-
PDF
- Split View
-
Views
-
Cite
Cite
Norman L Beatty, Harpreet Kaur, Kathryn Schlaffer, Kathryn Thompson, Preeti Manavalan, Zulmarie R Rijos, Abhinandan A Raman, H Richard Droghini, Elise M O’Connell, Subarachnoid Neurocysticercosis Case Series Reveals a Significant Delay in Diagnosis—Requiring a High Index of Suspicion Among Those at Risk, Open Forum Infectious Diseases, Volume 11, Issue 5, May 2024, ofae176, https://doi.org/10.1093/ofid/ofae176
Close - Share Icon Share
Abstract
Subarachnoid neurocysticercosis can be challenging to recognize, which often leads to a delay in diagnosis. We report 3 cases presenting as chronic headache disorders that highlight the unique manifestations seen with this form of neurocysticercosis and the role that the infectious diseases consultant can play in ensuring a timely diagnosis.
Neurocysticercosis (NCC) is an infection of the central nervous system with the larval stage of the cestode Taenia solium. The most common manifestation of NCC is seizures due to the parasite's location in the brain parenchyma. However, when the parasite is found in the subarachnoid spaces of the basilar cisterns, sylvian fissures, or spine, it often manifests as a relapsing meningitis with severe headaches and intracranial hypertension. Subarachnoid NCC is sometimes referred to as racemose NCC since the cysts may proliferate as grape-like clusters without a scolex. Consequences of long-standing subarachnoid NCC include cerebral vascular accident, subarachnoid hemorrhage, and communicating hydrocephalus. Here, we present 3 cases in which the diagnosis was delayed from 1 to 5 years after the patient presented with compatible imaging and clinical symptoms.
CASE 1
A 35-year-old female presented to the emergency department with the “worst headache of [her] life.” She had immigrated to the United States from Guatemala 12 years prior. Additional medical history included hypertension, diabetes, and chronic kidney disease.
Physical examination at the time did not reveal any neurologic deficits. Computed tomography (CT) imaging and lumbar puncture results are shown in Table 1. The headache resolved without any specific intervention, and she was discharged. She was diagnosed with migraines. However, CT at that time revealed a 2.8 × 1.4–cm cystic-appearing structure in the perimesencephalic cistern, interpreted as a “likely arachnoid cyst.” Over the next 5 years, she presented several times to the emergency department for evaluation of similar intermittent headache symptoms and was treated symptomatically. Repeat imaging during this time continued to show slow expansion of the cystic area and local mass effect but was still read as an arachnoid cyst.
CSF Parameters, Diagnostics, and MRI Findings Prior to and at the Time of Subarachnoid Neurocysticercosis Diagnosis.
| . | CSF Parameters . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Point . | WBC, Cells/mm3 . | Neutrophil, % . | Lymphocyte, % . | Eosinophil, % . | Protein, mg/dL . | Glucose, mg/dL . | T solium IgG ELISA . | QuantiFERON Gold . | Reported Brain MRI Findings . |
| Patient 1 | Negative | ||||||||
| 1: 5 y prior to diagnosis | 35 | … | … | … | 36 | 94 | … | Cystic CSF isointense process expanding the quadrigeminal, ambient, suprasellar, and central sylvian cisterns with displacement of the upper brainstem | |
| At diagnosis | 235 | 8 | 71 | 11 | 129 | 62 | Positive | Large multilobulated extra-axial cystic mass involving multiple cisterns; enlarging and consistent with epidermoid cyst | |
| Patient 2 | Negative | ||||||||
| 2 y prior to diagnosis | 68 | … | 79 | 8 | … | … | Positive | Basilar meningitis and communicating hydrocephalus | |
| At diagnosis | 27 | 0 | 80 | 10 | 123 | 43 | Negative | Stable basilar leptomeningeal enhancement | |
| Patient 3 | Positive | ||||||||
| 4 mo prior to diagnosis | 6 | 8 | 6 | 10 | 21 | 56 | … | Chronic hydrocephalus and papilledema | |
| At diagnosis | 56 | … | 58 | 22 | 42 | 52 | Positive | Leptomeningeal enhancement at basal cisterns; enlargement and possible septations of basal cisterns | |
| . | CSF Parameters . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Point . | WBC, Cells/mm3 . | Neutrophil, % . | Lymphocyte, % . | Eosinophil, % . | Protein, mg/dL . | Glucose, mg/dL . | T solium IgG ELISA . | QuantiFERON Gold . | Reported Brain MRI Findings . |
| Patient 1 | Negative | ||||||||
| 1: 5 y prior to diagnosis | 35 | … | … | … | 36 | 94 | … | Cystic CSF isointense process expanding the quadrigeminal, ambient, suprasellar, and central sylvian cisterns with displacement of the upper brainstem | |
| At diagnosis | 235 | 8 | 71 | 11 | 129 | 62 | Positive | Large multilobulated extra-axial cystic mass involving multiple cisterns; enlarging and consistent with epidermoid cyst | |
| Patient 2 | Negative | ||||||||
| 2 y prior to diagnosis | 68 | … | 79 | 8 | … | … | Positive | Basilar meningitis and communicating hydrocephalus | |
| At diagnosis | 27 | 0 | 80 | 10 | 123 | 43 | Negative | Stable basilar leptomeningeal enhancement | |
| Patient 3 | Positive | ||||||||
| 4 mo prior to diagnosis | 6 | 8 | 6 | 10 | 21 | 56 | … | Chronic hydrocephalus and papilledema | |
| At diagnosis | 56 | … | 58 | 22 | 42 | 52 | Positive | Leptomeningeal enhancement at basal cisterns; enlargement and possible septations of basal cisterns | |
Ellipses (…) indicate data not available/not reported.
Abbreviations: CSF, cerebral spinal fluid; ELISA, enzyme-linked immunosorbent assay; MRI, magnetic resonance imaging; T solium, Taenia solium; WBC, white blood cell.
CSF Parameters, Diagnostics, and MRI Findings Prior to and at the Time of Subarachnoid Neurocysticercosis Diagnosis.
| . | CSF Parameters . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Point . | WBC, Cells/mm3 . | Neutrophil, % . | Lymphocyte, % . | Eosinophil, % . | Protein, mg/dL . | Glucose, mg/dL . | T solium IgG ELISA . | QuantiFERON Gold . | Reported Brain MRI Findings . |
| Patient 1 | Negative | ||||||||
| 1: 5 y prior to diagnosis | 35 | … | … | … | 36 | 94 | … | Cystic CSF isointense process expanding the quadrigeminal, ambient, suprasellar, and central sylvian cisterns with displacement of the upper brainstem | |
| At diagnosis | 235 | 8 | 71 | 11 | 129 | 62 | Positive | Large multilobulated extra-axial cystic mass involving multiple cisterns; enlarging and consistent with epidermoid cyst | |
| Patient 2 | Negative | ||||||||
| 2 y prior to diagnosis | 68 | … | 79 | 8 | … | … | Positive | Basilar meningitis and communicating hydrocephalus | |
| At diagnosis | 27 | 0 | 80 | 10 | 123 | 43 | Negative | Stable basilar leptomeningeal enhancement | |
| Patient 3 | Positive | ||||||||
| 4 mo prior to diagnosis | 6 | 8 | 6 | 10 | 21 | 56 | … | Chronic hydrocephalus and papilledema | |
| At diagnosis | 56 | … | 58 | 22 | 42 | 52 | Positive | Leptomeningeal enhancement at basal cisterns; enlargement and possible septations of basal cisterns | |
| . | CSF Parameters . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Point . | WBC, Cells/mm3 . | Neutrophil, % . | Lymphocyte, % . | Eosinophil, % . | Protein, mg/dL . | Glucose, mg/dL . | T solium IgG ELISA . | QuantiFERON Gold . | Reported Brain MRI Findings . |
| Patient 1 | Negative | ||||||||
| 1: 5 y prior to diagnosis | 35 | … | … | … | 36 | 94 | … | Cystic CSF isointense process expanding the quadrigeminal, ambient, suprasellar, and central sylvian cisterns with displacement of the upper brainstem | |
| At diagnosis | 235 | 8 | 71 | 11 | 129 | 62 | Positive | Large multilobulated extra-axial cystic mass involving multiple cisterns; enlarging and consistent with epidermoid cyst | |
| Patient 2 | Negative | ||||||||
| 2 y prior to diagnosis | 68 | … | 79 | 8 | … | … | Positive | Basilar meningitis and communicating hydrocephalus | |
| At diagnosis | 27 | 0 | 80 | 10 | 123 | 43 | Negative | Stable basilar leptomeningeal enhancement | |
| Patient 3 | Positive | ||||||||
| 4 mo prior to diagnosis | 6 | 8 | 6 | 10 | 21 | 56 | … | Chronic hydrocephalus and papilledema | |
| At diagnosis | 56 | … | 58 | 22 | 42 | 52 | Positive | Leptomeningeal enhancement at basal cisterns; enlargement and possible septations of basal cisterns | |
Ellipses (…) indicate data not available/not reported.
Abbreviations: CSF, cerebral spinal fluid; ELISA, enzyme-linked immunosorbent assay; MRI, magnetic resonance imaging; T solium, Taenia solium; WBC, white blood cell.
Five years following her initial presentation, she developed a severe progressive headache over the course of 1 month with nausea, vomiting, and photophobia. She was afebrile and normotensive, and the remainder of the physical examination, including neurologic assessment, did not reveal any abnormality. Laboratory results were remarkable for a white blood cell count of 11.1 K cells/mcL (reference range, 4.5–11.0 K cells/mcL). CT and subsequent magnetic resonance imaging (MRI) suggested obstructive hydrocephalus due to mass effect from a large subarachnoid cyst burden, although this was later revealed to be communicating hydrocephalus. Ophthalmologic evaluation did not reveal papilledema, although the opening pressure was elevated at 28 cm H2O. Results from T solium quantitative polymerase chain reaction (qPCR) and antigen from the cerebrospinal fluid (CSF) were positive, confirming the diagnosis of subarachnoid NCC.
CASE 2
A 68-year-old male without a significant medical history presented with a brief period of night sweats, followed by 3 months of stilted gait, speech difficulty, incontinence, and headache. He was born and raised in Zacatecas, Mexico. He was initially thought to have Parkinson disease based on clinical symptoms. However, subsequent imaging demonstrated communicating hydrocephalus and basilar meningitis, and CSF showed a lymphocytic pleocytosis. A ventriculoperitoneal shunt was placed, with improvement in symptoms. CSF T solium antibody testing was positive at that time, but its significance was overlooked. He was followed clinically over the next 2 years for aseptic meningitis of unknown etiology with minimal symptoms aside from mild memory loss. Repeat CSF testing was done given continued MRI findings, and it revealed eosinophilic pleocytosis (Table 1). Interestingly, CSF at this time was negative for T solium antibody testing. Metagenomic sequencing was unable to determine an etiology. Diagnosis of subarachnoid NCC was confirmed by positive findings based on T solium qPCR and antigen from CSF.
CASE 3
A 24-year-old woman who was born in El Salvador with a history of chronic daily headaches for 16 years presented to the emergency department with an increase in headache severity. Head CT revealed hydrocephalus, confirmed 2 days later by MRI, but no intervention was performed. Six months later she presented again with a headache of increasing severity over 1 month accompanied by nausea and vomiting, and imaging findings revealed a chronic communicating hydrocephalus with papilledema. The patient underwent right endoscopic third ventriculostomy with initial relief of symptoms. However, over the course of 3 months, she was admitted 3 additional times for severe headaches with evidence of recurrent ventriculomegaly, requiring a shunt and subsequent shunt revisions each time. She ultimately underwent placement of a ventriculoatrial shunt, and the Infectious Diseases service was then consulted for workup. The patient was born in rural El Salvador and had immigrated to the United States 9 years earlier. CSF analysis revealed meningitis (Table 1). Yet, the infectious workup result was negative except for a positive QuantiFERON-TB Gold Plus result, and the patient started empiric treatment for tuberculous meningitis. Results from subsequent adenosine deaminase and Mycobacterium tuberculosis polymerase chain reaction testing of CSF were negative. Broad-based 16S ribosomal RNA sequencing for bacterial, fungal, and mycobacterium of CSF did not detect a pathogen. CSF was positive for T solium–specific IgG by a commercial enzyme-linked immunoassay, and the diagnosis was confirmed by positive findings based on T solium DNA qPCR and antigen in the CSF approximately 1 year after the initial finding of hydrocephalus. Given the alternative diagnosis, treatment for tuberculous meningitis was stopped after 8 weeks.
DISCUSSION
The diagnosis of subarachnoid NCC is typically based on the appearance of cysts in the subarachnoid spaces of the basilar cisterns of the brain, sylvian fissures, or spine. However, subarachnoid cysts can be missed by standard MRI until they are large enough to cause mass effect on adjacent brain parenchyma [1]. Moreover, since this is an uncommon diagnosis to make in a nonendemic setting, radiologists may be unfamiliar with subarachnoid NCC and simply overlook the diagnosis. In these cases, diagnosis requires a high index of suspicion from the clinician. In retrospect, each of these 3 cases had cysts visible in the basilar cisterns, which were missed or misinterpreted by the reading radiologists on multiple occasions (Figure 1). Two cases also presented with communicating hydrocephalus—a known sequelae of long-standing subarachnoid NCC. Case 1 illustrates this progression as the patient presented intermittently with headaches several times over a 5-year period, likely due to relapsing meningitis. Eventually, ventriculomegaly developed and further workup was pursued. In this case, a cyst was seen early in the presentation but was interpreted as a benign arachnoid cyst. The self-resolution of the episodic headaches, which is typical of this disease, likely prolonged the time to diagnosis. In all 3 of our patients, the absence of accompanying parenchymal disease (ie, cysts or calcifications) likely also led to a delay in recognition.
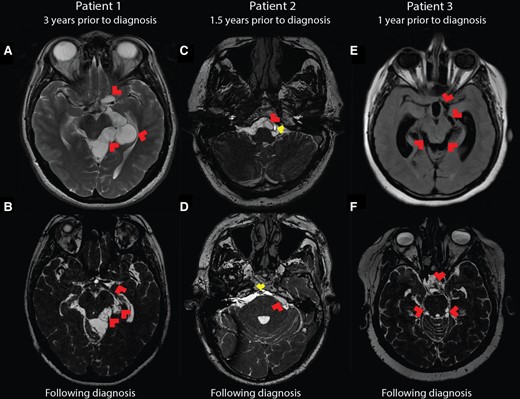
Brain imaging of patients over time. Earliest available MRI for each patient as compared with a BFFE sequence obtained following diagnosis demonstrates high resolution of the subarachnoid neurocysticercosis involvement. A, Patient 1 three years prior to diagnosis. T2 fast recovery fast spin echo sequence shows large cystic lesions throughout the left perimesencephalic space and along the left middle cerebral artery (red arrowheads), interpreted at the time as a benign arachnoid cyst. B, Patient 1 BFFE MRI following diagnosis shows partially collapsed membranes (red arrowheads) in the same locations as previously seen mass lesions. C, Following shunt placement, patient 2 did undergo a high-resolution MRI scan (fast imaging employing steady-state acquisition) 1.5 years prior to diagnosis, but cysts in the basilar cisterns were missed, including the cyst (red arrowhead) abutting the basilar artery (yellow arrowhead). D, Patient 2 following diagnosis underwent BFFE MRI, again showing a cyst near the basilar artery (yellow arrowhead) and others, including the cyst in the cerebellopontine angle (red arrowhead). E, T2 fluid-attenuated inversion recovery sequence of patient 3 one year prior to diagnosis illustrates ventriculomegaly and a subtle mass effect in the perimesencephalic space and around the origin of the left middle cerebral artery (red arrowheads). F, Patient 3 underwent shunt placement, and after the diagnosis was made, BFFE MRI revealed adhesions and cysts in the perimesencephalic space and the suprasellar cistern (red arrowheads). BFFE, balanced fast field echo; MRI, magnetic resonance imaging.
Utilization of MRI sequences with a high signal-to-noise ratio (balanced fast field echo, fast imaging employing steady-state acquisition, or 3-dimensional constructive interference in steady state) has been shown to improve visualization of cysts in the subarachnoid spaces of the brain [1], which can improve the chances of a radiologic diagnosis (Figure 1). One should consider the diagnosis of subarachnoid NCC in meningitis of unclear etiology in an immigrant from Latin America. While the CSF pleocytosis is typically lymphocytic, the presence of eosinophils should increase suspicion [2]. Additionally, a patient presenting with or having a history of hydrocephalus, ischemic stroke, or subarachnoid hemorrhage and inflammatory CSF should be evaluated for subarachnoid NCC, as these are well-described complications of this chronic meningitis [3]. While the use of metagenomic sequencing has been reported to detect subarachnoid NCC [4, 5], this highlights that a negative metagenomic sequencing report does not exclude the diagnosis. Additionally, the detection of T solium–directed antibodies in the CSF can be insensitive [2] and nonspecific for subarachnoid disease [6]. Antibody detection is also highly dependent on the type of assay used [6]. The enzyme-linked immunoelectrotransfer blood test that utilizes lentil lectin–purified parasite antigens is the gold standard serologic test and is most sensitive when testing serum in making any diagnosis of NCC [6]. As a serologic test, a positive antibody signals exposure to the parasite and does not always indicate active disease. A negative test result can effectively rule out extraparenchymal disease; in the setting of compatible brain imaging, however, it can help confirm the diagnosis. In recent years, T solium qPCR [7] and antigen detection [8–10] on CSF have emerged as highly sensitive and specific assays that can rule in or out the diagnosis of active extraparenchymal NCC with a high degree of confidence.
Treatment of subarachnoid NCC is prolonged, and treatment failures have occurred in those given shorter courses of antiparasitic therapy, which are routinely given to patients with parenchymal disease [11]. Most symptoms are due to the inflammation provoked by the degenerating cysts. A prolonged duration of anti-inflammatories, particularly glucocorticoids, is required to avoid intracranial hypertension, stroke, and other sequelae during treatment. While there are no prospective data to inform treatment, many experts favor the use of combination albendazole (15 mg/kg/d) and praziquantel (50 mg/kg/d) with high-dose glucocorticoids (eg, dexamethasone, 0.2 mg/kg/d, or prednisone-equivalent dose) with a slow taper [3]. While patient symptoms rapidly respond to high-dose corticosteroids, shunt surgery should be offered in those with hydrocephalus due to subarachnoid NCC [11]. Current clinical guidelines suggest the use of antiparasitic therapy until there is radiologic resolution of subarachnoid cysts [11]. However, imaging does not normalize for most patients with proven durable cure [12]. T solium qPCR or antigen levels have been shown to be reproducible, and when followed until negative in the CSF, they are highly predictive of cure [7–10]. Yet, these tests are available at only a single reference laboratory in the United States.
In summary, one should consider the diagnosis of subarachnoid NCC in patients from areas endemic for T solium with aseptic meningitis, particularly if eosinophils are present in the CSF or if meningitis is accompanied by hydrocephalus. Additionally, if arachnoid cysts are seen on imaging in a patient with severe recurrent headaches, the diagnosis should be considered. Specialized MRI sequences and biomarker detection in blood or CSF can confirm the diagnosis.
Notes
Patient consent statement. All patients gave written informed consent to complete treatment for subarachnoid neurocysticercosis at the NIH Clinical Center, and all were enrolled under the natural history clinical protocol (NCT00001205) of the NIH Intramural Research Program, which was approved by its institutional review board.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
Author notes
Potential conflicts of interests. All authors: No reported conflicts.





Comments