-
PDF
- Split View
-
Views
-
Cite
Cite
Nelson Iván Agudelo Higuita, Daniel B Chastain, Brian Scott, Syeda Sahra, Lilian Vargas Barahona, José Henao Cordero, Alfred L H Lee, Jose Tuells, Andrés F Henao-Martínez, Risk of Invasive Fungal Infections in Patients With Chronic Lymphocytic Leukemia Treated With Bruton Tyrosine Kinase Inhibitors: A Case-Control Propensity Score–Matched Analysis, Open Forum Infectious Diseases, Volume 11, Issue 6, June 2024, ofae115, https://doi.org/10.1093/ofid/ofae115
Close - Share Icon Share
Abstract
Prior reports have suggested a possible increase in the frequency of invasive fungal infections (IFIs) with use of a Bruton tyrosine kinase inhibitor (BTKi) for treatment of chronic lymphoid malignancies such as chronic lymphocytic leukemia (CLL), but precise estimates are lacking. We aim to characterize the prevalence of IFIs among patients with CLL, for whom a BTKi is now the first-line recommended therapy.
We queried TriNetX, a global research network database, to identify adult patients with CLL using the International Classification of Diseases, Tenth Revision code (C91.1) and laboratory results. We performed a case-control propensity score–matched analysis to determine IFIs events by BTKi use. We adjusted for age, sex, ethnicity, and clinical risk factors associated with an increased risk of IFIs.
Among 5358 matched patients with CLL, we found an incidence of 4.6% of IFIs in patients on a BTKi versus 3.5% among patients not on a BTKi at 5 years. Approximately 1% of patients with CLL developed an IFI while on a BTKi within this period. Our adjusted IFI event analysis found an elevated rate of Pneumocystis jirovecii pneumonia (PJP) (0.5% vs 0.3%, P = .02) and invasive candidiasis (3.5% vs 2.7%, P = .012) with the use of a BTKi. The number needed to harm for patients taking a BTKi was 120 and 358 for invasive candidiasis and PJP, respectively.
We found an adjusted elevated rate of PJP and invasive candidiasis with BTKi use. The rates are, however, low with a high number needed to harm. Additional studies stratifying other IFIs with specific BTKis are required to identify at-risk patients and preventive, cost-effective interventions.
Chronic lymphocytic leukemia (CLL) is the most common type of chronic leukemia in the United States (US), affecting approximately 200 000 people [1]. Novel and highly effective therapies targeting B-cell receptor (BCR) signaling pathways have revolutionized treatment and improved outcomes. Among these therapies, Bruton tyrosine kinase inhibitors (BTKis) irreversibly inhibit the Bruton tyrosine kinase, an enzyme involved in the BCR signaling pathway crucial in the pathogenesis of CLL [2].
The landmark study that led to the approval of ibrutinib, the first BTKi approved by the US Food and Drug Administration, did not identify an increased risk for invasive fungal infections (IFIs). However, subsequent reports emerged shortly after its approval, revealing atypical IFIs, such as central nervous system aspergillosis, initially in patients from Israel receiving concomitant glucocorticoids [3]. Moreover, observational data and postmarketing surveillance have corroborated an increased risk of severe infectious complications, including IFIs such as pulmonary and extrapulmonary aspergillosis, fusariosis, mucormycosis, cryptococcosis, and pneumocystosis [4–8].
A retrospective analysis of a case series of patients on ibrutinib found a 5% rate of IFIs, with Pneumocystis jirovecii pneumonia (PJP) accounting for 3.8% of cases [9]. Typically, IFIs associated with BTKi use manifest during the first 6 months of therapy, particularly in patients concurrently receiving glucocorticoids and those with a history of chemotherapy exposure [7]. Nevertheless, despite the reported increased risk for IFIs, the availability of high-quality data to determine their frequency and guide preventive strategies in this patient population remains limited.
Additionally, the underlying immunosuppression associated with CLL and the concomitant use of immunosuppressive medications confound the attribution of an increased risk of IFIs solely to treatment with a BTKi. Our study utilized a large multinational database to determine the prevalence of IFIs in patients with CLL treated with a BTKi and to identify associated risk factors for IFI development.
METHODS
Global Federated Research Network
We queried TriNetX, a global research network database (https://trinetx.com/), in August 2023 to identify adult patients with CLL based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) code (C91.1) and laboratory results over the previous decade. TriNetX datasets include clinical patient data such as demographics, diagnoses, procedures, laboratories, and medications—commonly referred to as real-world data. TriNetX has global data for approximately 100 million patients from >80 medical centers across the US, Canada, Europe, Australia, Indonesia, and various other countries. Our group has published several reports using the same methodology [10–13]. TriNetX, LLC complies with the Health Insurance Portability and Accountability Act (HIPAA), the US federal law protecting healthcare data’s privacy and security, and any additional data privacy regulations applicable to the contributing healthcare organization (HCO). Each HCO delivers electronic medical record systems data collected during patient care. Received data is either structured or unstructured data processed by Natural Language Processing Technology. Most participating HCOs are large academic medical institutions with inpatient and outpatient facilities, thereby offering comprehensive data representing the entire patient population under their care. Most HCOs provide an average of 7 years of historical data. TriNetX receives data directly from HCOs research repository into the TriNetX environment, or the HCO sends TriNetX data extracts in the form of CSV files coded in the TriNetX Data Dictionary. HCO and other data providers update their data at various times, with >80% refreshing in 1-, 2-, or 4-week frequency intervals. The average lag time for a HCOs source data to refresh is 1 month. TriNetX maps the data to a standard, controlled set of clinical terminologies and transforms it into a proprietary data model. This transformation process includes extensive data quality assessment that includes data cleaning that rejects records that do not meet the TriNetX quality standards. TriNetX is certified by the International Organization for Standardization (ISO) 27001:2013 standard and maintains an Information Security Management System to ensure the protection of the healthcare data it has access to and to meet the requirements of the HIPAA Security Rule.
Study Design and Population
The analysis comprised of a comparative assessment of the clinical characteristics between patients with CLL (identified through ICD-10 code C91.1 and a lymphocyte count ≥5.0 × 103 cells/µL) who were either on (n = 6548) or off (n = 36 164) a BTKi. We excluded individuals with any history of prior IFI within 3 months before the index event (Supplementary material). The earliest encounter was identified as the index event in patients with multiple encounters. Demographic characteristics, diagnoses, procedures, medications, complications, and measurements were captured without time constraints before the index event (eg, laboratory test results; see Supplementary Tables 1–4). Comorbidities were selected based on frequency and clinical importance. Results were reported after propensity score matching (PSM) was performed to control for differences between cases and controls. For the PSM analysis by BTKi use, we controlled for age, sex, ethnicity, human immunodeficiency virus (HIV) infection, chronic lower respiratory diseases, neoplasms, aplastic anemia, chronic kidney disease, systemic connective tissue disorders, CLL, transplant status, glucocorticoids (prednisone, dexamethasone, methylprednisolone, and prednisolone), immunosuppressants, antineoplastics, and trimethoprim-sulfamethoxazole. To estimate the 5-year relative risk of IFIs, we calculated the number needed to harm (NNH) using a previously described formula (NNH = 1 / [control event rate] − [experimental event rate]) [14].
Global Federated Research Network Outcome Measures
The primary outcome was the proportions of IFIs at 1 year and 5 years. Calculations of incidence rates were captured after PSM. The secondary outcomes included the need for hospitalization and mortality (Supplementary Table 4).
Statistical Analysis
All statistical analyses were conducted on the TriNetX platform. Descriptive statistics were presented as means and standard deviations for continuous variables and as frequency and proportions for categorical variables. Continuous data were compared using independent t tests, whereas categorical data were compared using χ2 or Fisher exact test, as appropriate. PSM was performed using a 1:1 greedy nearest-neighbor algorithm, utilizing a caliper width of 0.1 pooled standard deviation. Covariate balance comparing cases and controls was assessed using standardized mean difference, and absolute values >0.1 were considered positive for residual imbalance. The t test statistic compared the 2 cohorts after PSM to report differences between cohorts. A P value of <.05 was used to indicate statistical significance. Graphs were designed using GraphPad Prism version 8.0.0 for Windows software (GraphPad Software, San Diego, California).
Data Access
The corresponding author had full access to data in the study and had final responsibility for the decision to submit the manuscript for publication. The aggregated datasets generated and analyzed in the current study are available from the TriNetX platform with a subscription or through the corresponding author per a formal request.
Ethics Statement
Any data displayed on the TriNetX platform in aggregate form, or any patient-level data provided in a data set generated by the TriNetX platform, only contains de-identified data as per the de-identification standard defined in Section 164.514(a) of the HIPAA Privacy Rule. Geographic reporting at the regional level prevents potential reidentification through the localization of patients or HCOs. Research utilizing TriNetX does not require ethical approval because patient-identifiable information is not accessible to users. According to the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Denver, the current project is in HIPAA compliance. Analysis of clinical data was performed under an approved protocol (COMIRB Protocol 15-1340).
Patient Consent Statement
The study does not include factors necessitating patient consent.
RESULTS
Clinical Characteristics of Patients With CLL by BTKi Use
After balancing the cohorts for the selected covariates, TriNetX matched 5358 patients in each group (Table 1). Key demographic variables were well-balanced with more Hispanic patients but fewer African Americans with CLL not receiving a BTKi. Patients on a BTKi had higher rates of lymphadenopathy, malaise, dyspnea, and cough, while solid and other hematologic malignancies besides CLL were evenly distributed between both groups. However, overlapping nonfollicular lymphoma was more common in patients with CLL on a BTKi. In contrast, acute lymphoblastic leukemia and myeloid leukemia —although infrequent—were slightly more common in patients with CLL not on a BTKi. Anti-CD20 monoclonal antibodies were administered to <10% of patients, with slightly more frequent use observed among patients with CLL on a BTKi. Additionally, patients with CLL on a BTKi had higher total lymphocyte counts. Patients with CLL on BTKi received ibrutinib (84%) or other BTKi (16%).
Characteristics of Patients with Chronic Lymphocytic Leukemia, Stratified by Use of Bruton Tyrosine Kinase Inhibitors After Propensity Score Matching
| Characteristic . | CLL on BTKi (n = 5358) . | CLL off BTKi (n = 5358) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Age at index, y, mean ± SD | 69.54 ± 11.05 | 69.28 ± 13.08 | .268 |
| Race/ethnicity | |||
| White | 4428 (83) | 4393 (82) | .376 |
| Black or African American | 417 (8) | 302 (6) | <.001 |
| Hispanic or Latino | 96 (2) | 147 (3) | .001 |
| Asian | 81 (2) | 78 (1) | .811 |
| American Indian or Alaska Native | 10 (<1) | 15 (<1) | .317 |
| Male sex | 3311 (62) | 3310 (62) | .984 |
| Symptoms | |||
| Enlarged lymph nodes | 1834 (34) | 934 (17) | <.001 |
| Malaise and fatigue | 1579 (29) | 1265 (24) | <.001 |
| Dyspnea | 1320 (25) | 1180 (22) | .001 |
| Cough | 1265 (24) | 1074 (20) | <.001 |
| Pain in throat and chest | 1125 (21) | 1078 (20) | .261 |
| Nausea and vomiting | 686 (13) | 679 (13) | .839 |
| Diarrhea, unspecified | 659 (12) | 629 (12) | .373 |
| Fever, unspecified | 636 (12) | 514 (10) | <.001 |
| Headache | 562 (10) | 509 (10) | .088 |
| Cachexia | 46 (1) | 45 (1) | .916 |
| Comorbidities | |||
| Neoplasms | 5033 (94) | 5111 (95) | .001 |
| Malignant neoplasms of lymphoid tissue | 4986 (93) | 5004 (93) | .489 |
| Lymphoid leukemia | 4844 (90) | 4901 (91) | .055 |
| CLL of B-cell type | 4779 (89) | 4827 (90) | .128 |
| Connective tissue diseases | 2974 (56) | 3035 (57) | .235 |
| Hypertensive diseases | 2580 (48) | 2760 (52) | .001 |
| Chronic lower respiratory diseases | 1065 (20) | 1118 (21) | .204 |
| Diabetes mellitus | 1051 (20) | 1149 (21) | .019 |
| Nonfollicular lymphoma | 987 (18) | 549 (10) | <.001 |
| PV and myelodysplastic syndrome | 941 (18) | 859 (16) | .034 |
| Chronic kidney disease | 741 (14) | 765 (14) | .505 |
| Fever of unknown origin | 715 (13) | 593 (11) | <.001 |
| Melanoma | 646 (12) | 630 (12) | .633 |
| Neutropenia | 494 (9) | 470 (9) | .418 |
| Heart failure | 487 (9) | 576 (11) | .004 |
| Noninfective enteritis and colitis | 470 (9) | 513 (10) | .15 |
| Solitary pulmonary nodule | 435 (8) | 306 (6) | <.001 |
| Aplastic anemia | 396 (7) | 389 (7) | .795 |
| Acute lymphoblastic leukemia | 242 (5) | 318 (6) | .001 |
| Myeloid leukemia | 171 (3) | 414 (8) | <.001 |
| Transplanted organ and tissue status | 134 (3) | 134 (3) | 1 |
| Severe sepsis | 122 (2) | 168 (3) | .006 |
| Pulmonary fibrosis | 100 (2) | 112 (2) | .405 |
| Bone marrow transplant status | 78 (1) | 94 (2) | .219 |
| Fibrosis and cirrhosis of the liver | 64 (1) | 73 (1) | .439 |
| Sarcoidosis | 33 (1) | 22 (<1) | .137 |
| Systemic lupus erythematosus | 24 (<1) | 31 (1) | .344 |
| HIV | 15 (<1) | 15 (<1) | 1 |
| Medications | |||
| Glucocorticoids | 3056 (57) | 3127 (58) | .165 |
| Dexamethasone | 1513 (28) | 1481 (28) | .491 |
| Prednisone | 1323 (25) | 1364 (25) | .361 |
| Methylprednisolone | 1110 (21) | 1136 (21) | .537 |
| Prednisolone | 293 (5) | 328 (6) | .148 |
| Antineoplastics | 1988 (37) | 1918 (36) | .16 |
| Anti-CD20 monoclonal antibodies | 527 (10) | 444 (8) | .005 |
| Rituximab | 478 (9) | 391 (7) | .002 |
| Trimethoprim-sulfamethoxazole | 970 (18) | 983 (18) | .745 |
| Fluconazole | 454 (8) | 535 (10) | .007 |
| Immunosuppressantsa | 202 (4) | 209 (4) | .725 |
| Laboratory tests, mean ± SD | |||
| Leukocytes, 103/µL | 62.65 ± 90.27 | 40.33 ± 257.9 | <.001 |
| Hemoglobin, mg/dL | 11.73 ± 2.3 | 12.31 ± 2.39 | <.001 |
| Hematocrit, % | 36.38 ± 6.54 | 37.39 ± 6.93 | <.001 |
| Platelets, 103/µL | 158.12 ± 80.02 | 190.65 ± 125.09 | <.001 |
| Lymphocytes, (%) | 67.8 ± 27.7 | 41.6 ± 23.9 | <.001 |
| Neutrophils, 103/µL | 26.1 ± 130.5 | 5.6 ± 5.0 | <.001 |
| Creatinine, mg/dL | 1.1 ± 0.57 | 1.08 ± 0.63 | .067 |
| Lactate dehydrogenase, IU/mL | 290.89 ± 413.38 | 316.1 ± 535.84 | .043 |
| Hemoglobin A1c, % | 6.34 ± 1.6 | 6.41 ± 1.57 | .233 |
| Ferritin, μg/L | 315.03 ± 625.74 | 679.03 ± 2894.65 | <.001 |
| C-reactive protein, mg/dL | 28.97 ± 52.32 | 32.94 ± 55.92 | .157 |
| CD4 T cells/µL | 1077.5 ± 3705.62 | 2100.5 ± 10 483.5 | .263 |
| Galactomannan (index value) | 0.16 ± 0.48 | 0.13 ± 0.2 | .676 |
| 1,3-β-glucan in serum, pg/mL | 33.82 ± 48.36 | 21.32 ± 55.98 | .472 |
| Characteristic . | CLL on BTKi (n = 5358) . | CLL off BTKi (n = 5358) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Age at index, y, mean ± SD | 69.54 ± 11.05 | 69.28 ± 13.08 | .268 |
| Race/ethnicity | |||
| White | 4428 (83) | 4393 (82) | .376 |
| Black or African American | 417 (8) | 302 (6) | <.001 |
| Hispanic or Latino | 96 (2) | 147 (3) | .001 |
| Asian | 81 (2) | 78 (1) | .811 |
| American Indian or Alaska Native | 10 (<1) | 15 (<1) | .317 |
| Male sex | 3311 (62) | 3310 (62) | .984 |
| Symptoms | |||
| Enlarged lymph nodes | 1834 (34) | 934 (17) | <.001 |
| Malaise and fatigue | 1579 (29) | 1265 (24) | <.001 |
| Dyspnea | 1320 (25) | 1180 (22) | .001 |
| Cough | 1265 (24) | 1074 (20) | <.001 |
| Pain in throat and chest | 1125 (21) | 1078 (20) | .261 |
| Nausea and vomiting | 686 (13) | 679 (13) | .839 |
| Diarrhea, unspecified | 659 (12) | 629 (12) | .373 |
| Fever, unspecified | 636 (12) | 514 (10) | <.001 |
| Headache | 562 (10) | 509 (10) | .088 |
| Cachexia | 46 (1) | 45 (1) | .916 |
| Comorbidities | |||
| Neoplasms | 5033 (94) | 5111 (95) | .001 |
| Malignant neoplasms of lymphoid tissue | 4986 (93) | 5004 (93) | .489 |
| Lymphoid leukemia | 4844 (90) | 4901 (91) | .055 |
| CLL of B-cell type | 4779 (89) | 4827 (90) | .128 |
| Connective tissue diseases | 2974 (56) | 3035 (57) | .235 |
| Hypertensive diseases | 2580 (48) | 2760 (52) | .001 |
| Chronic lower respiratory diseases | 1065 (20) | 1118 (21) | .204 |
| Diabetes mellitus | 1051 (20) | 1149 (21) | .019 |
| Nonfollicular lymphoma | 987 (18) | 549 (10) | <.001 |
| PV and myelodysplastic syndrome | 941 (18) | 859 (16) | .034 |
| Chronic kidney disease | 741 (14) | 765 (14) | .505 |
| Fever of unknown origin | 715 (13) | 593 (11) | <.001 |
| Melanoma | 646 (12) | 630 (12) | .633 |
| Neutropenia | 494 (9) | 470 (9) | .418 |
| Heart failure | 487 (9) | 576 (11) | .004 |
| Noninfective enteritis and colitis | 470 (9) | 513 (10) | .15 |
| Solitary pulmonary nodule | 435 (8) | 306 (6) | <.001 |
| Aplastic anemia | 396 (7) | 389 (7) | .795 |
| Acute lymphoblastic leukemia | 242 (5) | 318 (6) | .001 |
| Myeloid leukemia | 171 (3) | 414 (8) | <.001 |
| Transplanted organ and tissue status | 134 (3) | 134 (3) | 1 |
| Severe sepsis | 122 (2) | 168 (3) | .006 |
| Pulmonary fibrosis | 100 (2) | 112 (2) | .405 |
| Bone marrow transplant status | 78 (1) | 94 (2) | .219 |
| Fibrosis and cirrhosis of the liver | 64 (1) | 73 (1) | .439 |
| Sarcoidosis | 33 (1) | 22 (<1) | .137 |
| Systemic lupus erythematosus | 24 (<1) | 31 (1) | .344 |
| HIV | 15 (<1) | 15 (<1) | 1 |
| Medications | |||
| Glucocorticoids | 3056 (57) | 3127 (58) | .165 |
| Dexamethasone | 1513 (28) | 1481 (28) | .491 |
| Prednisone | 1323 (25) | 1364 (25) | .361 |
| Methylprednisolone | 1110 (21) | 1136 (21) | .537 |
| Prednisolone | 293 (5) | 328 (6) | .148 |
| Antineoplastics | 1988 (37) | 1918 (36) | .16 |
| Anti-CD20 monoclonal antibodies | 527 (10) | 444 (8) | .005 |
| Rituximab | 478 (9) | 391 (7) | .002 |
| Trimethoprim-sulfamethoxazole | 970 (18) | 983 (18) | .745 |
| Fluconazole | 454 (8) | 535 (10) | .007 |
| Immunosuppressantsa | 202 (4) | 209 (4) | .725 |
| Laboratory tests, mean ± SD | |||
| Leukocytes, 103/µL | 62.65 ± 90.27 | 40.33 ± 257.9 | <.001 |
| Hemoglobin, mg/dL | 11.73 ± 2.3 | 12.31 ± 2.39 | <.001 |
| Hematocrit, % | 36.38 ± 6.54 | 37.39 ± 6.93 | <.001 |
| Platelets, 103/µL | 158.12 ± 80.02 | 190.65 ± 125.09 | <.001 |
| Lymphocytes, (%) | 67.8 ± 27.7 | 41.6 ± 23.9 | <.001 |
| Neutrophils, 103/µL | 26.1 ± 130.5 | 5.6 ± 5.0 | <.001 |
| Creatinine, mg/dL | 1.1 ± 0.57 | 1.08 ± 0.63 | .067 |
| Lactate dehydrogenase, IU/mL | 290.89 ± 413.38 | 316.1 ± 535.84 | .043 |
| Hemoglobin A1c, % | 6.34 ± 1.6 | 6.41 ± 1.57 | .233 |
| Ferritin, μg/L | 315.03 ± 625.74 | 679.03 ± 2894.65 | <.001 |
| C-reactive protein, mg/dL | 28.97 ± 52.32 | 32.94 ± 55.92 | .157 |
| CD4 T cells/µL | 1077.5 ± 3705.62 | 2100.5 ± 10 483.5 | .263 |
| Galactomannan (index value) | 0.16 ± 0.48 | 0.13 ± 0.2 | .676 |
| 1,3-β-glucan in serum, pg/mL | 33.82 ± 48.36 | 21.32 ± 55.98 | .472 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BTKi, Bruton tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; HIV, human immunodeficiency virus; PV, polycythemia vera; SD, standard deviation.
aIncludes tacrolimus, mycophenolate mofetil, mycophenolic acid, cyclosporine, azathioprine, sirolimus, infliximab, basiliximab, belatacept, omalizumab, siltuximab, belumosudil, ustekinumab.
Characteristics of Patients with Chronic Lymphocytic Leukemia, Stratified by Use of Bruton Tyrosine Kinase Inhibitors After Propensity Score Matching
| Characteristic . | CLL on BTKi (n = 5358) . | CLL off BTKi (n = 5358) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Age at index, y, mean ± SD | 69.54 ± 11.05 | 69.28 ± 13.08 | .268 |
| Race/ethnicity | |||
| White | 4428 (83) | 4393 (82) | .376 |
| Black or African American | 417 (8) | 302 (6) | <.001 |
| Hispanic or Latino | 96 (2) | 147 (3) | .001 |
| Asian | 81 (2) | 78 (1) | .811 |
| American Indian or Alaska Native | 10 (<1) | 15 (<1) | .317 |
| Male sex | 3311 (62) | 3310 (62) | .984 |
| Symptoms | |||
| Enlarged lymph nodes | 1834 (34) | 934 (17) | <.001 |
| Malaise and fatigue | 1579 (29) | 1265 (24) | <.001 |
| Dyspnea | 1320 (25) | 1180 (22) | .001 |
| Cough | 1265 (24) | 1074 (20) | <.001 |
| Pain in throat and chest | 1125 (21) | 1078 (20) | .261 |
| Nausea and vomiting | 686 (13) | 679 (13) | .839 |
| Diarrhea, unspecified | 659 (12) | 629 (12) | .373 |
| Fever, unspecified | 636 (12) | 514 (10) | <.001 |
| Headache | 562 (10) | 509 (10) | .088 |
| Cachexia | 46 (1) | 45 (1) | .916 |
| Comorbidities | |||
| Neoplasms | 5033 (94) | 5111 (95) | .001 |
| Malignant neoplasms of lymphoid tissue | 4986 (93) | 5004 (93) | .489 |
| Lymphoid leukemia | 4844 (90) | 4901 (91) | .055 |
| CLL of B-cell type | 4779 (89) | 4827 (90) | .128 |
| Connective tissue diseases | 2974 (56) | 3035 (57) | .235 |
| Hypertensive diseases | 2580 (48) | 2760 (52) | .001 |
| Chronic lower respiratory diseases | 1065 (20) | 1118 (21) | .204 |
| Diabetes mellitus | 1051 (20) | 1149 (21) | .019 |
| Nonfollicular lymphoma | 987 (18) | 549 (10) | <.001 |
| PV and myelodysplastic syndrome | 941 (18) | 859 (16) | .034 |
| Chronic kidney disease | 741 (14) | 765 (14) | .505 |
| Fever of unknown origin | 715 (13) | 593 (11) | <.001 |
| Melanoma | 646 (12) | 630 (12) | .633 |
| Neutropenia | 494 (9) | 470 (9) | .418 |
| Heart failure | 487 (9) | 576 (11) | .004 |
| Noninfective enteritis and colitis | 470 (9) | 513 (10) | .15 |
| Solitary pulmonary nodule | 435 (8) | 306 (6) | <.001 |
| Aplastic anemia | 396 (7) | 389 (7) | .795 |
| Acute lymphoblastic leukemia | 242 (5) | 318 (6) | .001 |
| Myeloid leukemia | 171 (3) | 414 (8) | <.001 |
| Transplanted organ and tissue status | 134 (3) | 134 (3) | 1 |
| Severe sepsis | 122 (2) | 168 (3) | .006 |
| Pulmonary fibrosis | 100 (2) | 112 (2) | .405 |
| Bone marrow transplant status | 78 (1) | 94 (2) | .219 |
| Fibrosis and cirrhosis of the liver | 64 (1) | 73 (1) | .439 |
| Sarcoidosis | 33 (1) | 22 (<1) | .137 |
| Systemic lupus erythematosus | 24 (<1) | 31 (1) | .344 |
| HIV | 15 (<1) | 15 (<1) | 1 |
| Medications | |||
| Glucocorticoids | 3056 (57) | 3127 (58) | .165 |
| Dexamethasone | 1513 (28) | 1481 (28) | .491 |
| Prednisone | 1323 (25) | 1364 (25) | .361 |
| Methylprednisolone | 1110 (21) | 1136 (21) | .537 |
| Prednisolone | 293 (5) | 328 (6) | .148 |
| Antineoplastics | 1988 (37) | 1918 (36) | .16 |
| Anti-CD20 monoclonal antibodies | 527 (10) | 444 (8) | .005 |
| Rituximab | 478 (9) | 391 (7) | .002 |
| Trimethoprim-sulfamethoxazole | 970 (18) | 983 (18) | .745 |
| Fluconazole | 454 (8) | 535 (10) | .007 |
| Immunosuppressantsa | 202 (4) | 209 (4) | .725 |
| Laboratory tests, mean ± SD | |||
| Leukocytes, 103/µL | 62.65 ± 90.27 | 40.33 ± 257.9 | <.001 |
| Hemoglobin, mg/dL | 11.73 ± 2.3 | 12.31 ± 2.39 | <.001 |
| Hematocrit, % | 36.38 ± 6.54 | 37.39 ± 6.93 | <.001 |
| Platelets, 103/µL | 158.12 ± 80.02 | 190.65 ± 125.09 | <.001 |
| Lymphocytes, (%) | 67.8 ± 27.7 | 41.6 ± 23.9 | <.001 |
| Neutrophils, 103/µL | 26.1 ± 130.5 | 5.6 ± 5.0 | <.001 |
| Creatinine, mg/dL | 1.1 ± 0.57 | 1.08 ± 0.63 | .067 |
| Lactate dehydrogenase, IU/mL | 290.89 ± 413.38 | 316.1 ± 535.84 | .043 |
| Hemoglobin A1c, % | 6.34 ± 1.6 | 6.41 ± 1.57 | .233 |
| Ferritin, μg/L | 315.03 ± 625.74 | 679.03 ± 2894.65 | <.001 |
| C-reactive protein, mg/dL | 28.97 ± 52.32 | 32.94 ± 55.92 | .157 |
| CD4 T cells/µL | 1077.5 ± 3705.62 | 2100.5 ± 10 483.5 | .263 |
| Galactomannan (index value) | 0.16 ± 0.48 | 0.13 ± 0.2 | .676 |
| 1,3-β-glucan in serum, pg/mL | 33.82 ± 48.36 | 21.32 ± 55.98 | .472 |
| Characteristic . | CLL on BTKi (n = 5358) . | CLL off BTKi (n = 5358) . | P Value . |
|---|---|---|---|
| Demographics | |||
| Age at index, y, mean ± SD | 69.54 ± 11.05 | 69.28 ± 13.08 | .268 |
| Race/ethnicity | |||
| White | 4428 (83) | 4393 (82) | .376 |
| Black or African American | 417 (8) | 302 (6) | <.001 |
| Hispanic or Latino | 96 (2) | 147 (3) | .001 |
| Asian | 81 (2) | 78 (1) | .811 |
| American Indian or Alaska Native | 10 (<1) | 15 (<1) | .317 |
| Male sex | 3311 (62) | 3310 (62) | .984 |
| Symptoms | |||
| Enlarged lymph nodes | 1834 (34) | 934 (17) | <.001 |
| Malaise and fatigue | 1579 (29) | 1265 (24) | <.001 |
| Dyspnea | 1320 (25) | 1180 (22) | .001 |
| Cough | 1265 (24) | 1074 (20) | <.001 |
| Pain in throat and chest | 1125 (21) | 1078 (20) | .261 |
| Nausea and vomiting | 686 (13) | 679 (13) | .839 |
| Diarrhea, unspecified | 659 (12) | 629 (12) | .373 |
| Fever, unspecified | 636 (12) | 514 (10) | <.001 |
| Headache | 562 (10) | 509 (10) | .088 |
| Cachexia | 46 (1) | 45 (1) | .916 |
| Comorbidities | |||
| Neoplasms | 5033 (94) | 5111 (95) | .001 |
| Malignant neoplasms of lymphoid tissue | 4986 (93) | 5004 (93) | .489 |
| Lymphoid leukemia | 4844 (90) | 4901 (91) | .055 |
| CLL of B-cell type | 4779 (89) | 4827 (90) | .128 |
| Connective tissue diseases | 2974 (56) | 3035 (57) | .235 |
| Hypertensive diseases | 2580 (48) | 2760 (52) | .001 |
| Chronic lower respiratory diseases | 1065 (20) | 1118 (21) | .204 |
| Diabetes mellitus | 1051 (20) | 1149 (21) | .019 |
| Nonfollicular lymphoma | 987 (18) | 549 (10) | <.001 |
| PV and myelodysplastic syndrome | 941 (18) | 859 (16) | .034 |
| Chronic kidney disease | 741 (14) | 765 (14) | .505 |
| Fever of unknown origin | 715 (13) | 593 (11) | <.001 |
| Melanoma | 646 (12) | 630 (12) | .633 |
| Neutropenia | 494 (9) | 470 (9) | .418 |
| Heart failure | 487 (9) | 576 (11) | .004 |
| Noninfective enteritis and colitis | 470 (9) | 513 (10) | .15 |
| Solitary pulmonary nodule | 435 (8) | 306 (6) | <.001 |
| Aplastic anemia | 396 (7) | 389 (7) | .795 |
| Acute lymphoblastic leukemia | 242 (5) | 318 (6) | .001 |
| Myeloid leukemia | 171 (3) | 414 (8) | <.001 |
| Transplanted organ and tissue status | 134 (3) | 134 (3) | 1 |
| Severe sepsis | 122 (2) | 168 (3) | .006 |
| Pulmonary fibrosis | 100 (2) | 112 (2) | .405 |
| Bone marrow transplant status | 78 (1) | 94 (2) | .219 |
| Fibrosis and cirrhosis of the liver | 64 (1) | 73 (1) | .439 |
| Sarcoidosis | 33 (1) | 22 (<1) | .137 |
| Systemic lupus erythematosus | 24 (<1) | 31 (1) | .344 |
| HIV | 15 (<1) | 15 (<1) | 1 |
| Medications | |||
| Glucocorticoids | 3056 (57) | 3127 (58) | .165 |
| Dexamethasone | 1513 (28) | 1481 (28) | .491 |
| Prednisone | 1323 (25) | 1364 (25) | .361 |
| Methylprednisolone | 1110 (21) | 1136 (21) | .537 |
| Prednisolone | 293 (5) | 328 (6) | .148 |
| Antineoplastics | 1988 (37) | 1918 (36) | .16 |
| Anti-CD20 monoclonal antibodies | 527 (10) | 444 (8) | .005 |
| Rituximab | 478 (9) | 391 (7) | .002 |
| Trimethoprim-sulfamethoxazole | 970 (18) | 983 (18) | .745 |
| Fluconazole | 454 (8) | 535 (10) | .007 |
| Immunosuppressantsa | 202 (4) | 209 (4) | .725 |
| Laboratory tests, mean ± SD | |||
| Leukocytes, 103/µL | 62.65 ± 90.27 | 40.33 ± 257.9 | <.001 |
| Hemoglobin, mg/dL | 11.73 ± 2.3 | 12.31 ± 2.39 | <.001 |
| Hematocrit, % | 36.38 ± 6.54 | 37.39 ± 6.93 | <.001 |
| Platelets, 103/µL | 158.12 ± 80.02 | 190.65 ± 125.09 | <.001 |
| Lymphocytes, (%) | 67.8 ± 27.7 | 41.6 ± 23.9 | <.001 |
| Neutrophils, 103/µL | 26.1 ± 130.5 | 5.6 ± 5.0 | <.001 |
| Creatinine, mg/dL | 1.1 ± 0.57 | 1.08 ± 0.63 | .067 |
| Lactate dehydrogenase, IU/mL | 290.89 ± 413.38 | 316.1 ± 535.84 | .043 |
| Hemoglobin A1c, % | 6.34 ± 1.6 | 6.41 ± 1.57 | .233 |
| Ferritin, μg/L | 315.03 ± 625.74 | 679.03 ± 2894.65 | <.001 |
| C-reactive protein, mg/dL | 28.97 ± 52.32 | 32.94 ± 55.92 | .157 |
| CD4 T cells/µL | 1077.5 ± 3705.62 | 2100.5 ± 10 483.5 | .263 |
| Galactomannan (index value) | 0.16 ± 0.48 | 0.13 ± 0.2 | .676 |
| 1,3-β-glucan in serum, pg/mL | 33.82 ± 48.36 | 21.32 ± 55.98 | .472 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BTKi, Bruton tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; HIV, human immunodeficiency virus; PV, polycythemia vera; SD, standard deviation.
aIncludes tacrolimus, mycophenolate mofetil, mycophenolic acid, cyclosporine, azathioprine, sirolimus, infliximab, basiliximab, belatacept, omalizumab, siltuximab, belumosudil, ustekinumab.
Proportion of IFIs in Patients With CLL by BTKi Use
There were 244 (4.6%) episodes of IFIs among patients with CLL on a BTKi compared to 188 (3.5%) in patients with CLL not on a BTKi. Figure 1 provides the incidence of IFIs for patients with CLL by BTKi exposure after PSM. Invasive candidiasis was more common in the BTKi group (3.5% vs 2.7%), with an excess of 45 cases, translating to an odds ratio (OR) of 1.3 and risk difference of 0.008 (P = .012). Invasive aspergillosis was less common in the BTKi group (0.2% vs 0.4%), with an OR of 0.5 and risk difference of −0.002 (P = .068). There was a nonsignificant excess of cryptococcosis in the BTKi group, with an OR of 1.6 and risk difference of 0.001 (P = .24). PJP was more prevalent in the BTKi group (0.5% vs 0.3%), with an excess of 15 cases and an OR of 2.1 (P = .02). The incidence of candidiasis, aspergillosis, cryptococcosis, and PJP was 705, 37, 60, and 108 episodes per 100 000 patients per year on BTKi treatment, respectively.
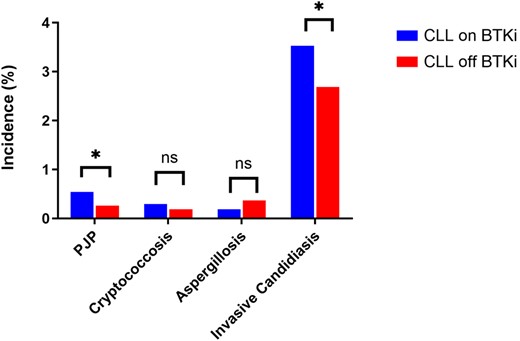
Proportions of invasive fungal infections among patients with chronic lymphocytic leukemia captured after propensity score matching at 5 years by use of Bruton tyrosine kinase inhibitor. *P < .05. Abbreviations: BTKi, Bruton tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; ns, nonsignificant; PJP, Pneumocystis jirovecii pneumonia.
Other Secondary Outcomes in Patients With CLL by BTKi Use
A total of 1142 patients with CLL in the BTKi group and 1177 patients with CLL not on a BTKi, died within 1 year. However, the 5-year mortality risk did not differ significantly between the 2 groups (OR, 0.96 [95% confidence interval {CI}, .87–1.04]; P = .289) (risk difference of −0.009). The log-rank test for survival was also nonsignificant (P = .504). More patients with CLL on a BTKi had been hospitalized at 5 years compared to those without BTKi (OR, 1.4 [XX% CI, 1.3–1.6]; P < .0001).
Assessment of Relative Risk and NNH for IFIs
BTKi use was associated with an NNH of 98 for IFIs. For specific IFIs, the NNH with BTKi use was 120 for invasive candidiasis, 536 for invasive aspergillosis, and 894 for cryptococcosis. PJP had an NNH of 358 (Table 2).
Relative Risk and Number Needed to Harm With Invasive Fungal Infections in Patients With Chronic Lmphocytic Leukemia on a Bruton Tyrosine Kinase Inhibitor at 5 Years
| Invasive Fungal Infection . | Episodesa, No. (%) . | Relative Risk (95% CI) . | NNH . |
|---|---|---|---|
| Pneumocystis jirovecii pneumonia | 29 (0.5%) | 2.1 (1.1 - 4.0) | 358 |
| Cryptococcosis | 16 (0.3%) | 1.6 (0.7 - 3.5) | 894 |
| Aspergillosis | 10 (0.2%) | 0.5 (0.2 - 1.1) | 536b |
| Invasive candidiasis | 189 (3.5%) | 1.3 (1.1 - 1.6) | 120 |
| Invasive Fungal Infection . | Episodesa, No. (%) . | Relative Risk (95% CI) . | NNH . |
|---|---|---|---|
| Pneumocystis jirovecii pneumonia | 29 (0.5%) | 2.1 (1.1 - 4.0) | 358 |
| Cryptococcosis | 16 (0.3%) | 1.6 (0.7 - 3.5) | 894 |
| Aspergillosis | 10 (0.2%) | 0.5 (0.2 - 1.1) | 536b |
| Invasive candidiasis | 189 (3.5%) | 1.3 (1.1 - 1.6) | 120 |
Abbreviation: NNH, number needed to harm.
aAmong 5358 matched patients with chronic lymphocytic leukemia on a Bruton tyrosine kinase inhibitor.
bNumber needed to treat.
Relative Risk and Number Needed to Harm With Invasive Fungal Infections in Patients With Chronic Lmphocytic Leukemia on a Bruton Tyrosine Kinase Inhibitor at 5 Years
| Invasive Fungal Infection . | Episodesa, No. (%) . | Relative Risk (95% CI) . | NNH . |
|---|---|---|---|
| Pneumocystis jirovecii pneumonia | 29 (0.5%) | 2.1 (1.1 - 4.0) | 358 |
| Cryptococcosis | 16 (0.3%) | 1.6 (0.7 - 3.5) | 894 |
| Aspergillosis | 10 (0.2%) | 0.5 (0.2 - 1.1) | 536b |
| Invasive candidiasis | 189 (3.5%) | 1.3 (1.1 - 1.6) | 120 |
| Invasive Fungal Infection . | Episodesa, No. (%) . | Relative Risk (95% CI) . | NNH . |
|---|---|---|---|
| Pneumocystis jirovecii pneumonia | 29 (0.5%) | 2.1 (1.1 - 4.0) | 358 |
| Cryptococcosis | 16 (0.3%) | 1.6 (0.7 - 3.5) | 894 |
| Aspergillosis | 10 (0.2%) | 0.5 (0.2 - 1.1) | 536b |
| Invasive candidiasis | 189 (3.5%) | 1.3 (1.1 - 1.6) | 120 |
Abbreviation: NNH, number needed to harm.
aAmong 5358 matched patients with chronic lymphocytic leukemia on a Bruton tyrosine kinase inhibitor.
bNumber needed to treat.
DISCUSSION
In our analysis of 5358 matched patients with CLL, we observed a 4.6% incidence of IFIs in patients on a BTKi, compared to 3.5% in patients not on a BTKi over 5 years, resulting in annual rates of 0.9% and 0.7%, respectively. Approximately 1% of patients with CLL developed an IFI while on a BTKi during this period. Our adjusted analysis revealed higher rates of PJP and invasive candidiasis with use of a BTKi, with 3.5% of BTKi patients experiencing invasive candidiasis, compared to 2.7% in those not using a BTKi. Additionally, 0.5% of BTKi patients developed PJP versus 0.3% of non-BTKi patients over 5 years, indicating an annual PJP risk of 0.1% per year.
The reported percentage of IFIs in patients with CLL on a BTKi varies from 0.7% to 1.6% [7, 15, 16] with invasive aspergillosis and cryptococcosis being the most common systemic fungal infections. In cases of IFIs among BTKi users, 40% of patients were also using glucocorticoids, and the median onset of infection was 45 days [17]. Aspergillus spp (22%), Cryptococcus spp (26%), Mucorales (6%), and Pneumocystis jirovecii (1%) were the most frequent IFIs described in the study. Another study that examined the frequency of PJP in 96 patients with CLL treated with ibrutinib found a 5% incidence, with an estimated risk of 2 cases per 100 patient-years, which significantly overestimates our annual PJP risk of 0.1% [18]. That estimation was limited by the small number of patients, the lack of a control group, and absence of adjustment for additional PJP risk factors. A recent systematic review found 14 cases of aspergillosis (3%), 1 case of cryptococcosis (0.2%), and 6 cases of PJP (1.2%) among 490 patients on BTKi combination therapy [6]. The increased risk of invasive candidiasis observed in our study had not been reported previously and needs confirmation through prospective studies. Ibrutinib is associated with dermatologic toxicities, including mucositis and mucocutaneous infection, which can both be predisposing conditions for invasive candidiasis [19].
BTKi predominantly affect the survival and function of B cells, but the increased risk of IFIs cannot be solely attributed to its effect on B cells. BTKi can alter the function of alveolar macrophages, neutrophils, T cells, natural killer cells, and the cytokine milieu needed to control infection [20, 21]. Additionally, other immunodeficiencies may result from off-target effects on pathways that regulate innate fungal immunity. For example, ibrutinib impairs neutrophil chemotaxis and macrophage activation through inhibition of both NFAT and NF-κB, and impairs production of tumor necrosis factor α [22]. Attribution of an increased risk of IFIs to BTKi alone is further confounded by the additional risk factors (eg, increasing age, previous lymphocyte-depleting therapy such as fludarabine and rituximab, glucocorticoid use) frequently found in patients with CLL.
Our study adjusted for known risk factors for IFIs between the 2 groups, allowing for more precise estimates. Notably, the NNH only reached double digits for the total IFI episodes, highlighting the safety-benefit of this medication class. In a cohort of patients with CLL treated with a BTKi, the estimated number needed to treat with trimethoprim-sulfamethoxazole to prevent 1 case of PJP was 42 [23]. Our adjusted IFI event analysis found an elevated rate of PJP, albeit at a low annual risk of 0.1% per year with use of a BTKi. The rates are, however, low with a high NNH. Our findings do not support routine PJP prophylaxis in this patient population, considering the historical PJP risk of >6.2% per person-year as the recommended threshold for primary prophylaxis in non-HIV immunocompromised patients [24].
Our study is limited by its retrospective design and the use of ICD-10 codes for diagnosis, which may introduce selection bias and the potential for misclassification. We did not have access to microbiologic or histologic data to identify the organism’s species and to use the consensus definitions of the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer and the Mycoses Study Group [25], which could have underestimated the rates of events. We could not estimate precise BTKi doses and exposure duration, limiting the extrapolation of the findings. In addition, missing data may impair the strength of the association. Furthermore, the risk of IFIs associated with newer and more selective BTKis, such as acalabrutinib [26] and zanubrutinib [27], could not be discerned from that of ibrutinib. The platform also limited a subgroup analysis by participating centers. Patients not on a BTKi may have undisclosed comorbidities or not have indications for treatment that could influence the results. Finally, the risk of IFIs in patients taking a BTKi for other indications (ie, non-Hodgkin lymphoma, Waldenström macroglobulinemia, and graft-vs-host disease) was not examined due to limitations of data extraction to 1 disease entity. Studies to better understand the risk factors associated with development of IFIs according to the BTKi indication are needed. This is, however, one of the largest studies of patients with CLL ever analyzed, with >10 000 subjects and with a specific and robust adjustment of comorbidities and other risk factors.
CONCLUSIONS
Bruton tyrosine kinase inhibitors offer an incredible therapeutic advantage in patients with CLL. While we found a 4.6% incidence of IFIs in patients on a BTKi, this translates to an estimated additional 1% risk of IFI in patients with CLL while on a BTKi over 5 years compared to those not on a BTKi. Although we observed higher rates of PJP and invasive candidiasis with BTKi use, the absolute rates are low, with a high NNH. Consequently, our findings do not support routine PJP prophylaxis in this patient population, considering the historical PJP risk threshold for primary prophylaxis in non-HIV immunocompromised patients. However, further studies are needed to identify at-risk patients and develop cost-effective preventive interventions for different IFIs associated with BTKi use.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Note
Potential conflicts of interest. The authors: No reported conflicts of interest.





Comments