-
PDF
- Split View
-
Views
-
Cite
Cite
Anne-Lise Beaumont, Femke Mestre, Sixtine Decaux, Chloé Bertin, Xavier Duval, Bernard Iung, François Rouzet, Nathalie Grall, Marylou Para, Michael Thy, Laurène Deconinck, Long-term Oral Suppressive Antimicrobial Therapy in Infective Endocarditis (SATIE Study): An Observational Study, Open Forum Infectious Diseases, Volume 11, Issue 5, May 2024, ofae194, https://doi.org/10.1093/ofid/ofae194
Close - Share Icon Share
Abstract
The role of suppressive antimicrobial therapy (SAT) in infective endocarditis (IE) management has yet to be defined. The objective of this study was to describe the use of SAT in an IE referral center and the patients’ outcomes.
We conducted a retrospective observational study in a French IE referral center (Paris). All patients with IE who received SAT between 2016 and 2022 were included.
Forty-two patients were included (36 male [86%]; median age [interquartile range {IQR}], 73 [61–82] years). The median Charlson Comorbidity Index score (IQR) was 3 (1–4). Forty patients (95%) had an intracardiac device. The most frequent microorganisms were Enterococcus faecalis (15/42, 36%) and Staphylococcus aureus (12/42, 29%). SAT indications were absence of surgery despite clinical indication (28/42, 67%), incomplete removal of prosthetic material (6/42, 14%), uncontrolled infection source (4/42, 10%), persistent abnormal uptake on nuclear imaging (1/42, 2%), or a combination of the previous indications (3/42, 7%). Antimicrobials were mainly doxycycline (19/42, 45%) and amoxicillin (19/42, 45%). The median follow-up time (IQR) was 398 (194–663) days. Five patients (12%) experienced drug adverse events. Five patients (12%) presented with a second IE episode during follow-up, including 2 reinfections (different bacterial species) and 3 possible relapses (same bacterial species). Fourteen patients (33%) in our cohort died during follow-up. Overall, the 1-year survival rate was 84.3% (73.5%–96.7%), and the 1-year survival rate without recurrence was 74.1% (61.4%–89.4%).
SAT was mainly prescribed to patients with cardiac devices because of the absence of surgery despite clinical indication. Five (12%) breakthrough second IE episodes were reported. Prospective comparative studies are required to guide this empirical practice.
Suppressive antimicrobial therapy (SAT) is a secondary prevention approach designed to decrease relapse or reinfection risks in severe infections. It consists of a long-term, sometimes life-long, antimicrobial treatment following an initial curative phase [1]. For instance, this therapeutic strategy is often recommended for opportunistic infections as long as immunosuppression persists, for example, in HIV-associated toxoplasmosis.
Regarding bacterial infections, SAT remains a rather rare and marginal practice. However, a growing body of evidence argues for its relevance for prosthetic joint infections [2], particularly when prosthesis removal is not feasible. In this clinical situation, SAT is recommended in Infectious Disease Society of America guidelines [3] and French guidelines [4]. Likewise, according to the American Heart Association guidelines for vascular graft infection [5], SAT may be considered for infections caused by multiresistant organisms or Candida species, in complex surgical cases, or for some patients who are poor candidates for redo surgery (Class IIb; Level of Evidence C).
Regarding infective endocarditis (IE), official guidelines concerning SAT are rare and of low level of evidence given the small number of clinical studies on the subject [6, 7]. In the 2023 European Society of Cardiology (ESC) guidelines for the management of endocarditis [8], SAT is only clearly recommended therapy for fungal endocarditis. In line with the European Heart Rhythm Association guidelines on cardiac implantable electronic device (CIED) infections (Expert Opinion) [9] and the AHA 2023 guidelines on CIED infection (Class IIb; Level of Evidence C) [10, 11], suppressive antimicrobial therapy for CIED infections is also mentioned when complete removal of the device is not possible as a “salvage treatment,” the duration of which should be “individualized.” Finally, SAT is mentioned in the ESC 2023 report as an option for “patients with IE who do not undergo cardiac surgery,” but the lack of evidence on SAT efficiency is also highlighted. Consequently, no clear indications or protocols are provided.
While its optimal position in the therapeutic strategy of IE remains undefined, this empirical practice meets a growing need for tailored treatment options. Indeed, IE epidemiology has changed greatly in recent decades [12], and IE is increasingly affecting an older population and/or patients with CIED or prosthetic valves. SAT appears to be a valuable therapeutic approach in selected patients with IE, considering its risk/benefit ratio. Various clinical scenarios may prompt the consideration of SAT. First, SAT becomes a viable option when surgery is indicated but the associated surgical risk is deemed unacceptably high. In such cases, SAT serves as a palliative approach to mitigate the elevated risk of relapse resulting from suboptimal initial treatment. Another circumstance arises when a patient has received optimal treatment yet the recurrence risk is very high (eg, because of a persistent portal of entry) or the prognosis is very poor (eg, because of multiple comorbidities). However, SAT indication relies to date on local expert opinion rather than clinical evidence.
The main objective of this study was to describe the indications of SAT for patients with IE in a referral center and their outcomes on this treatment.
METHODS
Study Design and Data Collection
We conducted a retrospective observational study in Bichat Hospital, Paris, France, which is an IE tertiary care referral center. A multidisciplinary Endocarditis Team meets weekly to evaluate complex medical cases and provide guidance for adequate medical and surgical management.
Thanks to meeting reports, a list of patients with IE for whom suppressive antimicrobial therapy was discussed by the Endocarditis Team between November 2016 and December 2022 was established. Of note, the recommended duration of SAT prescription advised by our Endocarditis Team was lifelong. During follow-up, the referring physician is free to reassess the risk/benefit ratio of this prescription, especially if new medical information becomes available.
Medical charts were reviewed by medical staff to check for inclusion criteria.
Inclusion criteria were the following: SAT recommended for definite or possible IE on a native or prosthetic valve or a CIED according to the 2023 Duke-ISCVID Criteria, SAT prescribed by the clinician in charge of the patient, at least 1 day of SAT intake, and follow-up in Bichat Hospital.
Exclusion criteria were the following: SAT prescribed for vascular graft infection without valve involvement, for ventricular assist device infection, for chronic bone infection, or for Q fever. If patients had several IE episodes, the episode treated by SAT was considered.
Data from the computerized medical record were collected in a case report form designed for the study, including information about patient medical history, characteristics of the infection, and follow-up. Vital status was collected from the medical record and was cross-checked on an online platform operated by the French National Institute of Statistics and Economic Studies (https://deces.matchid.io/search). This database is updated every month and records in- and out-hospital deaths; however, for data protection reasons, it does not provide any information on cause of death.
Variable Definition and Statistical Analysis
We defined IE relapse as a second episode of IE caused by the same species of microorganism (regardless of susceptibility pattern) and IE reinfection as a second episode of IE caused by a different species of microorganism, regardless of time interval. The term “recurrence” refers to either reinfection or relapse. As commonly defined, prosthetic valve endocarditis (PVE) was classified as either early or late depending on whether the infection occurred during or 12 months after valve surgery.
Data were described as median and interquartile range (IQR) for continuous variables and as absolute number and percentage for categorical variables. Cumulative survival was plotted with the Kaplan-Meier method. Time origin was the first day of SAT. The end point date was July 1, 2023, for every patient. The primary end point was defined as either death or IE relapse or reinfection. The date of last follow-up was the last time the patient was seen during a physician visit documented in the medical record.
Excel and Rstudio (version 2023.03.1) were used.
Patient Consent
The study was approved by the Ethics Committee of the French Infectious Diseases Society (SPILF, CER-MIT No. 2023–0902). The study is in accordance with the General Data Protection Regulation (GDPR, EU law) and was recorded in the Assistance Publique–Hôpitaux de Paris (AP-HP) register (No. 20230706192734). All living patients were informed in written form about the retrospective analysis of their anonymized data and were given the opportunity to opt out after receiving the information. Of note, in accordance with French law and the protocol submitted to the Ethics Committee, written consent was not necessary.
RESULTS
Long-term SAT was recommended for 91 patients by the multidisciplinary Endocarditis Team, of whom 42 met inclusion criteria and were included in the study (Figure 1).
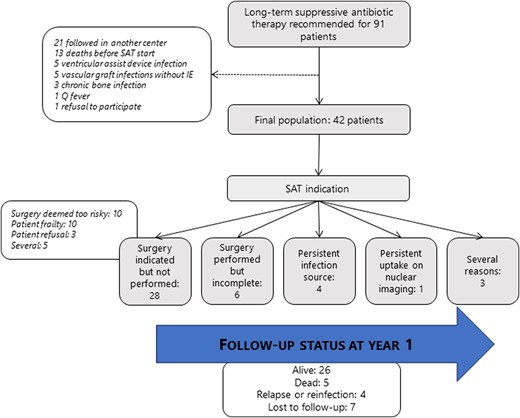
Flowchart with follow-up status at year 1. Abbreviation: SAT, suppressive antimicrobial therapy.
Study Population
Initial characteristics of the 42 included patients are shown in Table 1. Patients were majority male (36/42, 86%), with a median age (IQR) of 73 (61–82) years. Only 1 was a person who injects drugs. The median Charlson Comorbidity Index (unadjusted for age) (IQR) was 3 (1–4), reflecting the high prevalence of comorbidities in the cohort. Almost every patient had at least 1 prosthetic cardiac device (40/42, 95%). These were mainly prosthetic valves (27/42, 64%, percutaneously inserted in 5 cases and surgically in 22 cases), cardiac implantable electronic devices (13/42, 31%), or both (11/42, 26%). Eleven patients had a previous episode of IE (26%); in 64% (7/11), the episode of IE involved the same bacterial species as incident episode.
| Parameters . | Overall (n = 42) . |
|---|---|
| General characteristics | |
| Age, median (IQR), y | 73 (61–82) |
| Male sex | 36 (86) |
| BMI <18.5, kg/m2 | 4 (10) |
| Comorbidities | |
| Chronic renal failure | 7 (17) |
| Chronic congestive heart failure | 9 (21) |
| Chronic respiratory disease | 9 (21) |
| Chronic hepatic disease | 2 (5) |
| Dementia | 5 (12) |
| Stroke | 4 (9) |
| Diabetes mellitus | 12 (28) |
| Person who injects drugs | 1 (2) |
| Cancer | 14 (33) |
| History of sternotomya | 26 (62) |
| History of IE | 11 (26) |
| Charlson Comorbidity Index, unadjusted for age | |
| 0 | 6 (14) |
| 1–2 | 13 (31) |
| 3–4 | 14 (33) |
| ≥5 | 9 (21) |
| Functional independence | |
| Independent for activities of daily living | 29 (69) |
| Needs help for some activities of daily living | 10 (24) |
| Needs help for most activities of daily living | 3 (7) |
| Prosthetic cardiac device | |
| Any cardiac device | 40 (95) |
| Prosthetic valve—any kind | 27 (64) |
| Prosthetic valve—transcatheter implantation | 5 (12) |
| Ascending aorta prosthetic graft | 8 (19) |
| Cardiac implantable electronic device | 24 (57) |
| Parameters . | Overall (n = 42) . |
|---|---|
| General characteristics | |
| Age, median (IQR), y | 73 (61–82) |
| Male sex | 36 (86) |
| BMI <18.5, kg/m2 | 4 (10) |
| Comorbidities | |
| Chronic renal failure | 7 (17) |
| Chronic congestive heart failure | 9 (21) |
| Chronic respiratory disease | 9 (21) |
| Chronic hepatic disease | 2 (5) |
| Dementia | 5 (12) |
| Stroke | 4 (9) |
| Diabetes mellitus | 12 (28) |
| Person who injects drugs | 1 (2) |
| Cancer | 14 (33) |
| History of sternotomya | 26 (62) |
| History of IE | 11 (26) |
| Charlson Comorbidity Index, unadjusted for age | |
| 0 | 6 (14) |
| 1–2 | 13 (31) |
| 3–4 | 14 (33) |
| ≥5 | 9 (21) |
| Functional independence | |
| Independent for activities of daily living | 29 (69) |
| Needs help for some activities of daily living | 10 (24) |
| Needs help for most activities of daily living | 3 (7) |
| Prosthetic cardiac device | |
| Any cardiac device | 40 (95) |
| Prosthetic valve—any kind | 27 (64) |
| Prosthetic valve—transcatheter implantation | 5 (12) |
| Ascending aorta prosthetic graft | 8 (19) |
| Cardiac implantable electronic device | 24 (57) |
Percentages were rounded to the nearest whole number. Qualitative variables are reported as n (%). Activities of daily living are defined as in the ADL Barthel Index: walking, feeding, dressing, toileting, bathing, and transferring. There were no missing data except for body mass index (n = 39, 3 missing values).
Abbreviations: BMI, body mass index; IE, infective endocarditis.
aOf note, 4 patients had a history of sternotomy cardiac surgery, which was not related to valve replacement (coronary bypass surgery).
| Parameters . | Overall (n = 42) . |
|---|---|
| General characteristics | |
| Age, median (IQR), y | 73 (61–82) |
| Male sex | 36 (86) |
| BMI <18.5, kg/m2 | 4 (10) |
| Comorbidities | |
| Chronic renal failure | 7 (17) |
| Chronic congestive heart failure | 9 (21) |
| Chronic respiratory disease | 9 (21) |
| Chronic hepatic disease | 2 (5) |
| Dementia | 5 (12) |
| Stroke | 4 (9) |
| Diabetes mellitus | 12 (28) |
| Person who injects drugs | 1 (2) |
| Cancer | 14 (33) |
| History of sternotomya | 26 (62) |
| History of IE | 11 (26) |
| Charlson Comorbidity Index, unadjusted for age | |
| 0 | 6 (14) |
| 1–2 | 13 (31) |
| 3–4 | 14 (33) |
| ≥5 | 9 (21) |
| Functional independence | |
| Independent for activities of daily living | 29 (69) |
| Needs help for some activities of daily living | 10 (24) |
| Needs help for most activities of daily living | 3 (7) |
| Prosthetic cardiac device | |
| Any cardiac device | 40 (95) |
| Prosthetic valve—any kind | 27 (64) |
| Prosthetic valve—transcatheter implantation | 5 (12) |
| Ascending aorta prosthetic graft | 8 (19) |
| Cardiac implantable electronic device | 24 (57) |
| Parameters . | Overall (n = 42) . |
|---|---|
| General characteristics | |
| Age, median (IQR), y | 73 (61–82) |
| Male sex | 36 (86) |
| BMI <18.5, kg/m2 | 4 (10) |
| Comorbidities | |
| Chronic renal failure | 7 (17) |
| Chronic congestive heart failure | 9 (21) |
| Chronic respiratory disease | 9 (21) |
| Chronic hepatic disease | 2 (5) |
| Dementia | 5 (12) |
| Stroke | 4 (9) |
| Diabetes mellitus | 12 (28) |
| Person who injects drugs | 1 (2) |
| Cancer | 14 (33) |
| History of sternotomya | 26 (62) |
| History of IE | 11 (26) |
| Charlson Comorbidity Index, unadjusted for age | |
| 0 | 6 (14) |
| 1–2 | 13 (31) |
| 3–4 | 14 (33) |
| ≥5 | 9 (21) |
| Functional independence | |
| Independent for activities of daily living | 29 (69) |
| Needs help for some activities of daily living | 10 (24) |
| Needs help for most activities of daily living | 3 (7) |
| Prosthetic cardiac device | |
| Any cardiac device | 40 (95) |
| Prosthetic valve—any kind | 27 (64) |
| Prosthetic valve—transcatheter implantation | 5 (12) |
| Ascending aorta prosthetic graft | 8 (19) |
| Cardiac implantable electronic device | 24 (57) |
Percentages were rounded to the nearest whole number. Qualitative variables are reported as n (%). Activities of daily living are defined as in the ADL Barthel Index: walking, feeding, dressing, toileting, bathing, and transferring. There were no missing data except for body mass index (n = 39, 3 missing values).
Abbreviations: BMI, body mass index; IE, infective endocarditis.
aOf note, 4 patients had a history of sternotomy cardiac surgery, which was not related to valve replacement (coronary bypass surgery).
Endocarditis Initial Episode: Characteristics
All IE events were community-acquired (Table 2). The 2 most frequent microorganisms were Enterococcus faecalis (15/42, 36%) and Staphylococcus aureus (12/42, 29%, all methicillin-susceptible except 1). IE was left-sided in 26 cases (62%), CIED-associated in 9 (21%), right-sided in 4 (10%), both right- and left-sided in 1 case (2%), and IE location unknown in 2 (5%). Twenty-seven patients had PVE, among whom 19 had late PVE (70%) and 8 had early PVE (30%). Considering complications, 6 patients presented a perivalvular abscess (14%), 15 had an embolic complication (36%), and 3 had acute heart failure (7%). None presented with an atrioventricular block or septic shock.
| Parameters . | Overall (n = 42) . |
|---|---|
| IE localization | |
| CIED lead infection without proven valvular involvement | 9 (21) |
| Aortic valve | 19 (45) |
| Including ascending aorta prosthetic graft | 7 (17) |
| Mitral valve | 4 (9) |
| Including prosthetic mitral valve | 2 (5) |
| Pulmonary valve | 2 (5) |
| Including prosthetic pulmonary valve | 0 (0) |
| Tricuspid valve | 2 (5) |
| Including prosthetic tricuspid valve | 0 (0) |
| >1 valvular location | 6 (12) |
| Causative pathogen | |
| Enterococcus faecalis | 15 (36) |
| Staphylococcus aureus | 12 (29) |
| Including MRSA | 1 (2) |
| Streptococci spp. | 6 (14) |
| Coagulase-negative staphylococci | 4 (9) |
| Polymicrobial | 2 (5) |
| Candida albicans | 1 (2) |
| Enterobacter cloacae | 1 (2) |
| Uncertain | 1 (2) |
| Diagnosis classification (according to 2023 Duke modified criteria) | |
| Definite IE | 38 (91) |
| Possible IE | 4 (9) |
| Laboratory tests and imaging | |
| Positive blood cultures | 39 (93) |
| Transesophageal echocardiography performed | 33 (79) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment start | 38 (90) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment end | 20 (48) |
| Lesion type | |
| Vegetation | 20 (48) |
| Abscess | 6 (14) |
| Surgical indication | |
| Yes | 38 (90) |
| Heart failure | 1 (2) |
| Infection control including device infection | 34 (81) |
| Embolism prevention | 1 (2) |
| Several | 2 (5) |
| Complication | |
| Embolism | 15 (36) |
| Including cerebral embolism | 6 (14) |
| Acute heart failure | 3 (7) |
| Hemodynamic failure | 0 (0) |
| Atrioventricular block | 0 (0) |
| Parameters . | Overall (n = 42) . |
|---|---|
| IE localization | |
| CIED lead infection without proven valvular involvement | 9 (21) |
| Aortic valve | 19 (45) |
| Including ascending aorta prosthetic graft | 7 (17) |
| Mitral valve | 4 (9) |
| Including prosthetic mitral valve | 2 (5) |
| Pulmonary valve | 2 (5) |
| Including prosthetic pulmonary valve | 0 (0) |
| Tricuspid valve | 2 (5) |
| Including prosthetic tricuspid valve | 0 (0) |
| >1 valvular location | 6 (12) |
| Causative pathogen | |
| Enterococcus faecalis | 15 (36) |
| Staphylococcus aureus | 12 (29) |
| Including MRSA | 1 (2) |
| Streptococci spp. | 6 (14) |
| Coagulase-negative staphylococci | 4 (9) |
| Polymicrobial | 2 (5) |
| Candida albicans | 1 (2) |
| Enterobacter cloacae | 1 (2) |
| Uncertain | 1 (2) |
| Diagnosis classification (according to 2023 Duke modified criteria) | |
| Definite IE | 38 (91) |
| Possible IE | 4 (9) |
| Laboratory tests and imaging | |
| Positive blood cultures | 39 (93) |
| Transesophageal echocardiography performed | 33 (79) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment start | 38 (90) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment end | 20 (48) |
| Lesion type | |
| Vegetation | 20 (48) |
| Abscess | 6 (14) |
| Surgical indication | |
| Yes | 38 (90) |
| Heart failure | 1 (2) |
| Infection control including device infection | 34 (81) |
| Embolism prevention | 1 (2) |
| Several | 2 (5) |
| Complication | |
| Embolism | 15 (36) |
| Including cerebral embolism | 6 (14) |
| Acute heart failure | 3 (7) |
| Hemodynamic failure | 0 (0) |
| Atrioventricular block | 0 (0) |
Results are displayed as n (%). Percentages were rounded to the nearest whole number. Polymicrobial infections were traced in 1 case to Corynebacterium spp. + Staphylococcus epidermidis and in the other case to Streptococcus gallolyticus + Staphylococcus epidermidis.
Abbreviations: [18F] FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; CIED, cardiac implantable electronic device; MRSA, methicillin-resistant Staphylococcus aureus; WBC, white blood cell.
| Parameters . | Overall (n = 42) . |
|---|---|
| IE localization | |
| CIED lead infection without proven valvular involvement | 9 (21) |
| Aortic valve | 19 (45) |
| Including ascending aorta prosthetic graft | 7 (17) |
| Mitral valve | 4 (9) |
| Including prosthetic mitral valve | 2 (5) |
| Pulmonary valve | 2 (5) |
| Including prosthetic pulmonary valve | 0 (0) |
| Tricuspid valve | 2 (5) |
| Including prosthetic tricuspid valve | 0 (0) |
| >1 valvular location | 6 (12) |
| Causative pathogen | |
| Enterococcus faecalis | 15 (36) |
| Staphylococcus aureus | 12 (29) |
| Including MRSA | 1 (2) |
| Streptococci spp. | 6 (14) |
| Coagulase-negative staphylococci | 4 (9) |
| Polymicrobial | 2 (5) |
| Candida albicans | 1 (2) |
| Enterobacter cloacae | 1 (2) |
| Uncertain | 1 (2) |
| Diagnosis classification (according to 2023 Duke modified criteria) | |
| Definite IE | 38 (91) |
| Possible IE | 4 (9) |
| Laboratory tests and imaging | |
| Positive blood cultures | 39 (93) |
| Transesophageal echocardiography performed | 33 (79) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment start | 38 (90) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment end | 20 (48) |
| Lesion type | |
| Vegetation | 20 (48) |
| Abscess | 6 (14) |
| Surgical indication | |
| Yes | 38 (90) |
| Heart failure | 1 (2) |
| Infection control including device infection | 34 (81) |
| Embolism prevention | 1 (2) |
| Several | 2 (5) |
| Complication | |
| Embolism | 15 (36) |
| Including cerebral embolism | 6 (14) |
| Acute heart failure | 3 (7) |
| Hemodynamic failure | 0 (0) |
| Atrioventricular block | 0 (0) |
| Parameters . | Overall (n = 42) . |
|---|---|
| IE localization | |
| CIED lead infection without proven valvular involvement | 9 (21) |
| Aortic valve | 19 (45) |
| Including ascending aorta prosthetic graft | 7 (17) |
| Mitral valve | 4 (9) |
| Including prosthetic mitral valve | 2 (5) |
| Pulmonary valve | 2 (5) |
| Including prosthetic pulmonary valve | 0 (0) |
| Tricuspid valve | 2 (5) |
| Including prosthetic tricuspid valve | 0 (0) |
| >1 valvular location | 6 (12) |
| Causative pathogen | |
| Enterococcus faecalis | 15 (36) |
| Staphylococcus aureus | 12 (29) |
| Including MRSA | 1 (2) |
| Streptococci spp. | 6 (14) |
| Coagulase-negative staphylococci | 4 (9) |
| Polymicrobial | 2 (5) |
| Candida albicans | 1 (2) |
| Enterobacter cloacae | 1 (2) |
| Uncertain | 1 (2) |
| Diagnosis classification (according to 2023 Duke modified criteria) | |
| Definite IE | 38 (91) |
| Possible IE | 4 (9) |
| Laboratory tests and imaging | |
| Positive blood cultures | 39 (93) |
| Transesophageal echocardiography performed | 33 (79) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment start | 38 (90) |
| [18F]FDG PET/CT or radiolabeled WBC SPECT/CT at treatment end | 20 (48) |
| Lesion type | |
| Vegetation | 20 (48) |
| Abscess | 6 (14) |
| Surgical indication | |
| Yes | 38 (90) |
| Heart failure | 1 (2) |
| Infection control including device infection | 34 (81) |
| Embolism prevention | 1 (2) |
| Several | 2 (5) |
| Complication | |
| Embolism | 15 (36) |
| Including cerebral embolism | 6 (14) |
| Acute heart failure | 3 (7) |
| Hemodynamic failure | 0 (0) |
| Atrioventricular block | 0 (0) |
Results are displayed as n (%). Percentages were rounded to the nearest whole number. Polymicrobial infections were traced in 1 case to Corynebacterium spp. + Staphylococcus epidermidis and in the other case to Streptococcus gallolyticus + Staphylococcus epidermidis.
Abbreviations: [18F] FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; CIED, cardiac implantable electronic device; MRSA, methicillin-resistant Staphylococcus aureus; WBC, white blood cell.
The majority of patients (38/42, 90%) presented a theoretical indication for surgery according to the 2023 ESC guidelines. The surgical operation that was theoretically indicated was CIED extraction for 16 patients (16/38, 42%), valvular surgery for 19 patients (19/38, 50%), and both in 3 cases (3/38, 8%). Notably, CIED extraction was grouped with other surgical procedures as it was associated with a high risk of conversion to sternotomy, mostly due to the long duration of material implanted (>4 years in 75%, 12/14). Out of 38 cases for which surgery was indicated, surgery was not performed in 31 cases (79%). The main reason was that the surgical procedure was deemed too complex/risky (10/31), notably because of history of sternotomy (in 7 cases, including 2 patients who had already undergone reoperation), high surgical risk (11/31; due to functional dependence [6/11] or significant comorbidities despite functional independence [5/11]), patient refusal (3/31), or several of the previously mentioned reasons (7/31).
Almost every patient underwent nuclear imaging at the beginning of their treatment (38/42, 90%), with at least 1 pathological fixation being identified in 81% of cases (31/38). Of the patients in this group, 64% (20/31) underwent a second round of imaging at the end of curative treatment. The results showed that 35% (7/20) of these patients still had pathological fixation on the IE site.
Initial Treatment and Suppressive Antimicrobial Therapy
Initial antimicrobial regimens are detailed in Supplementary Table 1. Only 11 patients (26%) received initial treatment according to the ESC 2023 guidelines. In particular, more than half of the patients (52%, 22/42) had an initial treatment duration >60 days, the most common reason being an ascending aorta prosthetic graft infection (7/22, 32%). Most patients received only intravenous treatment during the initial treatment phase (74%, 31/42). For the 11 patients who were switched to the oral route during the initial treatment phase, the median delay before switching (IQR) was 45 (27–49) days.
The most frequent indication for SAT was surgery not performed despite theoretical indication (28/42, 67%). The others were incomplete removal of prosthetic cardiac device (6/42, 14%), uncontrolled infection source (4/42, 10%), persistent abnormal uptake on nuclear imaging at the end of curative treatment (1/42, 2%), or a combination (no surgery despite indication and persistent uptake on nuclear imaging in 3/42, 7%). Of note, persistent abnormal uptake on nuclear imaging was reported in 4 patients for whom SAT was initiated, but was not considered a main reason for SAT initiation. Previous IE with the same microorganism was also reported in 7 patients with SAT initiation, but previous IE was not considered the main reason to start SAT.
The antimicrobials prescribed for SAT were mainly doxycycline (19/42, 45%) and amoxicillin (19/42, 45%), followed by trimethoprim-sulfamethoxazole (2/42, 5%), fluconazole (1/42, 2%), and minocycline (1/42, 2%). Prescribed antimicrobials and their dosages are available in Supplementary Table 2.
Treatment and Outcomes
The median follow-up time (IQR) was 398 (194–663) days. The median follow-up time under SAT (IQR) was 326 (125–511) days.
The Kaplan-Meier estimator for adverse events during SAT at 1 year was 12.1% (0.2%–22.6%). In absolute terms, 5 patients (12%) experienced drug adverse events. An episode of acute kidney failure related to trimethoprim-sulfamethoxazole was the only severe adverse effect. It was resolved after antimicrobial interruption, and SAT was continued with a bitherapy (doxycycline + amoxicillin) without any further complications. Other events were minor: mild diarrhea, epigastric burning or moderate thrombocytopenia on doxycycline, blue coloration of scars on minocycline. SAT was maintained in all patients. Of note, no case of Clostridioides difficile colitis was reported.
The Kaplan-Meier estimator for 1-year SAT interruption was 13.1% (1.7%–23.2%). In absolute terms, 6 patients (14%) interrupted SAT. For 4 patients, SAT was interrupted unintentionally (prescription oversight in context of polymedication or interruption by a physician unaware of antimicrobial indication), whereas for the 2 other patients, interruption was intentional following infection source control (dental care or polypectomy). Poor treatment compliance was reported in 5 patients (12%) in medical records.
Five patients (12%) presented with relapse (n = 3) or reinfection (n = 2) during follow-up, within a median time (IQR) of 111 (93–335) days. All events led to rehospitalization. In all patients, the main reason for SAT prescription was the inability to perform surgery despite theoretical indication. No patient had stopped SAT at the time of recurrence. In 4 patients, a probable reason for recurrence was identified (sustained intravenous drug use, catheter infection during SAT, associated vascular graft infection, major colonic diverticulosis). Of the 3 patients who experienced a relapse, in 2 cases the documented strain remained susceptible to the molecule used for SAT according to disc diffusion antibiotic susceptibility testing, despite other differences in susceptibility (Table 3). In the third case, blood cultures remained negative, but the patient was treated as a relapse. Two of the 3 patients who experienced relapse underwent follow-up nuclear imaging before SAT initiation. Patient 6 showed CIED uptake, while patient 7 had normal results.
| . | Age, y . | Material . | First Bacterial species . | Initial Infection Source . | Initial Antibiotic Regimen (Molecules, Total Duration, Route) . | Indication for SAT . | SAT Molecule . | Second Bacterial species . | Delay Before Recurrence, da . | Clinical Context/Reason for Recurrence (if Identified) . | Follow-up Information . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| POSSIBLE RELAPSE | 67 | CIED | MSSA Doxycycline: S Rifampin: S Fosfomycin: S | Unknown | OXACILLIN -> CEFAZOLIN -> CEFAZOLIN + FOSFOMYCIN -> CEFAZOLIN + RIFAMPIN (51 d, IV only) | Surgery not performed despite indication | Doxycycline | MSSA Doxycycline: S Rifampin: R Fosfomycin: R | 32 | Possible endovascular femoral graft infection | 2 other possible relapses Hospitalized for 4th MSSA bacteriemia at end point time |
| 84 | CIED + prosthetic mitral, aortic, and tricuspid valves | E. faecalis | Colon cancer, cured | AMOXICILLIN + CEFTRIAXONE (46 d, IV only) | Severalb | Amoxicillin | Negative blood cultures, treated as E. faecalis | 111 | No additional reason identified | CIED fixation on nuclear imaging at the end of curative treatment; another relapse 6 mo later | |
| 84 | None | E. faecalis Amoxicillin: S Gentamicin: S | Colonic polyp, removed | AMOXICILLIN + CEFTRIAXONE -> AMOXICILLIN + GENTAMICIN (44 d, IV only) | Surgery not performed despite indication | Amoxicillin | E. faecalis Amoxicillin: S Gentamicin: R | 335 | Profuse sigmoid diverticulosis. | SAT dosage increase after relapse (amoxicillin 2 g to 3 g); no relapse at last follow-up | |
| REINFECTION | 31 | CIED + prosthetic aortic and mitral valves | S. epidermidis | IV drug use | DAPTOMYCIN + FOSFOMYCIN -> RIFAMPIN + LEVOFLOXACIN (105 d, IV followed by oral switch at day 49) | Surgery not performed despite indication | Trimethoprim-sulfamethoxazole | S. agalactiae | 93 | Unabated IV drug use | Death linked with recurrence |
| 48 | Prosthetic aortic valve + AAPG | MSSA | Pneumonia | CEFAZOLIN + GENTAMICIN -> DAPTOMYCIN + CEFTAROLINE -> CLINDAMYCIN + LEVOFLOXACIN -> DALBAVANCIN (95 d, IV only) | Surgery not performed despite indication | Doxycycline | MRSE | 829 | IV catheter infection; immunocompromised patient (lung transplant) | Switch SAT for minocycline; no relapse at last follow-up |
| . | Age, y . | Material . | First Bacterial species . | Initial Infection Source . | Initial Antibiotic Regimen (Molecules, Total Duration, Route) . | Indication for SAT . | SAT Molecule . | Second Bacterial species . | Delay Before Recurrence, da . | Clinical Context/Reason for Recurrence (if Identified) . | Follow-up Information . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| POSSIBLE RELAPSE | 67 | CIED | MSSA Doxycycline: S Rifampin: S Fosfomycin: S | Unknown | OXACILLIN -> CEFAZOLIN -> CEFAZOLIN + FOSFOMYCIN -> CEFAZOLIN + RIFAMPIN (51 d, IV only) | Surgery not performed despite indication | Doxycycline | MSSA Doxycycline: S Rifampin: R Fosfomycin: R | 32 | Possible endovascular femoral graft infection | 2 other possible relapses Hospitalized for 4th MSSA bacteriemia at end point time |
| 84 | CIED + prosthetic mitral, aortic, and tricuspid valves | E. faecalis | Colon cancer, cured | AMOXICILLIN + CEFTRIAXONE (46 d, IV only) | Severalb | Amoxicillin | Negative blood cultures, treated as E. faecalis | 111 | No additional reason identified | CIED fixation on nuclear imaging at the end of curative treatment; another relapse 6 mo later | |
| 84 | None | E. faecalis Amoxicillin: S Gentamicin: S | Colonic polyp, removed | AMOXICILLIN + CEFTRIAXONE -> AMOXICILLIN + GENTAMICIN (44 d, IV only) | Surgery not performed despite indication | Amoxicillin | E. faecalis Amoxicillin: S Gentamicin: R | 335 | Profuse sigmoid diverticulosis. | SAT dosage increase after relapse (amoxicillin 2 g to 3 g); no relapse at last follow-up | |
| REINFECTION | 31 | CIED + prosthetic aortic and mitral valves | S. epidermidis | IV drug use | DAPTOMYCIN + FOSFOMYCIN -> RIFAMPIN + LEVOFLOXACIN (105 d, IV followed by oral switch at day 49) | Surgery not performed despite indication | Trimethoprim-sulfamethoxazole | S. agalactiae | 93 | Unabated IV drug use | Death linked with recurrence |
| 48 | Prosthetic aortic valve + AAPG | MSSA | Pneumonia | CEFAZOLIN + GENTAMICIN -> DAPTOMYCIN + CEFTAROLINE -> CLINDAMYCIN + LEVOFLOXACIN -> DALBAVANCIN (95 d, IV only) | Surgery not performed despite indication | Doxycycline | MRSE | 829 | IV catheter infection; immunocompromised patient (lung transplant) | Switch SAT for minocycline; no relapse at last follow-up |
Abbreviations: AAPG, ascending aorta prosthetic graft; CIED, cardiac implantable electronic device; IV, intravenous; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSA, methicillin-susceptible Staphylococcus aureus; R, resistant (disc diffusion susceptibility testing); S, susceptible (disc diffusion susceptibility testing); SAT, suppressive antimicrobial therapy.
aAs SAT was a lifelong prescription and none of these 5 patients interrupted SAT before recurrence, delay before recurrence and duration of SAT intake are identical.
bSeveral reasons: Surgery not performed despite indication + persistent fixation on nuclear imaging at the end of curative treatment.
| . | Age, y . | Material . | First Bacterial species . | Initial Infection Source . | Initial Antibiotic Regimen (Molecules, Total Duration, Route) . | Indication for SAT . | SAT Molecule . | Second Bacterial species . | Delay Before Recurrence, da . | Clinical Context/Reason for Recurrence (if Identified) . | Follow-up Information . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| POSSIBLE RELAPSE | 67 | CIED | MSSA Doxycycline: S Rifampin: S Fosfomycin: S | Unknown | OXACILLIN -> CEFAZOLIN -> CEFAZOLIN + FOSFOMYCIN -> CEFAZOLIN + RIFAMPIN (51 d, IV only) | Surgery not performed despite indication | Doxycycline | MSSA Doxycycline: S Rifampin: R Fosfomycin: R | 32 | Possible endovascular femoral graft infection | 2 other possible relapses Hospitalized for 4th MSSA bacteriemia at end point time |
| 84 | CIED + prosthetic mitral, aortic, and tricuspid valves | E. faecalis | Colon cancer, cured | AMOXICILLIN + CEFTRIAXONE (46 d, IV only) | Severalb | Amoxicillin | Negative blood cultures, treated as E. faecalis | 111 | No additional reason identified | CIED fixation on nuclear imaging at the end of curative treatment; another relapse 6 mo later | |
| 84 | None | E. faecalis Amoxicillin: S Gentamicin: S | Colonic polyp, removed | AMOXICILLIN + CEFTRIAXONE -> AMOXICILLIN + GENTAMICIN (44 d, IV only) | Surgery not performed despite indication | Amoxicillin | E. faecalis Amoxicillin: S Gentamicin: R | 335 | Profuse sigmoid diverticulosis. | SAT dosage increase after relapse (amoxicillin 2 g to 3 g); no relapse at last follow-up | |
| REINFECTION | 31 | CIED + prosthetic aortic and mitral valves | S. epidermidis | IV drug use | DAPTOMYCIN + FOSFOMYCIN -> RIFAMPIN + LEVOFLOXACIN (105 d, IV followed by oral switch at day 49) | Surgery not performed despite indication | Trimethoprim-sulfamethoxazole | S. agalactiae | 93 | Unabated IV drug use | Death linked with recurrence |
| 48 | Prosthetic aortic valve + AAPG | MSSA | Pneumonia | CEFAZOLIN + GENTAMICIN -> DAPTOMYCIN + CEFTAROLINE -> CLINDAMYCIN + LEVOFLOXACIN -> DALBAVANCIN (95 d, IV only) | Surgery not performed despite indication | Doxycycline | MRSE | 829 | IV catheter infection; immunocompromised patient (lung transplant) | Switch SAT for minocycline; no relapse at last follow-up |
| . | Age, y . | Material . | First Bacterial species . | Initial Infection Source . | Initial Antibiotic Regimen (Molecules, Total Duration, Route) . | Indication for SAT . | SAT Molecule . | Second Bacterial species . | Delay Before Recurrence, da . | Clinical Context/Reason for Recurrence (if Identified) . | Follow-up Information . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| POSSIBLE RELAPSE | 67 | CIED | MSSA Doxycycline: S Rifampin: S Fosfomycin: S | Unknown | OXACILLIN -> CEFAZOLIN -> CEFAZOLIN + FOSFOMYCIN -> CEFAZOLIN + RIFAMPIN (51 d, IV only) | Surgery not performed despite indication | Doxycycline | MSSA Doxycycline: S Rifampin: R Fosfomycin: R | 32 | Possible endovascular femoral graft infection | 2 other possible relapses Hospitalized for 4th MSSA bacteriemia at end point time |
| 84 | CIED + prosthetic mitral, aortic, and tricuspid valves | E. faecalis | Colon cancer, cured | AMOXICILLIN + CEFTRIAXONE (46 d, IV only) | Severalb | Amoxicillin | Negative blood cultures, treated as E. faecalis | 111 | No additional reason identified | CIED fixation on nuclear imaging at the end of curative treatment; another relapse 6 mo later | |
| 84 | None | E. faecalis Amoxicillin: S Gentamicin: S | Colonic polyp, removed | AMOXICILLIN + CEFTRIAXONE -> AMOXICILLIN + GENTAMICIN (44 d, IV only) | Surgery not performed despite indication | Amoxicillin | E. faecalis Amoxicillin: S Gentamicin: R | 335 | Profuse sigmoid diverticulosis. | SAT dosage increase after relapse (amoxicillin 2 g to 3 g); no relapse at last follow-up | |
| REINFECTION | 31 | CIED + prosthetic aortic and mitral valves | S. epidermidis | IV drug use | DAPTOMYCIN + FOSFOMYCIN -> RIFAMPIN + LEVOFLOXACIN (105 d, IV followed by oral switch at day 49) | Surgery not performed despite indication | Trimethoprim-sulfamethoxazole | S. agalactiae | 93 | Unabated IV drug use | Death linked with recurrence |
| 48 | Prosthetic aortic valve + AAPG | MSSA | Pneumonia | CEFAZOLIN + GENTAMICIN -> DAPTOMYCIN + CEFTAROLINE -> CLINDAMYCIN + LEVOFLOXACIN -> DALBAVANCIN (95 d, IV only) | Surgery not performed despite indication | Doxycycline | MRSE | 829 | IV catheter infection; immunocompromised patient (lung transplant) | Switch SAT for minocycline; no relapse at last follow-up |
Abbreviations: AAPG, ascending aorta prosthetic graft; CIED, cardiac implantable electronic device; IV, intravenous; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSA, methicillin-susceptible Staphylococcus aureus; R, resistant (disc diffusion susceptibility testing); S, susceptible (disc diffusion susceptibility testing); SAT, suppressive antimicrobial therapy.
aAs SAT was a lifelong prescription and none of these 5 patients interrupted SAT before recurrence, delay before recurrence and duration of SAT intake are identical.
bSeveral reasons: Surgery not performed despite indication + persistent fixation on nuclear imaging at the end of curative treatment.
Fourteen patients (33%) from the cohort died during follow-up. The median time to death (IQR) was 406 (202–758) days. In 5 patients, death was documented in the medical record; death was related to IE in 1 case (proven reinfection), was IE-unrelated in 2 cases (clearly identified external cause), and in 2 patients it was impossible to formally exclude a participation of IE a posteriori although it was judged unlikely (end-stage heart failure, without any bacterial documentation on blood cultures). In the remaining 9 patients, the cause of death was unknown. Overall, the 1-year survival rate was 84.3% (73.5%–96.7%), and the 1-year survival rate without relapse or reinfection was 74.1% (61.4%–89.4%). Kaplan-Meier survival curves are displayed in Figure 2.
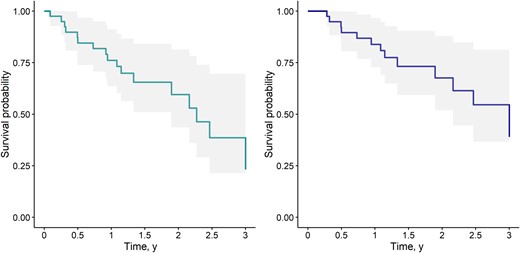
Kaplan-Meier curves. Confidence intervals are shown in shaded areas. Time is displayed in years. Right panel: Survival without reinfection or relapse until year 3 (composite end point: death, relapse, or reinfection). Left panel: Overall survival until year 3 (end point: death).
DISCUSSION
To our knowledge, this study reports the largest cohort of patients receiving long-term oral SAT for IE. This treatment was mostly prescribed to patients with prosthetic cardiac devices, often with a history of multiple previous surgical procedures. Contraindications to surgery as indicated in ESC guidelines were the main SAT indication. Adverse events related to SAT occurred in 12% of patients but were mainly minor. Despite SAT, the relapse or reinfection rate was substantial (12%), with 7% of possible relapse occurring with the same microorganism species. None of these breakthrough infections were clearly linked to emergence of antimicrobial resistance. Overall, the 1-year survival rate was 84.3% (73.5%–96.7%), and the 1-year survival rate without relapse or reinfection was 74.1% (61.4%–89.4%). The 1-year mortality rate (one-third) underscores the frailty of this population.
In 2021, Camazon et al. [7] published one of the first studies focusing on this topic. Beforehand, available data were case reports [13–16] and small case series focusing on specific contexts such as CIED infections [6] or aortic graft infections [17]. Camazon's cohort included 32 patients not undergoing surgery for IE despite indication. Out of these, 24 patients received SAT treatment after their initial curative therapy. In half of the cases, the initial treatment was prolonged compared with ESC guidelines, which is similar to our study. The patients’ baseline characteristics, clinical context, relapse rate (12.5%), and cumulative 3-year mortality (37%) are remarkably similar to our findings. This consistent high relapse rate underscores that breakthrough infections represent a substantial risk despite SAT. Recurrence risk is usually reported to be between 3% and 10% [18], which is slightly lower than our finding. Of note, this risk varies widely depending on several factors [19]. Notably, PVE and IE caused by S. aureus and Enterococcus spp. are associated with an increased rate of recurrence [8, 20]. These risk factors are common features in our population and add up to suboptimal treatment. The target population for SAT constitutes a “very high-risk” group for IE relapse, and SAT has proved insufficient to fully prevent this risk.
Lifelong SAT was prescribed for most patients in our study. However, no data currently support this strategy compared with time-limited SAT. Camazon et al. [7] used nuclear imaging to guide treatment duration: 9 patients underwent positron emission tomography/computed tomography (PET/CT), and SAT was stopped in all cases because of PET/CTs showing a reduction or termination of metabolic uptake. None of these patients experienced infection relapse after SAT interruption, highlighting the potential of PET/CT to predict when risk of recurrence is low enough to allow SAT interruption [21]. Although PET/CT is recommended in 2023 ESC guidelines for monitoring response to antimicrobial treatment in patients with SAT, few studies report follow-up data [22], although it is crucial for the interpretation of the results. Further prospective studies are therefore needed to provide evidence for possible future guidelines.
The absence of guidelines regarding SAT leads to heterogeneous protocols among centers, undermining its evaluation. While amoxicillin and doxycycline were mostly prescribed in our study, others reported the first-line usage of trimethoprim-sulfamethoxazole [6, 7], levofloxacin [7], or cephalexin [6]. The adverse events rate was albeit very close between studies. All these molecules are recommended for SAT in prosthetic joint infections [3]. Amoxicillin and doxycycline were favored in our center because of their relatively narrow spectrum, their good tolerance profile, their high oral bioavailability, and their low cost. We used trimethoprim-sulfamethoxazole as an alternative treatment because of its potential toxicity, but no adverse events were reported in these 2 patients. SAT’s ecological impact on gut microbiota, notably colonization/infection by Clostridioides difficile or multidrug-resistant bacteria, is one of the main concerns regarding SAT tolerance. While this impact was difficult to evaluate in our study, no clinical event related to such a phenomenon was reported. Furthermore, a study of the gut microbiota of 17 patients receiving SAT provides reassuring data [23]. Another Achilles heel of SAT therapeutic strategy is compliance, a fortiori in older patients receiving multiple medications. Thus, other options may deserve further examination, such as long-acting intravenous antibiotics. For example, dalbavacin has been used in left ventricular assist device infections [24] and in a case report of inoperable staphylococcal prosthetic valve endocarditis [14]. Their largely higher cost would be the main obstacle for implementation, but this strategy may prove to be cost-effective.
Several studies [25, 26] show that surgery improves the prognosis of older patients when their functional status is good. Nevertheless, comorbidities may lead to surgical hesitancy by referring physicians, surgeons, and patients themselves [27], leading to less frequent performance of curative surgery [28]. SAT is not an alternative option for surgery and remains a suboptimal and palliative approach. Although a default option, it may be the best one when surgery risk appears unacceptable for the surgeon or the patient, when implanted material removal is incomplete, or when the portal of entry cannot be cured. This approach targets a vulnerable population, as highlighted by the fact that 13 patients for whom SAT was prescribed died before initiating treatment. IE epidemiology is changing: The proportion of older patients is increasing [26, 29], and so is the rate of device-related infections. Clinical situations like those reported in our cohort are likely to become more and more frequent, highlighting the need to gather evidence on this strategy. These findings also highlight the importance of incorporating geriatricians into endocarditis teams to better guide therapeutic choices.
Our study has several limitations. First, because of its retrospective design, some information was unavailable such as the cause of out-hospital death. Thus, we cannot exclude that some of these deaths were due to IE recurrence, leading to an underestimation of recurrence rate. The indication for SAT depending mainly on local experts’ opinions, the results of this monocentric study may not be representative of practices in other centers. The reported decision to prescribe SAT was based solely on the reasons mentioned in the Endocarditis Team meeting reports. It is possible that other factors, such as a history of IE or persistent uptake on nuclear imaging, may have also influenced this decision. Furthermore, the number of patients was small, and there was no control group. Therefore, we cannot draw any conclusions on the efficacy of SAT to prevent IE. Our population exhibited considerable heterogeneity, notably due to the inclusion of patients requiring either CIED extraction or valvular surgery. We deemed this grouping acceptable, particularly considering the associated risk of conversion to sternotomy during CIED extraction.
Immediate implications can be drawn from our study, notably to guide physicians on this empirical practice. First, patients receiving SAT need to receive therapeutic education, and co-prescribers need to be informed of this treatment. A “wallet information card” in the model of those produced for IE primary prevention could be easily implemented for secondary prevention with SAT. This could prevent, at least in part, unintentional interruption. Possible adverse events, as well as signs evoking recurrence, should also be documented to enable early management. In our cohort, 3 out of 5 recurrences occurred >6 months after SAT initiation. This implies that close monitoring is essential and should be maintained for a prolonged time. We also suggest that follow-up should include regular biological surveillance, including blood cultures, to enable early detection of reinfection/relapse.
A randomized controlled trial, or by default a case–control study with propensity scores, is necessary to establish SAT efficiency and to refine its indication. Moreover, conducting prospective follow-up with whole-genome sequencing of isolated strains and comparative bacterial genomics could help distinguish between reinfection and relapse. Understanding the underlying mechanisms of recurrences is the first step toward identifying and preventing risk factors for recurrences. Our cohort can be used as a pilot study to build such trials. Together, these findings could help build evidence-based guidelines.
CONCLUSIONS
In this retrospective cohort of 42 patients receiving SAT for IE, the typical patient profile was a patient with a cardiac device who could not undergo surgery because of high surgical risk. Adverse events on SAT were reported for 12% of the patients but were mostly minor. The estimate of survival without recurrence was 74.1% (61.4%–89.4%) after 1 year. These findings need to be confirmed by larger prospective studies to standardize and guide this empirical practice, outside the scope of current official guidelines.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank the patients who agreed to be included in the study, as well as all the members of the multidisciplinary Endocarditis Team of Bichat Hospital for their work. Thanks to Dr. Empana for his helpful advice about survival analysis.
Author contributions. All authors are part of the Bichat Hospital multidisciplinary Endocarditis Team and take part in the clinical management of patients. L.D., M.T., C.B., F.M., S.D., and A.L.B. participated in the creation of the database. A.L.B. analyzed the data, and the results were discussed and interpreted with M.T. and L.D. A.L.B. drafted the manuscript. All authors read, revised, and approved the final manuscript.
Access to data. The corresponding author, A. L. Beaumont, has full access to the data set.
Financial support. No external funding was received.
References
Author notes
Potential conflicts of interest. L.D. was supported by Gilead for the attendance of 2 conferences. All other authors report no potential conflicts.





Comments