCV care or commencement of treatment. |
| Peer support | Structured peer mentor support program where mentors previously successfully treated for HCV regularly meet the “mentees” who are newly diagnosed with HCV to discuss and understand potential barriers to treatment success. Program comprised an initial face-to-face meeting with regular ongoing phone communication including check-in points before, during, and after treatment. |
| Motivational interviewing | A nurse-administered intervention focused on identifying barriers to commencing treatment among HCV-diagnosed patients and building behavioral and motivational skills to address these barriers. |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Defined Outcomes
HCV antibody testing uptake was defined as the proportion of people who received antibody testing out of the number eligible. In most studies, patients already having blood drawn were eligible for testing if they had no existing HCV diagnosis or positive antibody test in the prior year. Some studies only tested patients with risk factor(s) for HCV (eg, birth cohort, country of birth, or injecting drug use).
HCV RNA testing uptake was defined as the proportion of people who received RNA testing out of the number eligible. Eligibility criteria included a positive HCV antibody test and no recent positive RNA test or chronic HCV diagnosis in all studies except for one evaluating point of care RNA screening uptake where all patients were eligible for testing [16].
Linkage to care was defined as the proportion of people with an RNA-confirmed HCV diagnosis who attended an appointment with a DAA treatment prescriber within the study period.
Treatment commencement was defined as the proportion of people who received a DAA prescription out of the number eligible for treatment. In most studies, treatment eligibility criteria required a positive RNA test and no treatment within the prior year.
Sustained virological response (SVR) was defined as the proportion of people commencing DAAs who had undetectable HCV RNA 12 weeks or more after treatment completion.
Data Analysis
Studies were included in meta-analyses for specific outcomes if numerator and denominator data for the outcome were reported in both the intervention and comparator arms.
Primary Analyses
All analyses were conducted using Stata software (version 17.0). For each study, we calculated odds ratios (ORs) and corresponding 95% confidence intervals (CIs) from the reported numbers of eligible participants with and without each outcome in intervention and comparator arms. These ORs represented the association between receiving the intervention and the respective outcome in the corresponding study. We calculated separate ORs for each intervention in studies that assessed outcomes from 2 different interventions. Random-effects meta-analyses estimated pooled ORs of the association between study intervention and each outcome with SVR explored as a secondary outcome for treatment commencement studies. Heterogeneity across studies was measured using the I2 statistic.
Subgroup Analyses
For each outcome, primary subgroup analyses stratifying pooled OR by intervention type were conducted if >1 study was identified exploring the association between an intervention type and the outcome.
Secondary subgroup analyses stratifying pooled OR by publication year, country, setting, and patient population were also conducted for each outcome.
Sensitivity Analyses
We conducted sensitivity analyses for studies assessing antibody testing uptake exploring the impact of omitting the 2 studies with OR exceeding 15 on the overall association between intervention and outcome. Secondary subgroup analyses stratifying pooled OR by publication year, country, setting, and patient population were also repeated for studies assessing antibody testing uptake after omitting the 2 studies with OR exceeding 15.
Risk of Bias Analyses
Two reviewers (R. M. and C. S.) independently assessed risk of bias for each study using the revised Cochrane Collaboration Risk of Bias tool (RoB2) [17] for randomized studies, and the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool [18] for nonrandomized studies. Conflicts were resolved by consensus or a third reviewer (J. S. D.). Funnel plots assessed for publication bias.
RESULTS
Summary of Included Studies
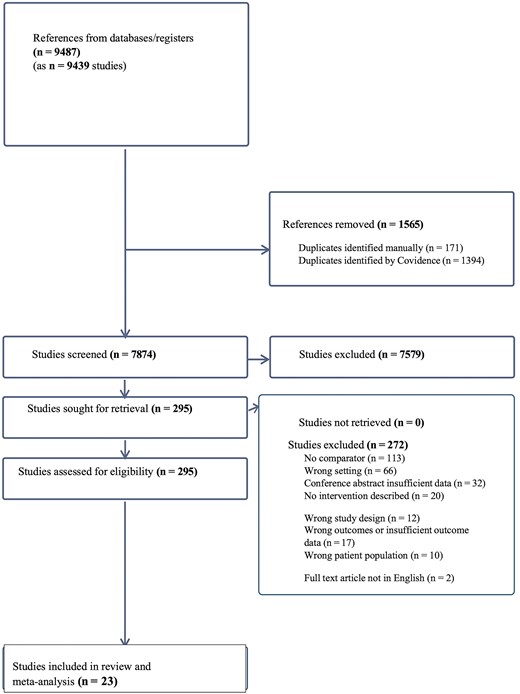
Overall, 9487 records were identified with 7872 unique abstracts screened after removing duplicates, and 295 full text studies assessed for eligibility (Figure 1). Twenty-three studies met criteria for inclusion in the review (Table 2).
| First Author (Publication Year) . | Country . | Study Design . | Setting . | Patient Population . | Outcome(s) . | Intervention(s) . | Comparator(s) . | Total Participants, No. . |
|---|---|---|---|---|---|---|---|---|
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Bundled testingb | HCV testing alone | 9317 (antibody testing) 1008 (linkage) |
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Universal screeningb | Targeted HCV and HIV testing | 21 027 (antibody testing) 1071 (linkage) |
| Fitch (2017) [20] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | No alert or automation | 9933 |
| Ford (2021) [21] | US | Nonrandomized | ED | All | RNA test uptake | Reflex RNA testingb | No reflex RNA testing | 1391 |
| Fortunato (2019)a [22] | US | Nonrandomized | IP | All | Linkage to care | Care coordination (in person) | Phone-based care coordination | 146 |
| Genova (2022)a [23] | US | Nonrandomized | OP | All | Antibody test uptake | Medical chart reminders | No medical chart reminders | 5638 |
| Geretti (2018) [16] | UK | Nonrandomized | ED | All | RNA test uptake | Bundled testing (POC) | HCV testing alone (POC) | 814 |
| Hsu (2020)a [24] | US | Nonrandomized | ED | All | Linkage to care | Universal screening | Targeted screening | 41 |
| Huang (2021) [25] | Taiwan | Nonrandomized | OP | All | RNA test uptake, treatment commencement | Reflex RNA testing, care coordination | No reflex RNA testing, No care coordination | 1521 (RNA testing) 1045 (treatment) |
| Lee (2020) [26] | US | Nonrandomized | OP | All | Linkage to care | Financial incentives for patients | No financial incentives | 243 |
| Manteuffel (2022) [27] | US | Nonrandomized | ED | Multiple HCV risk factors | RNA test uptake, linkage to care | Reflex RNA testingb | No reflex RNA testing | 748 (RNA testing) 315 (linkage) |
| Marks (2021) [28] | US | Nonrandomized | IP | Injecting drug use history | Antibody test uptake | Medical chart reminders | No medical chart reminders | 394 |
| Mehta (2022) [29] | US | RCT | IP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | Medical chart reminder, no automated testing | 7634 |
| Northrup (2022) [30] | US | Nonrandomized | IP | Multiple HCV risk factors | Antibody test uptake | Health provider education | No health provider education | 190 |
| Oji (2023) [31] | US | Nonrandomized | IP | All | Antibody test uptake | Health provider education | No health provider education | 173 |
| Schechter-Perkins (2018) [32] | US | Nonrandomized | ED | All | RNA test uptake, linkage to care | Reflex RNA testingb, care coordination | Opt-in reflex RNA testing, No care coordination | 513 (RNA testing) 295 (linkage) |
| Smout (2022) [33] | UK | Nonrandomized | ED | All | Antibody test uptake | Automated opt-out screeningb | No automated testing | 50 131 |
| Starbird (2020) [34] | US | RCT | OP | HIV coinfected | Linkage to care, treatment commencement, SVR | Care coordination | One appointment reminder and HCV patient information brochure | 66 (linkage), 24 (treatment commencement), 12 (SVR; 4 = intervention; 8 = comparatora) |
| Wang (2018)a [35] | US | Nonrandomized | ED | Multiple HCV risk factors | Antibody test uptake | Automated opt-out screeningb | No automated testing | 14 451 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Financial incentives for patients | Nurse-led multidisciplinary case management | 90 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Peer support | Nurse-led multidisciplinary case management | 90 |
| Wasti (2019) [37] | US | Nonrandomized | ED | All | Antibody test uptake | Universal screeningb | Targeted screening | 7841 |
| Weiss (2017) [38] | US | RCT | OP | HIV coinfected | Treatment commencement | Motivational interviewing | Attention control | 53 |
| White (2018) [39] | US | Nonrandomized | ED | All | Antibody testing uptake | Automated opt-out screeningb | Nurse-ordered screening | 40 862 |
| Yeboah- Korang (2018) [40] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody testing uptake | Medical chart reminders | No medical chart reminders | 55 277 |
| First Author (Publication Year) . | Country . | Study Design . | Setting . | Patient Population . | Outcome(s) . | Intervention(s) . | Comparator(s) . | Total Participants, No. . |
|---|---|---|---|---|---|---|---|---|
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Bundled testingb | HCV testing alone | 9317 (antibody testing) 1008 (linkage) |
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Universal screeningb | Targeted HCV and HIV testing | 21 027 (antibody testing) 1071 (linkage) |
| Fitch (2017) [20] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | No alert or automation | 9933 |
| Ford (2021) [21] | US | Nonrandomized | ED | All | RNA test uptake | Reflex RNA testingb | No reflex RNA testing | 1391 |
| Fortunato (2019)a [22] | US | Nonrandomized | IP | All | Linkage to care | Care coordination (in person) | Phone-based care coordination | 146 |
| Genova (2022)a [23] | US | Nonrandomized | OP | All | Antibody test uptake | Medical chart reminders | No medical chart reminders | 5638 |
| Geretti (2018) [16] | UK | Nonrandomized | ED | All | RNA test uptake | Bundled testing (POC) | HCV testing alone (POC) | 814 |
| Hsu (2020)a [24] | US | Nonrandomized | ED | All | Linkage to care | Universal screening | Targeted screening | 41 |
| Huang (2021) [25] | Taiwan | Nonrandomized | OP | All | RNA test uptake, treatment commencement | Reflex RNA testing, care coordination | No reflex RNA testing, No care coordination | 1521 (RNA testing) 1045 (treatment) |
| Lee (2020) [26] | US | Nonrandomized | OP | All | Linkage to care | Financial incentives for patients | No financial incentives | 243 |
| Manteuffel (2022) [27] | US | Nonrandomized | ED | Multiple HCV risk factors | RNA test uptake, linkage to care | Reflex RNA testingb | No reflex RNA testing | 748 (RNA testing) 315 (linkage) |
| Marks (2021) [28] | US | Nonrandomized | IP | Injecting drug use history | Antibody test uptake | Medical chart reminders | No medical chart reminders | 394 |
| Mehta (2022) [29] | US | RCT | IP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | Medical chart reminder, no automated testing | 7634 |
| Northrup (2022) [30] | US | Nonrandomized | IP | Multiple HCV risk factors | Antibody test uptake | Health provider education | No health provider education | 190 |
| Oji (2023) [31] | US | Nonrandomized | IP | All | Antibody test uptake | Health provider education | No health provider education | 173 |
| Schechter-Perkins (2018) [32] | US | Nonrandomized | ED | All | RNA test uptake, linkage to care | Reflex RNA testingb, care coordination | Opt-in reflex RNA testing, No care coordination | 513 (RNA testing) 295 (linkage) |
| Smout (2022) [33] | UK | Nonrandomized | ED | All | Antibody test uptake | Automated opt-out screeningb | No automated testing | 50 131 |
| Starbird (2020) [34] | US | RCT | OP | HIV coinfected | Linkage to care, treatment commencement, SVR | Care coordination | One appointment reminder and HCV patient information brochure | 66 (linkage), 24 (treatment commencement), 12 (SVR; 4 = intervention; 8 = comparatora) |
| Wang (2018)a [35] | US | Nonrandomized | ED | Multiple HCV risk factors | Antibody test uptake | Automated opt-out screeningb | No automated testing | 14 451 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Financial incentives for patients | Nurse-led multidisciplinary case management | 90 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Peer support | Nurse-led multidisciplinary case management | 90 |
| Wasti (2019) [37] | US | Nonrandomized | ED | All | Antibody test uptake | Universal screeningb | Targeted screening | 7841 |
| Weiss (2017) [38] | US | RCT | OP | HIV coinfected | Treatment commencement | Motivational interviewing | Attention control | 53 |
| White (2018) [39] | US | Nonrandomized | ED | All | Antibody testing uptake | Automated opt-out screeningb | Nurse-ordered screening | 40 862 |
| Yeboah- Korang (2018) [40] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody testing uptake | Medical chart reminders | No medical chart reminders | 55 277 |
Abbreviations: ED, emergency department; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IP, inpatient unit; OP, outpatient clinic; POC, point of care; RCT, randomized controlled trial; SVR, sustained virological response; UK, United Kingdom; US, United States.
aConference abstract.
bTesting delivered to patients already undergoing venipuncture as part of care.
| First Author (Publication Year) . | Country . | Study Design . | Setting . | Patient Population . | Outcome(s) . | Intervention(s) . | Comparator(s) . | Total Participants, No. . |
|---|---|---|---|---|---|---|---|---|
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Bundled testingb | HCV testing alone | 9317 (antibody testing) 1008 (linkage) |
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Universal screeningb | Targeted HCV and HIV testing | 21 027 (antibody testing) 1071 (linkage) |
| Fitch (2017) [20] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | No alert or automation | 9933 |
| Ford (2021) [21] | US | Nonrandomized | ED | All | RNA test uptake | Reflex RNA testingb | No reflex RNA testing | 1391 |
| Fortunato (2019)a [22] | US | Nonrandomized | IP | All | Linkage to care | Care coordination (in person) | Phone-based care coordination | 146 |
| Genova (2022)a [23] | US | Nonrandomized | OP | All | Antibody test uptake | Medical chart reminders | No medical chart reminders | 5638 |
| Geretti (2018) [16] | UK | Nonrandomized | ED | All | RNA test uptake | Bundled testing (POC) | HCV testing alone (POC) | 814 |
| Hsu (2020)a [24] | US | Nonrandomized | ED | All | Linkage to care | Universal screening | Targeted screening | 41 |
| Huang (2021) [25] | Taiwan | Nonrandomized | OP | All | RNA test uptake, treatment commencement | Reflex RNA testing, care coordination | No reflex RNA testing, No care coordination | 1521 (RNA testing) 1045 (treatment) |
| Lee (2020) [26] | US | Nonrandomized | OP | All | Linkage to care | Financial incentives for patients | No financial incentives | 243 |
| Manteuffel (2022) [27] | US | Nonrandomized | ED | Multiple HCV risk factors | RNA test uptake, linkage to care | Reflex RNA testingb | No reflex RNA testing | 748 (RNA testing) 315 (linkage) |
| Marks (2021) [28] | US | Nonrandomized | IP | Injecting drug use history | Antibody test uptake | Medical chart reminders | No medical chart reminders | 394 |
| Mehta (2022) [29] | US | RCT | IP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | Medical chart reminder, no automated testing | 7634 |
| Northrup (2022) [30] | US | Nonrandomized | IP | Multiple HCV risk factors | Antibody test uptake | Health provider education | No health provider education | 190 |
| Oji (2023) [31] | US | Nonrandomized | IP | All | Antibody test uptake | Health provider education | No health provider education | 173 |
| Schechter-Perkins (2018) [32] | US | Nonrandomized | ED | All | RNA test uptake, linkage to care | Reflex RNA testingb, care coordination | Opt-in reflex RNA testing, No care coordination | 513 (RNA testing) 295 (linkage) |
| Smout (2022) [33] | UK | Nonrandomized | ED | All | Antibody test uptake | Automated opt-out screeningb | No automated testing | 50 131 |
| Starbird (2020) [34] | US | RCT | OP | HIV coinfected | Linkage to care, treatment commencement, SVR | Care coordination | One appointment reminder and HCV patient information brochure | 66 (linkage), 24 (treatment commencement), 12 (SVR; 4 = intervention; 8 = comparatora) |
| Wang (2018)a [35] | US | Nonrandomized | ED | Multiple HCV risk factors | Antibody test uptake | Automated opt-out screeningb | No automated testing | 14 451 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Financial incentives for patients | Nurse-led multidisciplinary case management | 90 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Peer support | Nurse-led multidisciplinary case management | 90 |
| Wasti (2019) [37] | US | Nonrandomized | ED | All | Antibody test uptake | Universal screeningb | Targeted screening | 7841 |
| Weiss (2017) [38] | US | RCT | OP | HIV coinfected | Treatment commencement | Motivational interviewing | Attention control | 53 |
| White (2018) [39] | US | Nonrandomized | ED | All | Antibody testing uptake | Automated opt-out screeningb | Nurse-ordered screening | 40 862 |
| Yeboah- Korang (2018) [40] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody testing uptake | Medical chart reminders | No medical chart reminders | 55 277 |
| First Author (Publication Year) . | Country . | Study Design . | Setting . | Patient Population . | Outcome(s) . | Intervention(s) . | Comparator(s) . | Total Participants, No. . |
|---|---|---|---|---|---|---|---|---|
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Bundled testingb | HCV testing alone | 9317 (antibody testing) 1008 (linkage) |
| Cave (2022) [19] | US | Nonrandomized | ED | All | Antibody test uptake, linkage to care | Universal screeningb | Targeted HCV and HIV testing | 21 027 (antibody testing) 1071 (linkage) |
| Fitch (2017) [20] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | No alert or automation | 9933 |
| Ford (2021) [21] | US | Nonrandomized | ED | All | RNA test uptake | Reflex RNA testingb | No reflex RNA testing | 1391 |
| Fortunato (2019)a [22] | US | Nonrandomized | IP | All | Linkage to care | Care coordination (in person) | Phone-based care coordination | 146 |
| Genova (2022)a [23] | US | Nonrandomized | OP | All | Antibody test uptake | Medical chart reminders | No medical chart reminders | 5638 |
| Geretti (2018) [16] | UK | Nonrandomized | ED | All | RNA test uptake | Bundled testing (POC) | HCV testing alone (POC) | 814 |
| Hsu (2020)a [24] | US | Nonrandomized | ED | All | Linkage to care | Universal screening | Targeted screening | 41 |
| Huang (2021) [25] | Taiwan | Nonrandomized | OP | All | RNA test uptake, treatment commencement | Reflex RNA testing, care coordination | No reflex RNA testing, No care coordination | 1521 (RNA testing) 1045 (treatment) |
| Lee (2020) [26] | US | Nonrandomized | OP | All | Linkage to care | Financial incentives for patients | No financial incentives | 243 |
| Manteuffel (2022) [27] | US | Nonrandomized | ED | Multiple HCV risk factors | RNA test uptake, linkage to care | Reflex RNA testingb | No reflex RNA testing | 748 (RNA testing) 315 (linkage) |
| Marks (2021) [28] | US | Nonrandomized | IP | Injecting drug use history | Antibody test uptake | Medical chart reminders | No medical chart reminders | 394 |
| Mehta (2022) [29] | US | RCT | IP | Birth cohort 1945–1965 | Antibody test uptake | Automated opt-out screening | Medical chart reminder, no automated testing | 7634 |
| Northrup (2022) [30] | US | Nonrandomized | IP | Multiple HCV risk factors | Antibody test uptake | Health provider education | No health provider education | 190 |
| Oji (2023) [31] | US | Nonrandomized | IP | All | Antibody test uptake | Health provider education | No health provider education | 173 |
| Schechter-Perkins (2018) [32] | US | Nonrandomized | ED | All | RNA test uptake, linkage to care | Reflex RNA testingb, care coordination | Opt-in reflex RNA testing, No care coordination | 513 (RNA testing) 295 (linkage) |
| Smout (2022) [33] | UK | Nonrandomized | ED | All | Antibody test uptake | Automated opt-out screeningb | No automated testing | 50 131 |
| Starbird (2020) [34] | US | RCT | OP | HIV coinfected | Linkage to care, treatment commencement, SVR | Care coordination | One appointment reminder and HCV patient information brochure | 66 (linkage), 24 (treatment commencement), 12 (SVR; 4 = intervention; 8 = comparatora) |
| Wang (2018)a [35] | US | Nonrandomized | ED | Multiple HCV risk factors | Antibody test uptake | Automated opt-out screeningb | No automated testing | 14 451 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Financial incentives for patients | Nurse-led multidisciplinary case management | 90 |
| Ward (2019) [36] | US | RCT | OP | Multiple HCV risk factors | Treatment commencement, SVR | Peer support | Nurse-led multidisciplinary case management | 90 |
| Wasti (2019) [37] | US | Nonrandomized | ED | All | Antibody test uptake | Universal screeningb | Targeted screening | 7841 |
| Weiss (2017) [38] | US | RCT | OP | HIV coinfected | Treatment commencement | Motivational interviewing | Attention control | 53 |
| White (2018) [39] | US | Nonrandomized | ED | All | Antibody testing uptake | Automated opt-out screeningb | Nurse-ordered screening | 40 862 |
| Yeboah- Korang (2018) [40] | US | Nonrandomized | OP | Birth cohort 1945–1965 | Antibody testing uptake | Medical chart reminders | No medical chart reminders | 55 277 |
Abbreviations: ED, emergency department; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IP, inpatient unit; OP, outpatient clinic; POC, point of care; RCT, randomized controlled trial; SVR, sustained virological response; UK, United Kingdom; US, United States.
aConference abstract.
bTesting delivered to patients already undergoing venipuncture as part of care.
Studies were from 19 journal articles and 4 conference abstracts. Four studies were RCTs, and 19 were nonrandomized studies. Most studies were conducted in single centers (n = 19) with all from high-income countries including 19 from the United States, 2 from the United Kingdom, and 1 from Taiwan.
Nine studies were conducted in EDs, 9 in outpatient clinics, and 5 in inpatient units. More than half of the included studies (n = 13) examined all adults engaged in hospital care. The others only examined patient populations with 1 or more risk factors for HCV including birth cohort 1945–1965, HIV coinfection, history of substance use including injecting drug use, or mixed populations with several risk factors for HCV (Table 2). Most studies (n = 18) had interventions with multiple components. No study examined the impact of 1 intervention across all 4 HCV care cascade outcomes.
Primary Analyses
HCV Antibody Testing Uptake
Twelve studies (assessing 13 interventions and 5 intervention types) with a total of 222 868 participants reported HCV antibody testing uptake. Overall, these interventions were associated with an increase in antibody testing uptake when data were pooled and assessed against comparators (pooled OR, 5.83 [95% confidence interval [CI], 2.49–13.61]); however, heterogeneity across studies was high (I2 = 99.9%, P < .001) (Figure 2).

Forest plot examining association between intervention type and hepatitis C antibody testing uptake. Weights are from random-effects analysis. aI2 not calculated as too few studies for pooled analysis. bNo P value calculated as I2 was not calculated. Abbreviations: CI, confidence interval; OR, odds ratio.
HCV RNA Testing Uptake
Five studies with a total of 4987 participants reported HCV RNA testing uptake. Overall, the interventions were associated with an increase in RNA testing uptake when data were pooled and assessed against comparators (pooled OR, 10.65 [95% CI, 1.70–66.50]), with high heterogeneity across studies (I2 = 97.9%, P < .001) (Figure 3).
![Forest plot examining the association between intervention type and hepatitis C RNA testing uptake. Weights are from random-effects analysis. aI2 not calculated as too few studies for pooled analysis. bNo P value calculated as I2 was not calculated. *Comparison data cited in Cunningham et al [13], not primary study publication. Abbreviations: CI, confidence interval; OR, odds ratio.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/12/2/10.1093_ofid_ofaf056/2/m_ofaf056f3.jpeg?Expires=1747897118&Signature=XjcWBukbVOab6wnMZk-llw~JSCTRXmpSYmqnMaIOfWGfRRcPiaUfDB9aVhbGtrsJfG42Y19qAtHK3ckouV5Pz99~zF49E5JmcyGviPHQUkxZoyk5Eynlk-DYbwdFmEwJJh1SH00X~KT~zamHZ15lDzWOB9P26g2Q3mUlXmLHWJm3baQ4e5SP~vYsYq-p3a5t74Qby8q1PCjq0fzVPSB5wsmBk9vUtJpGkFZ-RD1rVl2ZcOVmkGULiGhSCUcFqFXxFelxFTz5zhD-yBtHMAQA~OHO~-trLVSTgG5gLzbstTX7YXvI7cMkl1Uou9MYX968HhcnvFNqwSXXEYf9ebaJvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Forest plot examining the association between intervention type and hepatitis C RNA testing uptake. Weights are from random-effects analysis. aI2 not calculated as too few studies for pooled analysis. bNo P value calculated as I2 was not calculated. *Comparison data cited in Cunningham et al [13], not primary study publication. Abbreviations: CI, confidence interval; OR, odds ratio.
Linkage to Care
Seven studies (assessing 8 interventions) with a total of 3185 participants reported linkage to care. Overall, the interventions were associated with an increase in linkage to care when data were pooled and assessed against comparators (7 studies: pooled OR, 1.75 [95% CI, 1.10–2.79]); however, heterogeneity was high (I2 = 79.9, P < .001) (Figure 4).
![Forest plot examining the association between intervention type and linkage to hepatitis C care. *Comparison data cited in Cunningham et al [13], not primary study publication. Abbreviations: CI, confidence interval; OR, odds ratio.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/12/2/10.1093_ofid_ofaf056/2/m_ofaf056f4.jpeg?Expires=1747897118&Signature=AMOdacFhb1RxRbm5OuFteKglQvbfomQDA-nQcjQxzkbhws2wcgEv6Y3qnfsuYXmXNYmC0I-0MYre4vD43tCPqtkf8k-s9BaDHA-Wn1PpEOHJ8gYSOJeAw4LqVjSJFctWNNeM5An9qEhUUrR6gI0VRYevw5O3JJ2RI3J1Ze5gSBzrX6HbZHOFhFfrG2Y2HhvWnRsn8VZidobahgdkh4~Og4oHjINaiUvaaLMzSzoDUVfnOuySnYBTbp2TEvvJWzpSnKU~TKyR~6I4Ohtibc0JGvLGA7FCM9llPhAZLzr~xb1KpXOnHI25~mqjlWgEA1F4KUieHbinzPRxLFN0wi~KAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Forest plot examining the association between intervention type and linkage to hepatitis C care. *Comparison data cited in Cunningham et al [13], not primary study publication. Abbreviations: CI, confidence interval; OR, odds ratio.
Treatment Commencement and SVR
Four studies (assessing 5 interventions) with a total of 1344 participants reported treatment commencement. Overall, the interventions were associated with no changes to treatment commencement when data were pooled and assessed against comparators (pooled OR, 1.06 [95% CI, .35–3.25]); however, heterogeneity across studies was high (I2 = 84.9, P < .001) (Figure 5).
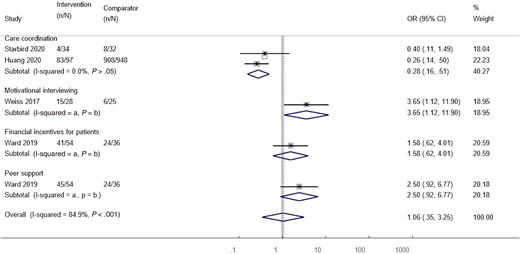
Forest plot examining the association between intervention type and treatment commencement. Weights are from random-effects analysis. aI2 not calculated as too few studies for pooled analysis. bNo P value calculated as I2 was not calculated. Abbreviations: CI, confidence interval; OR, odds ratio.
Only 2 studies (assessing 3 interventions) with a total of 146 participants reported SVR; therefore, pooled meta-analysis was not performed for this outcome. In 1 study, all patients receiving care coordination who commenced treatment achieved SVR, so ORs and CIs could not be calculated. In the other study, financial incentives and peer support had no effect on SVR compared to standard care (OR, 0.85 [95% CI, .07–6.45] and 0.93 [95% CI, .08–7.11], respectively).
Primary Subgroup Analyses
HCV Antibody Testing Uptake
Subgroup analyses exploring differences in antibody testing uptake by intervention type showed that automated opt-out testing had the greatest impact on uptake (5 studies: pooled OR, 16.13 [95% CI, 3.35–77.66]), followed by clinician education (2 studies: pooled OR, 5.41 [95% CI, 2.49–11.74]) and universal screening (2 studies: pooled OR, 3.39 [95% CI, 1.84–2.64]). Automated opt-out testing was associated with increased antibody testing uptake in studies from ED [18–20], inpatient [21], and outpatient settings [22]. Medical chart reminders had no impact on antibody testing uptake in pooled analyses (3 studies: pooled OR, 4.13 [95% CI, .49–35.21]). One study examined bundled testing, finding a potential association with reduced antibody testing uptake (OR, 0.35 [95% CI, .32–.38]).
HCV RNA Testing Uptake
Reflex RNA testing was the only intervention type with sufficient data to be pooled for subgroup analyses exploring differences in RNA testing uptake by intervention type. Across 4 studies, reflex RNA testing was found to be associated with increased RNA test uptake compared to nonreflexive testing (pooled OR, 25.04 [95% CI, 3.63–172.79]). One study explored bundled HCV and HIV point of care testing and found that it was associated with lower RNA test uptake compared to HCV point of care testing alone (OR, 0.51 [95% CI, .39–.69]).
Linkage to Care
Two categories of interventions (patient-level interventions and testing-level interventions) had sufficient data for pooled primary subgroup analyses exploring differences in linkage to care by intervention type. Patient-level interventions including care coordination and financial incentives were associated with significantly increased linkage to care relative to comparators in pooled analyses (4 studies: pooled OR, 2.73 [95% CI, 1.85–4.03]). Testing-level interventions including universal screening, reflex RNA testing, and bundled testing were not associated with increased linkage in pooled analyses (4 studies: pooled OR, 1.19 [95% CI, .71–1.98]).
Treatment Commencement and SVR
Primary subgroup analyses by intervention type could not be conducted for treatment commencement or SVR outcomes due to insufficient data.
Secondary Subgroup Analyses
Forest plots showing secondary subgroup analyses are reported in the Supplementary material.
Sensitivity Analyses
Sensitivity analyses for studies reporting antibody testing uptake showed an attenuated but statistically significant association between intervention and uptake after omitting the 2 studies with OR exceeding 15 (pooled OR, 2.37 [95% CI, 1.32–4.27]), with persistently high heterogeneity (I2 = 99.7%, P < .001).
Secondary subgroup analyses found that antibody testing uptake was higher in studies conducted within inpatient (pooled OR, 3.51 [95% CI, 2.26–5.46]) compared to outpatient settings (pooled OR, 1.83 [95% CI, 1.70–1.97]) in analyses that omitted studies with OR exceeding 15.
Risk of Bias Analyses
Among randomized studies, the overall risk of bias was assessed as having some concerns primarily related to randomization processes and deviations from intended interventions in the study reporting HCV antibody testing uptake, 2 of 3 reporting treatment commencement, and 1 of 2 reporting SVR (see Supplementary material). The other RCTs had low risk of bias.
The overall risk of bias was serious in most of the nonrandomized studies due to uncontrolled confounding including for 10 of 11 studies (91%) and 4 of 5 (80%) reporting antibody and RNA testing uptake, respectively; 5 of 6 (83%) reporting linkage to care; and 1 (100%) reporting treatment commencement (see Supplementary material). The other studies had moderate risk of bias. Funnel plots showed no clear evidence of publication bias in either direction within the limits of the heterogeneous studies included.
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis exclusively of hospital-led interventions to increase HCV testing, linkage to care, and treatment. Other reviews have predominantly examined primary or community healthcare [13] and drug and alcohol treatment settings [41–43], or predated DAA treatment [44].
Our review found that interventions at the system level increased HCV testing uptake, while those at the patient level including care coordination and financial incentives increased linkage to care. Interventions associated with increased HCV testing uptake did not appear to increase linkage to care or treatment commencement, and no single intervention type increased uptake at all HCV care cascade steps.
Automated opt-out testing delivered through electronic health record modifications had the most impact on HCV antibody testing uptake. Clinician education and universal screening had the next greatest impact, while medical chart reminders had no impact.
Primary studies in ED and inpatient settings from other systematic reviews have also shown increased HCV antibody testing uptake with opt-out automated testing [8, 29, 45], even in contexts with preexisting medical chart reminders [29, 45]. A systematic review predominantly of primary care– and community health–delivered interventions also showed that automated opt-out testing was associated with increased HCV antibody testing uptake [13]. However, in contrast to our review, they found increased testing uptake in association with medical chart reminders but no effect of universal screening.
Automated opt-out testing likely improves uptake through streamlining and simplifying testing processes. Universal screening may be more important in normalizing testing and increasing its acceptability and uptake in hospitals where the patient is likely unknown to the clinician [8, 46–48]. Medical chart reminders may have less impact on testing uptake in hospital because hospital clinicians may be more susceptible to “alert fatigue” and ignore the testing recommendations compared to primary and community care counterparts [49–51].
Reflex RNA testing clearly increased RNA testing uptake in our systematic review, confirming findings in different contexts [13, 45, 52] and supporting World Health Organization recommendations [53]. By simultaneously testing for HCV antibody and RNA, the patient can receive both test results during 1 hospital encounter, mitigating the risks of loss to follow-up associated with nonreflexive testing. In hospitals, reflex RNA testing can quickly diagnose HCV in all patients and is especially useful to capture vulnerable people disproportionately affected by yet underdiagnosed with HCV [42, 54, 55] who might otherwise be lost to follow-up [56].
Our meta-analysis found that testing-level interventions, including universal screening, reflex RNA testing, and bundled testing, collectively made no difference to linkage to HCV care. These testing-level interventions were mostly delivered in ED settings. Patient-level interventions including care coordination and financial incentives increased linkage in inpatient and outpatient settings in our review, consistent with findings in primary care and community health studies [13]. Linkage to care failure following ED-based HCV testing is common [8, 57], particularly among people with comorbid psychiatric disorders, with substance use, or who are homeless [57, 58]. Inpatient units and outpatient clinics offering greater continuity of patient care than EDs typically show superior linkage [59–61].
Few published studies exist exploring hospital-led interventions to increase HCV treatment commencement. An RCT published outside our search publication date criterion showed faster HCV treatment uptake and superior completion among people with a history of injecting drug use who were receiving HCV treatment while inpatients, compared to standard outpatient care [62]. This is consistent with studies in psychiatric inpatient wards showing excellent outcomes from inpatient HCV treatment among people with comorbid mental health conditions [60], who may be considered too complicated for HCV treatment in other healthcare settings.
Our review had limitations. First, most studies were single center and exclusively from high-income countries, predominantly the United States. Hence, review findings may have limited generalizability beyond these contexts. Since low-income countries have the highest prevalence of undiagnosed and untreated HCV [63], further research to examine interventions that optimally increase testing and treatment in these hospital contexts is essential.
Second, studies had high statistical heterogeneity likely due to the highly variable comparators, populations, and interventions assessed by the included studies. Third, among the predominantly nonrandomized studies reviewed, many were at serious risk of bias, primarily due to uncontrolled confounding and unbalanced cointerventions. The latter related to the interventions in most studies having multiple components. Even though the intervention type was defined from a primary intervention component, other components may still have affected outcomes as cointerventions and could not be adjusted for in analyses.
Finally, there were not enough studies in our review to identify the most effective intervention types in specific hospital settings and populations and to disentangle possible effects of setting on outcomes according to intervention type.
Our review has implications for research, policy, and clinical practice. Given that no single hospital-led intervention increased HCV antibody and RNA testing uptake, linkage to care, and treatment commencement, multifaceted interventions targeting hospital, clinician, and patient are necessary to progress patients from HCV testing to treatment commencement. Electronic health record modifications that automate HCV antibody and reflex RNA testing together show great potential to increase testing uptake in hospitals in ED, inpatient, and outpatient contexts. Complementary patient-level interventions are also needed to facilitate linkage to HCV care and treatment commencement, otherwise increased testing will not translate to increased treatment.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. S. D., H. M. O., C. R., and M. E. H. conceptualized the original study with later input from R. M. and C. S.; H. M. O. and C. R. drafted the review protocol and developed the search strategy. R. M. and C. S., with guidance from J. S. D., searched literature databases, screened search results, and extracted data from eligible studies. R. M., C. S., and J. S. D. accessed and verified all data included in this study. M. W. T., with input from R. M., J. S. D., and C. S., conducted the meta-analysis. C. S. and R. M. performed risk of bias assessments for eligible studies. R. M. wrote article drafts, with input from J. S. D., M. E. H., M. W. T., C. S., H. M. O., and C. R. All authors reviewed and approved the final version of the manuscript before submission. J. S. D. supervised the study. All authors had access to all the data used in this study and accepted responsibility for the decision to submit the manuscript for publication.
Patient consent. Because this study was a systematic review and meta-analysis, there were no factors necessitating patient consent.
References
Author notes
Potential conflicts of interest. M. W. T. has received investigator-initiated research grants, speakers' honoraria, and consultancy fees from Gilead Sciences, unrelated to the submitted work. C. R. has received investigator-initiated research grants from Gilead Sciences and Deakin University, unrelated to the submitted work. M. E. H. and J. S. D.'s institute receives funding from Gilead Sciences and AbbVie for investigator-initiated research, unrelated to the submitted work. All other authors report no potential conflicts.





Comments