-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth Bell, Jennifer Pisano, Manasa Brown, Daniel Friedman, An Unexpectedly High Incidence of Invasive Fungal Diseases in Solid Organ Transplant Recipients Taking Belatacept for Organ Rejection Prophylaxis: A Single-Center Retrospective Cohort Study, Open Forum Infectious Diseases, Volume 11, Issue 6, June 2024, ofae158, https://doi.org/10.1093/ofid/ofae158
Close - Share Icon Share
Abstract
Among solid organ transplant recipients taking belatacept, 15% developed invasive fungal diseases. The most common invasive fungal diseases were aspergillosis (56%) and candidiasis (22%). The infected cohort was more likely to receive basiliximab, undergo lung transplantation, or identify as White. Higher rates of aspergillosis were seen in this lung cohort than previously reported.
Solid organ transplantation (SOT) is an important option for patients with end-stage organ dysfunction. Despite advances in patient selection, surgical technique, and patient education, opportunistic infections, particularly invasive fungal diseases (IFDs), remain serious causes of morbidity and mortality following transplantation [1, 2]. Though variable, the overall incidence of IFD in the first year post-SOT is 3.1%, with lung transplantation having a much higher incidence (8.6%) and kidney recipients having the lowest incidence (1.3%) [2].
Belatacept is a CTLA-4 inhibitor (cytotoxic T lymphocyte–associated antigen 4) that diminishes immune response by blocking the stimulation of T cells. Belatacept was initially approved for organ rejection prophylaxis in kidney recipients and has become an essential alternative to calcineurin inhibitors (CNIs), given its lower risk of nephrotoxicity, decreased pill burden, and lack of therapeutic monitoring [3, 4]. Other organ programs are now adopting belatacept for similar reasons, in addition to its benefits in recipients who are highly sensitized [5–7].
Viral infections are well-characterized infectious adverse events related to belatacept use. Although data are conflicting, some studies have demonstrated higher incidences of viral infections with belatacept than CNIs, with one study being prematurely stopped due to increased mortality [4, 8–12]. The prevalence of fungal infections in this population is less well studied. One small study documented a 4%–15% range of fungal infections on belatacept-containing regimens, and there are several case reports of cutaneous fungal infections and invasive mycoses [10, 11, 13]. Perceiving a higher frequency of IFD in this population at our transplant center, we conducted this study to better evaluate the epidemiology of IFDs in SOT recipients receiving belatacept.
METHODS
We conducted a retrospective cohort analysis of all adult SOT recipients who received at least 1 dose of belatacept between 1 June 2011 and 30 September 2022 at the University of Chicago Medical Center. This start date corresponds to belatacept’s approval by the Food and Drug Administration. Patients were identified by ICD-10 codes corresponding to SOT procedures and cross-referenced with pharmacy medication administrative records. Chart review was performed to confirm the presence of IFD based on the most recent consensus definition, including the probability of diagnosis [14–16], and to collect patient characteristics, therapeutics, and outcomes. Induction therapy was classified as lymphocyte depleting if the patient received alemtuzumab or antithymocyte globulin. Concomitant immunosuppression was defined as immunosuppression received in the 6 months preceding fungal infection (if present), study conclusion, or death (if before 30 September 22). Rejection in the last year was defined as 1 year preceding IFD, study conclusion, or death as applicable. Patient data were deidentified and stored in REDCap [17, 18]. We compared cases of IFD with those without IFD after receipt of belatacept.
Categorical variables are expressed as percentages and compared by χ2 or Fisher exact test. Continuous variables are expressed as mean (SD) or median (IQR) and are compared by Student t test. A 2-sided P < .05 was considered significant. Statistics were calculated with Stata version 17 (StataCorp).
RESULTS
A total of 215 SOT recipients received at least 1 dose of belatacept during the study period, and 33 patients (15.3%) developed 36 IFDs. The most common IFDs were invasive aspergillosis (n = 20, 56%) and invasive candidiasis (n = 8, 22%; Figure 1). Most cases of aspergillosis were pulmonary infections (n = 18, 90%), though 1 patient developed peritonitis and 1 developed sinusitis with concomitant skull base osteomyelitis and endophthalmitis. The latter 2 were mixed fungal infections with Candida; however, Aspergillus was favored to be the driving pathogen and thus counted as such. Lung transplant recipients (LTRs) accounted for most aspergillosis cases (17 patients), though 1 received simultaneous heart and kidney transplants and 1 was a kidney recipient. Two invasive candidiasis cases were candidemia, 3 were esophageal candidiasis, and 3 were candida osteomyelitis. Eighteen (50%) cases were proven IFDs, 8 (22%) were probable, and 10 (27%) were possible. The median time to diagnosis was 19.8 months (IQR, 8.6–93.8) from transplant.
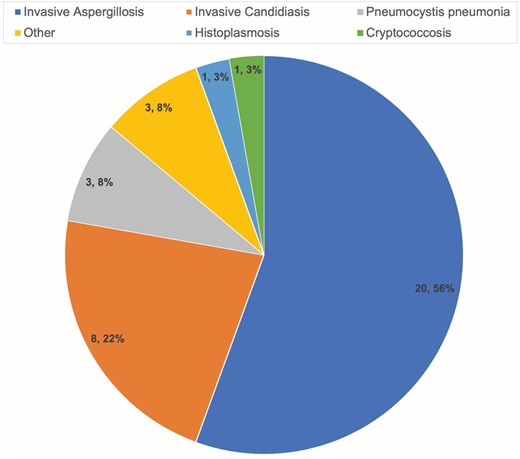
Thirty-three patients developed 36 distinct invasive fungal diseases, delineated by diagnosis.
A total of 57 (27%) patients died during the review period, 17 (30%) of whom were diagnosed with at least 1 IFD. Eleven (33%) had complete resolution of their IFDs on follow-up, and 2 (66%) who developed a second IFD had complete resolution. Seven patients were undergoing systemic antifungal prophylaxis at the time of initial diagnosis. Patients with IFD had a normal mean leukocyte count of 8.5 × 103/μL and mean absolute neutrophil count of 6.7 × 103/μL at the time of diagnosis, though 2 were severely neutropenic. Most were lymphopenic, with a mean absolute lymphocyte count of 0.76 × 103/μL.
Table 1 summarizes the characteristics of SOT recipients by group. Of those with IFDs, there was a significantly higher proportion of White patients (67% vs 34%, P < .001) and lower proportion of Black patients (30% vs 56%, P = .006) as compared with those without infection. The transplanted organ was more commonly lung for patients with IFDs (70% vs 18%, P < .001) and less commonly kidney (24% vs 66%, P < .001). There was a trend toward higher rates of recent rejection in those with IFD vs those without (30% vs 17%, P = .076). A total of 28 patients underwent retransplantation, with a similar likelihood of developing an IFD (12% vs 13%, P = .867).
| . | Infected (n = 33) . | Noninfected (n = 182) . | P Value . |
|---|---|---|---|
| Age, y, mean (SD) | 60.4 (10.0) | 55.5 (13.6) | .018 |
| Female sex | 14 (42) | 70 (38) | .668 |
| Comorbidity | |||
| Diabetes mellitus, type 1 or 2 | 10 (30) | 72 (40) | .314 |
| Rheumatologic autoimmune condition | 7 (21) | 19 (10) | .081 |
| Person living with HIV | 0 | 3 (2) | .458 |
| Obesity | 3 (9) | 65 (36) | .002 |
| Malignancy, active or in remissiona | 5 (15) | 26 (14) | .896 |
| Chronic obstructive pulmonary disease | 5 (15) | 12 (7) | .094 |
| End-stage renal disease | 3 (9) | 17 (9) | .964 |
| None | 8(24) | 32 (18) | .366 |
| Race | .006 | ||
| Asian | 1 (3) | 5 (3) | .928 |
| Black/African American | 10 (30) | 102 (56) | .006 |
| White/Caucasian | 22 (67) | 61 (34) | <.001 |
| >1 race | 0 | 12 (7) | .129 |
| Other | 0 | 2 (1) | .545 |
| Transplanted organ | <.001 | ||
| Lungb | 23 (70) | 33 (18) | <.001 |
| Heart | 1 (3) | 20 (11) | .157 |
| Kidney | 8 (24) | 121 (66) | <.001 |
| Simultaneous heart/kidney | 1 (3) | 4 (2) | .770 |
| Simultaneous pancreas/kidney | 0 | 3 (2) | .458 |
| Simultaneous heart/liver | 0 | 1 (0.6) | .670 |
| Retransplanted | 4 (12) | 24 (13) | .867 |
| Rejection in last 1 yc | 10 (30) | 30 (17) | .076 |
| Induction therapy | <.001 | ||
| Lymphocyte depleting | 3 (9) | 80 (44) | <.001 |
| Basiliximab | 25 (76) | 64 (35) | <.001 |
| Combination therapyd | 1 (3) | 8 (4) | .719 |
| None | 0 | 6 (3) | .290 |
| Other, including unknown | 4 (12) | 24(13) | .867 |
| Immunosuppressione | |||
| Belatacept-containing regimen | 23 (70) | 129 (71) | .891 |
| CNI-containing regimen | 25 (76) | 94 (52) | .010 |
| Corticosteroid-containing regimen | 32 (97) | 170 (93) | .429 |
| Antimetabolite-containing regimen | 21 (64) | 122 (67) | .704 |
| mTOR-containing regimen | 3 (9) | 13 (7) | .695 |
| 3 concomitant immunosuppressants | 17 (52) | 126 (69) | .047 |
| 4 concomitant immunosuppressants | 11 (33) | 30 (16) | .023 |
| 5 concomitant immunosuppressants | 0 | 3 (2) | .458 |
| Median total belatacept doses, No. (IQR) | 9 (4–27) | 13 (4–40) | .139 |
| . | Infected (n = 33) . | Noninfected (n = 182) . | P Value . |
|---|---|---|---|
| Age, y, mean (SD) | 60.4 (10.0) | 55.5 (13.6) | .018 |
| Female sex | 14 (42) | 70 (38) | .668 |
| Comorbidity | |||
| Diabetes mellitus, type 1 or 2 | 10 (30) | 72 (40) | .314 |
| Rheumatologic autoimmune condition | 7 (21) | 19 (10) | .081 |
| Person living with HIV | 0 | 3 (2) | .458 |
| Obesity | 3 (9) | 65 (36) | .002 |
| Malignancy, active or in remissiona | 5 (15) | 26 (14) | .896 |
| Chronic obstructive pulmonary disease | 5 (15) | 12 (7) | .094 |
| End-stage renal disease | 3 (9) | 17 (9) | .964 |
| None | 8(24) | 32 (18) | .366 |
| Race | .006 | ||
| Asian | 1 (3) | 5 (3) | .928 |
| Black/African American | 10 (30) | 102 (56) | .006 |
| White/Caucasian | 22 (67) | 61 (34) | <.001 |
| >1 race | 0 | 12 (7) | .129 |
| Other | 0 | 2 (1) | .545 |
| Transplanted organ | <.001 | ||
| Lungb | 23 (70) | 33 (18) | <.001 |
| Heart | 1 (3) | 20 (11) | .157 |
| Kidney | 8 (24) | 121 (66) | <.001 |
| Simultaneous heart/kidney | 1 (3) | 4 (2) | .770 |
| Simultaneous pancreas/kidney | 0 | 3 (2) | .458 |
| Simultaneous heart/liver | 0 | 1 (0.6) | .670 |
| Retransplanted | 4 (12) | 24 (13) | .867 |
| Rejection in last 1 yc | 10 (30) | 30 (17) | .076 |
| Induction therapy | <.001 | ||
| Lymphocyte depleting | 3 (9) | 80 (44) | <.001 |
| Basiliximab | 25 (76) | 64 (35) | <.001 |
| Combination therapyd | 1 (3) | 8 (4) | .719 |
| None | 0 | 6 (3) | .290 |
| Other, including unknown | 4 (12) | 24(13) | .867 |
| Immunosuppressione | |||
| Belatacept-containing regimen | 23 (70) | 129 (71) | .891 |
| CNI-containing regimen | 25 (76) | 94 (52) | .010 |
| Corticosteroid-containing regimen | 32 (97) | 170 (93) | .429 |
| Antimetabolite-containing regimen | 21 (64) | 122 (67) | .704 |
| mTOR-containing regimen | 3 (9) | 13 (7) | .695 |
| 3 concomitant immunosuppressants | 17 (52) | 126 (69) | .047 |
| 4 concomitant immunosuppressants | 11 (33) | 30 (16) | .023 |
| 5 concomitant immunosuppressants | 0 | 3 (2) | .458 |
| Median total belatacept doses, No. (IQR) | 9 (4–27) | 13 (4–40) | .139 |
Data are presented as No. (%) unless noted otherwise.
Abbreviations: CNI, calcineurin inhibitor; mTOR, mammalian target of rapamycin.
aMalignancy included all solid and hematologic malignancies, active and in remission.
bOne patient received a lung transplant and then years later received a kidney transplant and is counted as a lung transplant here.
cLast 1 year preceding infection, death, or study conclusion as applicable.
dCombination induction therapy included 2 patients with basiliximab and antithymocyte globulin, 6 with antithymocyte globulin and another agent (eg, intravenous immunoglobulin or belatacept), and 1 with basiliximab and belatacept.
eImmunosuppression used in the 6 months preceding infection, study conclusion, or death, as applicable. Antimetabolite regimen includes azathioprine, mycophenolate mofetil, or mycophenolic acid. CNI regimen includes tacrolimus and cyclosporine. mTOR inhibitors include sirolimus and everolimus.
| . | Infected (n = 33) . | Noninfected (n = 182) . | P Value . |
|---|---|---|---|
| Age, y, mean (SD) | 60.4 (10.0) | 55.5 (13.6) | .018 |
| Female sex | 14 (42) | 70 (38) | .668 |
| Comorbidity | |||
| Diabetes mellitus, type 1 or 2 | 10 (30) | 72 (40) | .314 |
| Rheumatologic autoimmune condition | 7 (21) | 19 (10) | .081 |
| Person living with HIV | 0 | 3 (2) | .458 |
| Obesity | 3 (9) | 65 (36) | .002 |
| Malignancy, active or in remissiona | 5 (15) | 26 (14) | .896 |
| Chronic obstructive pulmonary disease | 5 (15) | 12 (7) | .094 |
| End-stage renal disease | 3 (9) | 17 (9) | .964 |
| None | 8(24) | 32 (18) | .366 |
| Race | .006 | ||
| Asian | 1 (3) | 5 (3) | .928 |
| Black/African American | 10 (30) | 102 (56) | .006 |
| White/Caucasian | 22 (67) | 61 (34) | <.001 |
| >1 race | 0 | 12 (7) | .129 |
| Other | 0 | 2 (1) | .545 |
| Transplanted organ | <.001 | ||
| Lungb | 23 (70) | 33 (18) | <.001 |
| Heart | 1 (3) | 20 (11) | .157 |
| Kidney | 8 (24) | 121 (66) | <.001 |
| Simultaneous heart/kidney | 1 (3) | 4 (2) | .770 |
| Simultaneous pancreas/kidney | 0 | 3 (2) | .458 |
| Simultaneous heart/liver | 0 | 1 (0.6) | .670 |
| Retransplanted | 4 (12) | 24 (13) | .867 |
| Rejection in last 1 yc | 10 (30) | 30 (17) | .076 |
| Induction therapy | <.001 | ||
| Lymphocyte depleting | 3 (9) | 80 (44) | <.001 |
| Basiliximab | 25 (76) | 64 (35) | <.001 |
| Combination therapyd | 1 (3) | 8 (4) | .719 |
| None | 0 | 6 (3) | .290 |
| Other, including unknown | 4 (12) | 24(13) | .867 |
| Immunosuppressione | |||
| Belatacept-containing regimen | 23 (70) | 129 (71) | .891 |
| CNI-containing regimen | 25 (76) | 94 (52) | .010 |
| Corticosteroid-containing regimen | 32 (97) | 170 (93) | .429 |
| Antimetabolite-containing regimen | 21 (64) | 122 (67) | .704 |
| mTOR-containing regimen | 3 (9) | 13 (7) | .695 |
| 3 concomitant immunosuppressants | 17 (52) | 126 (69) | .047 |
| 4 concomitant immunosuppressants | 11 (33) | 30 (16) | .023 |
| 5 concomitant immunosuppressants | 0 | 3 (2) | .458 |
| Median total belatacept doses, No. (IQR) | 9 (4–27) | 13 (4–40) | .139 |
| . | Infected (n = 33) . | Noninfected (n = 182) . | P Value . |
|---|---|---|---|
| Age, y, mean (SD) | 60.4 (10.0) | 55.5 (13.6) | .018 |
| Female sex | 14 (42) | 70 (38) | .668 |
| Comorbidity | |||
| Diabetes mellitus, type 1 or 2 | 10 (30) | 72 (40) | .314 |
| Rheumatologic autoimmune condition | 7 (21) | 19 (10) | .081 |
| Person living with HIV | 0 | 3 (2) | .458 |
| Obesity | 3 (9) | 65 (36) | .002 |
| Malignancy, active or in remissiona | 5 (15) | 26 (14) | .896 |
| Chronic obstructive pulmonary disease | 5 (15) | 12 (7) | .094 |
| End-stage renal disease | 3 (9) | 17 (9) | .964 |
| None | 8(24) | 32 (18) | .366 |
| Race | .006 | ||
| Asian | 1 (3) | 5 (3) | .928 |
| Black/African American | 10 (30) | 102 (56) | .006 |
| White/Caucasian | 22 (67) | 61 (34) | <.001 |
| >1 race | 0 | 12 (7) | .129 |
| Other | 0 | 2 (1) | .545 |
| Transplanted organ | <.001 | ||
| Lungb | 23 (70) | 33 (18) | <.001 |
| Heart | 1 (3) | 20 (11) | .157 |
| Kidney | 8 (24) | 121 (66) | <.001 |
| Simultaneous heart/kidney | 1 (3) | 4 (2) | .770 |
| Simultaneous pancreas/kidney | 0 | 3 (2) | .458 |
| Simultaneous heart/liver | 0 | 1 (0.6) | .670 |
| Retransplanted | 4 (12) | 24 (13) | .867 |
| Rejection in last 1 yc | 10 (30) | 30 (17) | .076 |
| Induction therapy | <.001 | ||
| Lymphocyte depleting | 3 (9) | 80 (44) | <.001 |
| Basiliximab | 25 (76) | 64 (35) | <.001 |
| Combination therapyd | 1 (3) | 8 (4) | .719 |
| None | 0 | 6 (3) | .290 |
| Other, including unknown | 4 (12) | 24(13) | .867 |
| Immunosuppressione | |||
| Belatacept-containing regimen | 23 (70) | 129 (71) | .891 |
| CNI-containing regimen | 25 (76) | 94 (52) | .010 |
| Corticosteroid-containing regimen | 32 (97) | 170 (93) | .429 |
| Antimetabolite-containing regimen | 21 (64) | 122 (67) | .704 |
| mTOR-containing regimen | 3 (9) | 13 (7) | .695 |
| 3 concomitant immunosuppressants | 17 (52) | 126 (69) | .047 |
| 4 concomitant immunosuppressants | 11 (33) | 30 (16) | .023 |
| 5 concomitant immunosuppressants | 0 | 3 (2) | .458 |
| Median total belatacept doses, No. (IQR) | 9 (4–27) | 13 (4–40) | .139 |
Data are presented as No. (%) unless noted otherwise.
Abbreviations: CNI, calcineurin inhibitor; mTOR, mammalian target of rapamycin.
aMalignancy included all solid and hematologic malignancies, active and in remission.
bOne patient received a lung transplant and then years later received a kidney transplant and is counted as a lung transplant here.
cLast 1 year preceding infection, death, or study conclusion as applicable.
dCombination induction therapy included 2 patients with basiliximab and antithymocyte globulin, 6 with antithymocyte globulin and another agent (eg, intravenous immunoglobulin or belatacept), and 1 with basiliximab and belatacept.
eImmunosuppression used in the 6 months preceding infection, study conclusion, or death, as applicable. Antimetabolite regimen includes azathioprine, mycophenolate mofetil, or mycophenolic acid. CNI regimen includes tacrolimus and cyclosporine. mTOR inhibitors include sirolimus and everolimus.
The highest rates of IFD were seen with basiliximab as induction therapy (76% vs 35%, P < .001). CNI-containing regimens were more common in those with IFD than those without (76% vs 52%, P = .010). Although all patients received at least 1 dose of belatacept in our cohort, about 71% were still taking belatacept at the time of evaluation (infection, death, or study conclusion); 94% of whom were taking concomitant steroids, 68% concomitant CNI, and 53% concomitant mycophenolate. Most patients, with and without IFD, were taking 3 concomitant immunosuppressants at the time of evaluation (52% vs 69%, P = .047). More patients with IFD were receiving 4 concomitant immunosuppressants vs those without (33% vs 16%, P = .023). For those with IFD, the median (IQR) number of total belatacept doses received was 9 (4–27), and the median number of doses received prior to onset of IFD was 6 (2–12). For patients who were uninfected, the median number of doses was 13 (4–40). Most patients in our cohort started taking belatacept due to CNI toxicity (n = 148, 69%), which allowed patients to either discontinue CNI therapy or accommodate lower CNI troughs. Nineteen (9%) patients started taking belatacept for antirejection or desensitization purposes.
DISCUSSION
In our cohort of SOT recipients, 15% of patients were diagnosed with an IFD after receiving at least 1 dose of belatacept, most of whom (70%) were LTRs. Furthermore, 41% of all LTRs in our cohort were diagnosed with an IFD. While LTRs are known to be at heightened risk for pulmonary IFD due to the constant exposure of the allograft with the environment, reported rates of invasive aspergillosis are around 6% on average [1], approximately 7 times lower than that of our cohort.
With rare exception at our institution, LTRs receive basiliximab as induction therapy; this likely explains the higher incidence of IFD associated with this induction regimen as compared with others. Similarly, the trend toward patients with chronic obstructive pulmonary disease being more likely to be infected is likely confounded by the higher infection risk of lung transplantation itself. Most LTRs in our cohort identified as White (60%), likely accounting for the higher rates of infection, as compared with most kidney transplant recipients identifying as Black (72%), likely accounting for the significantly lower incidence of IFD in these populations.
A major impact on SOT recipient infection risk is concomitant immunosuppression. Our cohort had a variety of regimens, including 18 distinct regimens among 33 infected patients alone. Though concomitant immunosuppression was measured at different times (6 months preceding infection, study conclusion, or death), overall the regimens were similar—the only exception being that those who developed infection had higher rates of CNI-containing regimens. Overlapping transitions of therapy during the 6-month time frame may partially explain this, as well as increasing the net state of immunosuppression by adding belatacept rather than replacing another medication. Though only 1 of 33 patients with IFD were switched to belatacept for desensitization in our cohort, our institution's protocol to incorporate belatacept in LTRs with worsening renal function to accommodate lower CNI goals still supports the idea of increasing patients’ overall immunosuppression. This likely explains the higher number of concomitant immunosuppressants in the infected group. In addition, several patients were prescribed belatacept due to significant acute kidney injuries during hospitalization, which may be a surrogate indicator of these patients’ overall morbidity.
There was a trend toward fewer total belatacept doses in the infected group. However, this is likely explained by survivorship bias, as a patient who developed an IFD after starting belatacept may have discontinued it or succumbed to the IFD itself.
Our study has several limitations. The retrospective nature, single center, and relatively small sample size limit the ability to quantify the effect of belatacept on developing IFD. However, this cohort provides some insight into the rates and type of fungal infections encountered among SOT recipients taking belatacept and that LTRs may need particular review due to the higher association with infection. Further investigation is needed to determine the true risk of developing IFDs with belatacept.
Notes
Author contributions. Study design: E. B. and D. F. Data analysis: E. B. Data collection: E. B. and M.B. Manuscript writing and decision to submit: all authors.
Patient consent statement. This study does not include factors necessitating patient consent.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts.





Comments