-
PDF
- Split View
-
Views
-
Cite
Cite
Takahiro Matsuo, Sebastian Wurster, Ying Jiang, Jeffrey Tarrand, Scott E Evans, Dimitrios P Kontoyiannis, Determinant of 30-Day Mortality of Pulmonary Legionellosis: Do Coinfections Matter?, Open Forum Infectious Diseases, Volume 11, Issue 9, September 2024, ofae529, https://doi.org/10.1093/ofid/ofae529
Close - Share Icon Share
Abstract
We retrospectively reviewed 64 cases of cancer with pulmonary legionellosis (Legionella pneumophila in 73%). Nearly all patients received Legionella-active antibiotics, yet 30-day mortality was 23%. Independent predictors of 30-day mortality were hyponatremia, bilateral lung involvement, and Sequential Organ Failure Assessment score ≥5. Lung coinfections were common (31%) but did not significantly increase mortality.
Pulmonary legionellosis (PL) is a global opportunistic infection caused by the inhalation of aerosolized water particles laden with Legionella spp. Patients who are immunocompromised, especially those with hematologic malignancies or recipients of hematopoietic stem cell transplantation or solid organ transplantation, are at a heightened risk for developing PL [1–6], with mortality rates ranging from 27% to 65% [1–4].
Recent advances in diagnostic technologies, such as matrix-assisted laser desorption ionization–time of flight mass spectrometry and 16S ribosomal RNA sequencing, have resulted in an increasing number of reports that identify Legionella species other than Legionella pneumophila serogroup 1 as the causative agent of PL, especially among patients who are immunosuppressed [2, 4, 6]. Additionally, there has been a growing body of literature reporting various viral, bacterial, and fungal coinfections in patients with PL [4, 6, 7]. Therefore, we reviewed the epidemiology, risk factors, clinical characteristics, and outcomes of PL, including the impact of coinfections, in a contemporary cohort of patients with cancer and PL at a tertiary cancer center.
METHODS
We retrospectively reviewed the records of all adult patients with cancer (≥18 years old) who were diagnosed with PL at The University of Texas MD Anderson Cancer Center from January 2009 to October 2021. PL diagnosis was confirmed through either a Legionella urine antigen test (UAT; BinaxNOW [Alere Inc]) or culture from sputum, tracheal aspirates, or bronchoalveolar lavage samples with selective and nonselective buffered charcoal yeast extract agar plates. Species identification was performed with either matrix-assisted laser desorption ionization–time of flight mass spectrometry (Vitek MS; BioMérieux) or 16S ribosomal RNA gene sequencing [8]. Collected data, definitions of variables, diagnostic criteria/workup for lung coinfections, and statistical methods are provided in the supplementary methods and Supplementary Table 1.
RESULTS
We identified 64 cases of cancer with PL: 45 patients (70%) were male and the median age was 60 years. Most patients (n = 42, 66%) had hematologic malignancies. A history of hematopoietic stem cell transplantation was present in 20 patients (31%). Lymphopenia was common at PL diagnosis (n = 48, 75%), whereas neutropenia was noted in only 16 patients (25%; Table 1).
| Characteristic . | Survived (n = 49) . | Died (n = 15) . | Total (n = 64) . | P Value . |
|---|---|---|---|---|
| Male sex | 35 (71) | 10 (67) | 45 (70) | .753 |
| Age, y | 64 (53–69) | 58 (45–61) | 60 (52–68) | .068 |
| Race and ethnicity | >.999 | |||
| Non-Hispanic White | 35 (71) | 11 (73) | 46 (72) | |
| Hispanic | 9 (18) | 3 (20) | 12 (19) | |
| African American | 2 (4) | 1 (7) | 3 (5) | |
| Asian | 3 (6) | 0 (0) | 3 (5) | |
| Body mass index | 27 (25–31) | 26 (20–33) | 27 (24–31) | .379 |
| Smoking | >.999 | |||
| None | 27 (55) | 8 (53) | 35 (55) | |
| Current | 5 (10) | 1 (7) | 6 (9) | |
| Former | 17 (35) | 6 (40) | 23 (36) | |
| Alcohol | .043 | |||
| None | 30 (61) | 14 (93) | 44 (69) | |
| Current | 18 (37) | 1 (7) | 19 (30) | |
| Former | 1 (2) | 0 (0) | 1 (2) | |
| Malignancy type | .473 | |||
| Hematologic | 31 (63) | 11 (73) | 42 (66) | |
| Solid | 18 (37) | 4 (27) | 22 (34) | |
| Active malignancy status at diagnosis of PL | 45 (92) | 13 (87) | 58 (91) | .618 |
| Active chemotherapya | 31 (63) | 12 (80) | 43 (67) | .348 |
| Corticosteroid use | 10 (20) | 5 (33) | 15 (23) | .315 |
| History of HSCT | 14 (29) | 6 (40) | 20 (31) | .526 |
| Type of HSCT | .778 | |||
| Allogenic | 9/14 (64) | 4/6 (67) | 13/20 (65) | |
| Autologous | 5/14 (36) | 2/6 (33) | 7/20 (35) | |
| Graft-vs-host disease | 5/9 (56) | 2/4 (50) | 7/13 (54) | >.999 |
| Other underlying risk factors | ||||
| Diabetes | 9 (18) | 1 (7) | 10 (16) | .429 |
| Chronic lung disease | 6 (12) | 1 (7) | 7 (11) | >.999 |
| Chronic kidney disease | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Chronic heart failure | 2 (4) | 0 (0) | 2 (3) | >.999 |
| History of splenectomy | 1 (2) | 0 (0) | 1 (2) | >.999 |
| Neutropenia at diagnosis, neutrophils/µL | 10 (20) | 6 (40) | 16 (25) | .173 |
| 500–1000 | 2 (4) | 2 (13) | 4 (6) | |
| 100–500 | 5 (10) | 3 (20) | 8 (13) | |
| <100 | 3 (6) | 1 (7) | 4 (6) | |
| Lymphopenia at diagnosis, lymphocytes/µL | 38 (78) | 10 (67) | 48 (75) | .498 |
| 500–1000 | 4 (8) | 0 (0) | 4 (6) | |
| 100–500 | 28 (57) | 8 (53) | 36 (56) | |
| <100 | 6 (12) | 2 (13) | 8 (13) | |
| Clinical presentation of PL | ||||
| Cough | 42 (86) | 12 (80) | 54 (84) | .687 |
| Fever | 40 (82) | 12 (80) | 52 (81) | >.999 |
| Dyspnea | 32 (65) | 12 (80) | 44 (69) | .354 |
| Hypoxia | 31 (63) | 11 (73) | 42 (66) | .473 |
| Headache | 8 (16) | 0 (0) | 8 (13) | .181 |
| Diarrhea | 8 (16) | 0 (0) | 8 (13) | .181 |
| Nausea/vomiting | 6 (12) | 0 (0) | 6 (9) | .322 |
| Confusion | 5 (10) | 1 (7) | 6 (9) | >.999 |
| Pleuritic chest pain | 5 (10) | 0 (0) | 5 (8) | .329 |
| Clinical laboratory findings | ||||
| Elevated liver enzyme | 27 (55) | 10 (67) | 37 (58) | .427 |
| Hyponatremia | 15 (31) | 10 (67) | 25 (39) | .012 |
| Acute kidney injury | 11 (22) | 6 (40) | 17 (27) | .197 |
| Computed tomography findingsb | 47 | 12 | 59 | |
| Airspace consolidation | 42 (89) | 11 (92) | 53 (90) | >.999 |
| Ground glass opacities | 34 (72) | 10 (83) | 44 (75) | .712 |
| Multilobar infiltrate | 32 (68) | 10 (83) | 42 (71) | .478 |
| Adenopathy | 30 (64) | 8 (67) | 38 (64) | >.999 |
| Nodules | 30 (64) | 6 (50) | 36 (61) | .510 |
| Pleural effusions | 26 (55) | 8 (67) | 34 (58) | .478 |
| Mass-like consolidation | 13 (28) | 4 (33) | 17 (29) | .729 |
| Halo sign | 5 (11) | 0 (0) | 5 (9) | .573 |
| Cavitation | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Reversed halo sign | 2 (4) | 0 (0) | 2 (3) | >.999 |
| Bilateral | 27 (57) | 11 (92) | 38 (64) | .041 |
| Legionella type | .740 | |||
| L pneumophila | 35 (71) | 12 (80) | 47 (73) | |
| Legionella, not pneumophila | 14 (29) | 3 (20) | 17 (27) | |
| Coinfectionsc | 16 (33) | 4 (27) | 20 (31) | .759 |
| Bacterial | 7 (14) | 2 (13) | 9 (14) | >.999 |
| Viral | 9 (18) | 2 (13) | 11 (17) | >.999 |
| Fungal | 1 (2) | 2 (13) | 3 (5) | .134 |
| SOFA at PL diagnosis | 3 (2–5) | 6 (4–7) | 4 (2–6) | .051 |
| Time to PL diagnosis from admission, median (range) | 0 (1–13) | 2 (0–10) | 2.5 (0–13) | .055 |
| Infectious diseases consultation | 46 (94) | 13 (87) | 59 (92) | .583 |
| Treatment for PLd | .487 | |||
| Monotherapy | 35 (71) | 12/14 (86) | 47/63 (75) | |
| Combination | 14 (29) | 2/14 (14) | 16/63 (25) | |
| Levofloxacin-containing regimen | 30 (61) | 10 (67) | 40 (63) | .546 |
| Time to initiation of appropriate antibioticsd from admission, d | 0 (0–1) | 0 (0–1) | 0 (0–1) | .268 |
| Intensive care unit stay within 1 wk of PL diagnosis | 6 (12) | 11 (73) | 17 (27) | <.0001 |
| Characteristic . | Survived (n = 49) . | Died (n = 15) . | Total (n = 64) . | P Value . |
|---|---|---|---|---|
| Male sex | 35 (71) | 10 (67) | 45 (70) | .753 |
| Age, y | 64 (53–69) | 58 (45–61) | 60 (52–68) | .068 |
| Race and ethnicity | >.999 | |||
| Non-Hispanic White | 35 (71) | 11 (73) | 46 (72) | |
| Hispanic | 9 (18) | 3 (20) | 12 (19) | |
| African American | 2 (4) | 1 (7) | 3 (5) | |
| Asian | 3 (6) | 0 (0) | 3 (5) | |
| Body mass index | 27 (25–31) | 26 (20–33) | 27 (24–31) | .379 |
| Smoking | >.999 | |||
| None | 27 (55) | 8 (53) | 35 (55) | |
| Current | 5 (10) | 1 (7) | 6 (9) | |
| Former | 17 (35) | 6 (40) | 23 (36) | |
| Alcohol | .043 | |||
| None | 30 (61) | 14 (93) | 44 (69) | |
| Current | 18 (37) | 1 (7) | 19 (30) | |
| Former | 1 (2) | 0 (0) | 1 (2) | |
| Malignancy type | .473 | |||
| Hematologic | 31 (63) | 11 (73) | 42 (66) | |
| Solid | 18 (37) | 4 (27) | 22 (34) | |
| Active malignancy status at diagnosis of PL | 45 (92) | 13 (87) | 58 (91) | .618 |
| Active chemotherapya | 31 (63) | 12 (80) | 43 (67) | .348 |
| Corticosteroid use | 10 (20) | 5 (33) | 15 (23) | .315 |
| History of HSCT | 14 (29) | 6 (40) | 20 (31) | .526 |
| Type of HSCT | .778 | |||
| Allogenic | 9/14 (64) | 4/6 (67) | 13/20 (65) | |
| Autologous | 5/14 (36) | 2/6 (33) | 7/20 (35) | |
| Graft-vs-host disease | 5/9 (56) | 2/4 (50) | 7/13 (54) | >.999 |
| Other underlying risk factors | ||||
| Diabetes | 9 (18) | 1 (7) | 10 (16) | .429 |
| Chronic lung disease | 6 (12) | 1 (7) | 7 (11) | >.999 |
| Chronic kidney disease | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Chronic heart failure | 2 (4) | 0 (0) | 2 (3) | >.999 |
| History of splenectomy | 1 (2) | 0 (0) | 1 (2) | >.999 |
| Neutropenia at diagnosis, neutrophils/µL | 10 (20) | 6 (40) | 16 (25) | .173 |
| 500–1000 | 2 (4) | 2 (13) | 4 (6) | |
| 100–500 | 5 (10) | 3 (20) | 8 (13) | |
| <100 | 3 (6) | 1 (7) | 4 (6) | |
| Lymphopenia at diagnosis, lymphocytes/µL | 38 (78) | 10 (67) | 48 (75) | .498 |
| 500–1000 | 4 (8) | 0 (0) | 4 (6) | |
| 100–500 | 28 (57) | 8 (53) | 36 (56) | |
| <100 | 6 (12) | 2 (13) | 8 (13) | |
| Clinical presentation of PL | ||||
| Cough | 42 (86) | 12 (80) | 54 (84) | .687 |
| Fever | 40 (82) | 12 (80) | 52 (81) | >.999 |
| Dyspnea | 32 (65) | 12 (80) | 44 (69) | .354 |
| Hypoxia | 31 (63) | 11 (73) | 42 (66) | .473 |
| Headache | 8 (16) | 0 (0) | 8 (13) | .181 |
| Diarrhea | 8 (16) | 0 (0) | 8 (13) | .181 |
| Nausea/vomiting | 6 (12) | 0 (0) | 6 (9) | .322 |
| Confusion | 5 (10) | 1 (7) | 6 (9) | >.999 |
| Pleuritic chest pain | 5 (10) | 0 (0) | 5 (8) | .329 |
| Clinical laboratory findings | ||||
| Elevated liver enzyme | 27 (55) | 10 (67) | 37 (58) | .427 |
| Hyponatremia | 15 (31) | 10 (67) | 25 (39) | .012 |
| Acute kidney injury | 11 (22) | 6 (40) | 17 (27) | .197 |
| Computed tomography findingsb | 47 | 12 | 59 | |
| Airspace consolidation | 42 (89) | 11 (92) | 53 (90) | >.999 |
| Ground glass opacities | 34 (72) | 10 (83) | 44 (75) | .712 |
| Multilobar infiltrate | 32 (68) | 10 (83) | 42 (71) | .478 |
| Adenopathy | 30 (64) | 8 (67) | 38 (64) | >.999 |
| Nodules | 30 (64) | 6 (50) | 36 (61) | .510 |
| Pleural effusions | 26 (55) | 8 (67) | 34 (58) | .478 |
| Mass-like consolidation | 13 (28) | 4 (33) | 17 (29) | .729 |
| Halo sign | 5 (11) | 0 (0) | 5 (9) | .573 |
| Cavitation | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Reversed halo sign | 2 (4) | 0 (0) | 2 (3) | >.999 |
| Bilateral | 27 (57) | 11 (92) | 38 (64) | .041 |
| Legionella type | .740 | |||
| L pneumophila | 35 (71) | 12 (80) | 47 (73) | |
| Legionella, not pneumophila | 14 (29) | 3 (20) | 17 (27) | |
| Coinfectionsc | 16 (33) | 4 (27) | 20 (31) | .759 |
| Bacterial | 7 (14) | 2 (13) | 9 (14) | >.999 |
| Viral | 9 (18) | 2 (13) | 11 (17) | >.999 |
| Fungal | 1 (2) | 2 (13) | 3 (5) | .134 |
| SOFA at PL diagnosis | 3 (2–5) | 6 (4–7) | 4 (2–6) | .051 |
| Time to PL diagnosis from admission, median (range) | 0 (1–13) | 2 (0–10) | 2.5 (0–13) | .055 |
| Infectious diseases consultation | 46 (94) | 13 (87) | 59 (92) | .583 |
| Treatment for PLd | .487 | |||
| Monotherapy | 35 (71) | 12/14 (86) | 47/63 (75) | |
| Combination | 14 (29) | 2/14 (14) | 16/63 (25) | |
| Levofloxacin-containing regimen | 30 (61) | 10 (67) | 40 (63) | .546 |
| Time to initiation of appropriate antibioticsd from admission, d | 0 (0–1) | 0 (0–1) | 0 (0–1) | .268 |
| Intensive care unit stay within 1 wk of PL diagnosis | 6 (12) | 11 (73) | 17 (27) | <.0001 |
Data are presented as No. (%) and median (IQR) unless noted otherwise. Bold P values indicate P < .05.
Abbreviations: HSCT, hematopoietic stem cell transplantation; PL, pulmonary legionellosis; SOFA, Sequential Organ Failure Assessment.
aActive chemotherapies were administered within 90 days of PL diagnosis.
bComputed tomography findings were obtained within 7 days before or after PL diagnosis.
cThree patients had >1 category of copathogens (2 with bacteria and virus, 1 with virus and fungus).
dAntibiotics having activity against Legionella, including levofloxacin, azithromycin, and doxycycline.
| Characteristic . | Survived (n = 49) . | Died (n = 15) . | Total (n = 64) . | P Value . |
|---|---|---|---|---|
| Male sex | 35 (71) | 10 (67) | 45 (70) | .753 |
| Age, y | 64 (53–69) | 58 (45–61) | 60 (52–68) | .068 |
| Race and ethnicity | >.999 | |||
| Non-Hispanic White | 35 (71) | 11 (73) | 46 (72) | |
| Hispanic | 9 (18) | 3 (20) | 12 (19) | |
| African American | 2 (4) | 1 (7) | 3 (5) | |
| Asian | 3 (6) | 0 (0) | 3 (5) | |
| Body mass index | 27 (25–31) | 26 (20–33) | 27 (24–31) | .379 |
| Smoking | >.999 | |||
| None | 27 (55) | 8 (53) | 35 (55) | |
| Current | 5 (10) | 1 (7) | 6 (9) | |
| Former | 17 (35) | 6 (40) | 23 (36) | |
| Alcohol | .043 | |||
| None | 30 (61) | 14 (93) | 44 (69) | |
| Current | 18 (37) | 1 (7) | 19 (30) | |
| Former | 1 (2) | 0 (0) | 1 (2) | |
| Malignancy type | .473 | |||
| Hematologic | 31 (63) | 11 (73) | 42 (66) | |
| Solid | 18 (37) | 4 (27) | 22 (34) | |
| Active malignancy status at diagnosis of PL | 45 (92) | 13 (87) | 58 (91) | .618 |
| Active chemotherapya | 31 (63) | 12 (80) | 43 (67) | .348 |
| Corticosteroid use | 10 (20) | 5 (33) | 15 (23) | .315 |
| History of HSCT | 14 (29) | 6 (40) | 20 (31) | .526 |
| Type of HSCT | .778 | |||
| Allogenic | 9/14 (64) | 4/6 (67) | 13/20 (65) | |
| Autologous | 5/14 (36) | 2/6 (33) | 7/20 (35) | |
| Graft-vs-host disease | 5/9 (56) | 2/4 (50) | 7/13 (54) | >.999 |
| Other underlying risk factors | ||||
| Diabetes | 9 (18) | 1 (7) | 10 (16) | .429 |
| Chronic lung disease | 6 (12) | 1 (7) | 7 (11) | >.999 |
| Chronic kidney disease | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Chronic heart failure | 2 (4) | 0 (0) | 2 (3) | >.999 |
| History of splenectomy | 1 (2) | 0 (0) | 1 (2) | >.999 |
| Neutropenia at diagnosis, neutrophils/µL | 10 (20) | 6 (40) | 16 (25) | .173 |
| 500–1000 | 2 (4) | 2 (13) | 4 (6) | |
| 100–500 | 5 (10) | 3 (20) | 8 (13) | |
| <100 | 3 (6) | 1 (7) | 4 (6) | |
| Lymphopenia at diagnosis, lymphocytes/µL | 38 (78) | 10 (67) | 48 (75) | .498 |
| 500–1000 | 4 (8) | 0 (0) | 4 (6) | |
| 100–500 | 28 (57) | 8 (53) | 36 (56) | |
| <100 | 6 (12) | 2 (13) | 8 (13) | |
| Clinical presentation of PL | ||||
| Cough | 42 (86) | 12 (80) | 54 (84) | .687 |
| Fever | 40 (82) | 12 (80) | 52 (81) | >.999 |
| Dyspnea | 32 (65) | 12 (80) | 44 (69) | .354 |
| Hypoxia | 31 (63) | 11 (73) | 42 (66) | .473 |
| Headache | 8 (16) | 0 (0) | 8 (13) | .181 |
| Diarrhea | 8 (16) | 0 (0) | 8 (13) | .181 |
| Nausea/vomiting | 6 (12) | 0 (0) | 6 (9) | .322 |
| Confusion | 5 (10) | 1 (7) | 6 (9) | >.999 |
| Pleuritic chest pain | 5 (10) | 0 (0) | 5 (8) | .329 |
| Clinical laboratory findings | ||||
| Elevated liver enzyme | 27 (55) | 10 (67) | 37 (58) | .427 |
| Hyponatremia | 15 (31) | 10 (67) | 25 (39) | .012 |
| Acute kidney injury | 11 (22) | 6 (40) | 17 (27) | .197 |
| Computed tomography findingsb | 47 | 12 | 59 | |
| Airspace consolidation | 42 (89) | 11 (92) | 53 (90) | >.999 |
| Ground glass opacities | 34 (72) | 10 (83) | 44 (75) | .712 |
| Multilobar infiltrate | 32 (68) | 10 (83) | 42 (71) | .478 |
| Adenopathy | 30 (64) | 8 (67) | 38 (64) | >.999 |
| Nodules | 30 (64) | 6 (50) | 36 (61) | .510 |
| Pleural effusions | 26 (55) | 8 (67) | 34 (58) | .478 |
| Mass-like consolidation | 13 (28) | 4 (33) | 17 (29) | .729 |
| Halo sign | 5 (11) | 0 (0) | 5 (9) | .573 |
| Cavitation | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Reversed halo sign | 2 (4) | 0 (0) | 2 (3) | >.999 |
| Bilateral | 27 (57) | 11 (92) | 38 (64) | .041 |
| Legionella type | .740 | |||
| L pneumophila | 35 (71) | 12 (80) | 47 (73) | |
| Legionella, not pneumophila | 14 (29) | 3 (20) | 17 (27) | |
| Coinfectionsc | 16 (33) | 4 (27) | 20 (31) | .759 |
| Bacterial | 7 (14) | 2 (13) | 9 (14) | >.999 |
| Viral | 9 (18) | 2 (13) | 11 (17) | >.999 |
| Fungal | 1 (2) | 2 (13) | 3 (5) | .134 |
| SOFA at PL diagnosis | 3 (2–5) | 6 (4–7) | 4 (2–6) | .051 |
| Time to PL diagnosis from admission, median (range) | 0 (1–13) | 2 (0–10) | 2.5 (0–13) | .055 |
| Infectious diseases consultation | 46 (94) | 13 (87) | 59 (92) | .583 |
| Treatment for PLd | .487 | |||
| Monotherapy | 35 (71) | 12/14 (86) | 47/63 (75) | |
| Combination | 14 (29) | 2/14 (14) | 16/63 (25) | |
| Levofloxacin-containing regimen | 30 (61) | 10 (67) | 40 (63) | .546 |
| Time to initiation of appropriate antibioticsd from admission, d | 0 (0–1) | 0 (0–1) | 0 (0–1) | .268 |
| Intensive care unit stay within 1 wk of PL diagnosis | 6 (12) | 11 (73) | 17 (27) | <.0001 |
| Characteristic . | Survived (n = 49) . | Died (n = 15) . | Total (n = 64) . | P Value . |
|---|---|---|---|---|
| Male sex | 35 (71) | 10 (67) | 45 (70) | .753 |
| Age, y | 64 (53–69) | 58 (45–61) | 60 (52–68) | .068 |
| Race and ethnicity | >.999 | |||
| Non-Hispanic White | 35 (71) | 11 (73) | 46 (72) | |
| Hispanic | 9 (18) | 3 (20) | 12 (19) | |
| African American | 2 (4) | 1 (7) | 3 (5) | |
| Asian | 3 (6) | 0 (0) | 3 (5) | |
| Body mass index | 27 (25–31) | 26 (20–33) | 27 (24–31) | .379 |
| Smoking | >.999 | |||
| None | 27 (55) | 8 (53) | 35 (55) | |
| Current | 5 (10) | 1 (7) | 6 (9) | |
| Former | 17 (35) | 6 (40) | 23 (36) | |
| Alcohol | .043 | |||
| None | 30 (61) | 14 (93) | 44 (69) | |
| Current | 18 (37) | 1 (7) | 19 (30) | |
| Former | 1 (2) | 0 (0) | 1 (2) | |
| Malignancy type | .473 | |||
| Hematologic | 31 (63) | 11 (73) | 42 (66) | |
| Solid | 18 (37) | 4 (27) | 22 (34) | |
| Active malignancy status at diagnosis of PL | 45 (92) | 13 (87) | 58 (91) | .618 |
| Active chemotherapya | 31 (63) | 12 (80) | 43 (67) | .348 |
| Corticosteroid use | 10 (20) | 5 (33) | 15 (23) | .315 |
| History of HSCT | 14 (29) | 6 (40) | 20 (31) | .526 |
| Type of HSCT | .778 | |||
| Allogenic | 9/14 (64) | 4/6 (67) | 13/20 (65) | |
| Autologous | 5/14 (36) | 2/6 (33) | 7/20 (35) | |
| Graft-vs-host disease | 5/9 (56) | 2/4 (50) | 7/13 (54) | >.999 |
| Other underlying risk factors | ||||
| Diabetes | 9 (18) | 1 (7) | 10 (16) | .429 |
| Chronic lung disease | 6 (12) | 1 (7) | 7 (11) | >.999 |
| Chronic kidney disease | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Chronic heart failure | 2 (4) | 0 (0) | 2 (3) | >.999 |
| History of splenectomy | 1 (2) | 0 (0) | 1 (2) | >.999 |
| Neutropenia at diagnosis, neutrophils/µL | 10 (20) | 6 (40) | 16 (25) | .173 |
| 500–1000 | 2 (4) | 2 (13) | 4 (6) | |
| 100–500 | 5 (10) | 3 (20) | 8 (13) | |
| <100 | 3 (6) | 1 (7) | 4 (6) | |
| Lymphopenia at diagnosis, lymphocytes/µL | 38 (78) | 10 (67) | 48 (75) | .498 |
| 500–1000 | 4 (8) | 0 (0) | 4 (6) | |
| 100–500 | 28 (57) | 8 (53) | 36 (56) | |
| <100 | 6 (12) | 2 (13) | 8 (13) | |
| Clinical presentation of PL | ||||
| Cough | 42 (86) | 12 (80) | 54 (84) | .687 |
| Fever | 40 (82) | 12 (80) | 52 (81) | >.999 |
| Dyspnea | 32 (65) | 12 (80) | 44 (69) | .354 |
| Hypoxia | 31 (63) | 11 (73) | 42 (66) | .473 |
| Headache | 8 (16) | 0 (0) | 8 (13) | .181 |
| Diarrhea | 8 (16) | 0 (0) | 8 (13) | .181 |
| Nausea/vomiting | 6 (12) | 0 (0) | 6 (9) | .322 |
| Confusion | 5 (10) | 1 (7) | 6 (9) | >.999 |
| Pleuritic chest pain | 5 (10) | 0 (0) | 5 (8) | .329 |
| Clinical laboratory findings | ||||
| Elevated liver enzyme | 27 (55) | 10 (67) | 37 (58) | .427 |
| Hyponatremia | 15 (31) | 10 (67) | 25 (39) | .012 |
| Acute kidney injury | 11 (22) | 6 (40) | 17 (27) | .197 |
| Computed tomography findingsb | 47 | 12 | 59 | |
| Airspace consolidation | 42 (89) | 11 (92) | 53 (90) | >.999 |
| Ground glass opacities | 34 (72) | 10 (83) | 44 (75) | .712 |
| Multilobar infiltrate | 32 (68) | 10 (83) | 42 (71) | .478 |
| Adenopathy | 30 (64) | 8 (67) | 38 (64) | >.999 |
| Nodules | 30 (64) | 6 (50) | 36 (61) | .510 |
| Pleural effusions | 26 (55) | 8 (67) | 34 (58) | .478 |
| Mass-like consolidation | 13 (28) | 4 (33) | 17 (29) | .729 |
| Halo sign | 5 (11) | 0 (0) | 5 (9) | .573 |
| Cavitation | 3 (6) | 0 (0) | 3 (5) | >.999 |
| Reversed halo sign | 2 (4) | 0 (0) | 2 (3) | >.999 |
| Bilateral | 27 (57) | 11 (92) | 38 (64) | .041 |
| Legionella type | .740 | |||
| L pneumophila | 35 (71) | 12 (80) | 47 (73) | |
| Legionella, not pneumophila | 14 (29) | 3 (20) | 17 (27) | |
| Coinfectionsc | 16 (33) | 4 (27) | 20 (31) | .759 |
| Bacterial | 7 (14) | 2 (13) | 9 (14) | >.999 |
| Viral | 9 (18) | 2 (13) | 11 (17) | >.999 |
| Fungal | 1 (2) | 2 (13) | 3 (5) | .134 |
| SOFA at PL diagnosis | 3 (2–5) | 6 (4–7) | 4 (2–6) | .051 |
| Time to PL diagnosis from admission, median (range) | 0 (1–13) | 2 (0–10) | 2.5 (0–13) | .055 |
| Infectious diseases consultation | 46 (94) | 13 (87) | 59 (92) | .583 |
| Treatment for PLd | .487 | |||
| Monotherapy | 35 (71) | 12/14 (86) | 47/63 (75) | |
| Combination | 14 (29) | 2/14 (14) | 16/63 (25) | |
| Levofloxacin-containing regimen | 30 (61) | 10 (67) | 40 (63) | .546 |
| Time to initiation of appropriate antibioticsd from admission, d | 0 (0–1) | 0 (0–1) | 0 (0–1) | .268 |
| Intensive care unit stay within 1 wk of PL diagnosis | 6 (12) | 11 (73) | 17 (27) | <.0001 |
Data are presented as No. (%) and median (IQR) unless noted otherwise. Bold P values indicate P < .05.
Abbreviations: HSCT, hematopoietic stem cell transplantation; PL, pulmonary legionellosis; SOFA, Sequential Organ Failure Assessment.
aActive chemotherapies were administered within 90 days of PL diagnosis.
bComputed tomography findings were obtained within 7 days before or after PL diagnosis.
cThree patients had >1 category of copathogens (2 with bacteria and virus, 1 with virus and fungus).
dAntibiotics having activity against Legionella, including levofloxacin, azithromycin, and doxycycline.
Cough (84%), fever (81%), dyspnea (69%), and hypoxia (66%) were common. Headache (13%), diarrhea (13%), nausea/vomiting (9%), and confusion (9%) were less frequent. Hyponatremia and acute kidney injury were observed in 39% and 27% of cases, respectively. Elevated liver enzymes were common (58%). The commonest computed tomography findings were airspace consolidation (90%), ground glass opacities (75%), and multilobar infiltrates (71%). Bilateral lung involvement was also frequent (64%).
Forty-seven PL cases (73%) were caused by L pneumophila (Figure 1A). The commonest non-pneumophila species isolated were L longbeachae (6%) and L anisa (5%). Notably, the proportion of non-pneumophila species was 34%. The median time to diagnosis of PL was 2.5 days overall (range, 0–13); it was shorter for L pneumophila than for non-pneumophila species (median, 2 vs 13 days; P < .01).
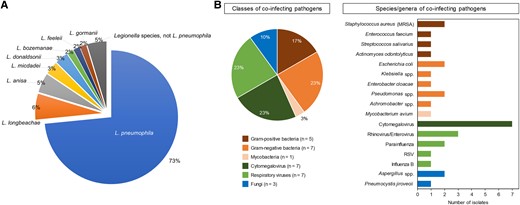
A, Types of Legionella spp. B, Types of coinfections (20 patients with 30 copathogens isolated). MRSA, methicillin-resistant Staphylococcus aureus; RSV, respiratory syncytial virus.
Twenty patients (31%) had coinfections: bacterial (14%, mainly gram-negative rods), viral (17%, mainly respiratory viruses and cytomegalovirus), and fungal (5%; Figure 1B). Of those, 5 patients (25%) had >1 copathogen. We compared the clinical characteristics and outcomes of patients with and without lung coinfections (Supplementary Table 2). No significant differences between the cohorts were found, except for younger age (median, 56 vs 64 years; P = .034), a higher proportion of Hispanic patients (40% vs 9%, P = .016), and patients with a history of hematopoietic stem cell transplantation (55% vs 20%, P = .009) in the coinfection group. Of note, the presence of coinfections did not significantly affect 30-day mortality (P = .759).
We further compared the clinical characteristics and outcomes of patients with hematologic malignancies and solid tumors (Supplementary Table 3). A higher Sequential Organ Failure Assessment (SOFA) score was found in the hematologic malignancy group as compared with the solid tumor group (median, 5 vs 3; P = .001).
All but 1 patient received appropriate empiric antibiotics with activity against Legionella species after admission (median, 0 days; range, 0–7). Forty-seven patients (73%) were treated with monotherapy, including fluoroquinolones (n = 33), azithromycin (n = 9), or tetracyclines (n = 5). The remaining 16 patients (25%) received antimicrobial combination therapy, consisting of fluoroquinolone and azithromycin (n = 7), fluoroquinolone and tetracycline (n = 7), or azithromycin and tetracycline (n = 2).
Fifteen patients (23%) died within 30 days after PL diagnosis. In univariate analysis, hyponatremia (P = .012), bilateral lung involvement (P = .041), and intensive care unit admission within 1 week of PL diagnosis (P < .0001) were significantly associated with 30-day mortality (Table 1). In contrast, malignancy type (P = .473), Legionella species (P = .740), neutropenia (P = .173) or lymphopenia (P = .498) at PL diagnosis, coinfections (P = .759), levofloxacin-containing regimen (P = .546), and combination therapy (P = .487) did not significantly affect 30-day mortality. Multivariable analysis identified the following as independent risk factors for 30-day mortality: hyponatremia (adjusted odds ratio [aOR], 3.03; 95% CI, 1.21–10.43; P = .013), bilateral lung involvement (aOR, 3.78; 95% CI, 1.16–28.98; P = .019), and SOFA score ≥5 at PL diagnosis (aOR, 2.76; 95% CI, 1.08–9.44; P = .029; Supplementary Table 4).
Finally, we compared the clinical characteristics and outcomes of patients with L pneumophila and non-pneumophila species (Supplementary Table 5). Interestingly, a higher proportion of viral coinfection was found in the non-pneumophila group as compared with the pneumophila group (24% vs 4%, P = .038). Patients with non-pneumophila species were more likely to receive antibiotic combination therapy than those with L pneumophila (47% vs 17%, P = .010). Thirty-day mortality was comparable between patients with non-pneumophila species and those with L pneumophila (18% vs 26%, P = .740), although the time to appropriate empiric treatment after admission for the non-pneumophila group (median, 1 day; IQR, 0–4) was longer than for the pneumophila group (median, 0 days; IQR, 0–1; P = .007).
DISCUSSION
To our knowledge, this study represents one of the largest contemporary cohorts of patients with cancer and PL. Despite appropriate empiric antimicrobial treatment, the high mortality rate of 23% aligns with previous studies and underscores the persistence of poor outcomes of PL in this vulnerable population [1, 2]. Thirty-nine percent of our patients had hyponatremia; however, it is unclear if this is a differentiating feature of PL in our population, because hyponatremia is commonly observed in patients with various malignancies [9]. Moreover, it is commonly associated with PL in patients with community-acquired pneumonia [10]. Although a combination of inappropriate antidiuretic hormone secretion, interstitial nephritis, and Fanconi syndrome has been described as a cause of hyponatremia in patients with PL [11, 12], the detailed pathophysiologic mechanisms have never been conclusively identified [13, 14].
We also identified hyponatremia, bilateral lung involvement, and a SOFA score >5 as poor prognostic factors. Given that hyponatremia has been associated with the severity of PL [15], it may be a marker for increased mortality. However, hyponatremia as prognostic factor in PL is questionable, as low serum sodium is a well-recognized poor prognostic factor in patients with malignancies [9]. The increased mortality of patients with bilateral lung involvement aligns with previous research on community-acquired pneumonia [16].
Notably, our study identified a high prevalence of lung coinfections in patients with cancer and PL (31%). This observation has implications for clinical management of PL, as coinfections such as gram-negative bacteria, respiratory viruses, and cytomegalovirus add complexity to the diagnosis and assessment of treatment response in PL. Although the frequent coinfections could reflect the severe net state of immunosuppression in our patients, Legionella is known to have pleiotropic effects on pulmonary and systemic host immune defense [17], including epithelial damage, changes in airway function, airway leakage, and delayed tissue repair, as well as altered cellular recognition, inflammatory responses, and immune cell recruitment to the airways [18, 19]. Whether these pathophysiologic changes could predispose patients to excess coinfections remains unclear.
As is the case in patients without cancer and consistent with previous reports [2, 4, 6], L pneumophila serogroup 1 was the predominant Legionella species in our cohort of patients with cancer and can be readily identified with UATs. In contrast, the majority of UATs cannot detect serogroups of L pneumophila other than serogroup 1 and non-pneumophila species [20]. We found that 25% of patients with PL had non-pneumophila species, underscoring a need for clinicians to consider further workup with culture or molecular techniques if PL is a consideration [20]. This is especially important in high-risk cases with hematologic malignancies, as previous research suggested a significantly higher proportion of non-pneumophila PL in patients with hematologic malignancies [6]. Consistent with our previous institutional cohort [2], the mortality associated with non-pneumophila species was similar to that of the L pneumophila group. However, the time to appropriate treatment was longer for the non-pneumophila group than for L pneumophila, likely due to delayed diagnosis.
Furthermore, the observation that the majority of patients (59%) had a low SOFA score challenges the classical view of PL as a severe form of community-acquired pneumonia, suggesting that immunosuppression might modulate the inflammatory drivers of presentation, leading to milder clinical manifestations than traditionally expected, although the underlying mechanisms remain unclear.
Our study has several limitations. First, its single-center nature may affect the generalizability of our findings. Second, not all patients underwent bronchoalveolar lavage, which could have affected the detection of copathogens. Third, this study arises from the potential selection bias present in our data set, which was skewed toward more severe cases of PL, due to the exclusion of outpatients who were empirically treated with quinolones or macrolides without a diagnostic workup. Fourth, we acknowledge that true copathogens and colonizers cannot always be distinguished reliably in a retrospective study. Although some pathogens, such as Actinomyces, viridans group streptococci, and Enterococcus, might be bystanders instead of true copathogens, we included these as copathogens based on reports that they were causative pathogens in patients with cancer [21–23]. Last, given the diagnostic gap of UAT, PL due to non-pneumophila species has likely been underdiagnosed and thus underrepresented in this study, as bronchoalveolar lavage or sputum studies were not routinely performed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. P. K. was responsible for study concept and design. T. M. and J. T. were responsible for acquisition of the data. T. M. wrote the first draft of the manuscript. Y. J. contributed to the data analysis. S. W. and S. E. E. provided critical comments. All authors were responsible for interpretation of the results and revision of the manuscript.
Previous presentation. Parts of our results were presented during a poster presentation at IDWeek, 19–23 October 2023, Washington, DC.
Financial support. This work was supported by the Robert C. Hickey Chair in Clinical Care endowment (to D. P. K).
References
Author notes
Potential conflicts of interest. D. P. K. reports honoraria and research support from Gilead Sciences and Astellas Pharma. He also received consultant fees from Astellas Pharma, Merck, and Gilead Sciences and is a member of the Data Review Committee for Cidara Therapeutics, AbbVie, Scynexis, and the Mycoses Study Group. All other authors report no potential conflicts.





Comments