-
PDF
- Split View
-
Views
-
Cite
Cite
Chloe Lahoud, Andres E Franceschi, Fabiola Reyes, Dimitrios G Moshovitis, Angela Achkar, Antara Chakraborty, Manu Balusu, Jawad Safiia, Eliezer Zachary Nussbaum, Alyssa R Letourneau, Sarah P Hammond, Camille N Kotton, Sophia Koo, 2715. Toxicities and clinical consequences of valganciclovir prophylaxis in solid organ transplant recipients, Open Forum Infectious Diseases, Volume 10, Issue Supplement_2, December 2023, ofad500.2326, https://doi.org/10.1093/ofid/ofad500.2326
Close - Share Icon Share
Abstract
Cytomegalovirus (CMV) commonly affects solid organ transplant (SOT) recipients, with increased risks of rejection, graft failure, and other infections. While valganciclovir (vGCV) prophylaxis is effective in preventing CMV, its use is sometimes hindered by toxicity. We examined the association between vGCV exposure and adverse outcomes in SOT recipients.
We performed a retrospective cohort study in two large academic transplant centers in Boston, with 1650 sequential adult (≥ 18 years of age) lung (343), heart (200), and kidney (1107) transplant recipients from 2010-2016. CMV D+ and/or CMV R+ SOT recipients generally received 3-12 months of vGCV prophylaxis. We used Fisher’s exact tests, Wilcoxon rank-sum tests, and Cox models to examine the relationship between vGCV exposure and adverse events potentially related to vGCV exposure.
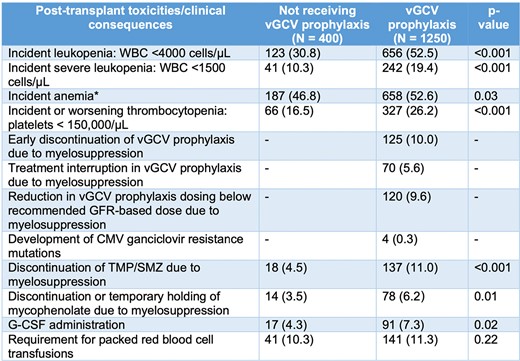
Potential toxicities associated with vGCV exposure. *Hemoglobin < 12 g/dL for women and < 13.5 g/dL for men or worsening anemia (drop >1 g/dL from baseline chronic anemia).
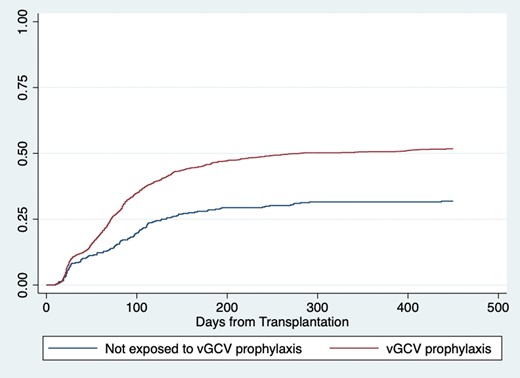
All transplant recipients, Kaplan-Meier cumulative incidence of leukopenia < 4,000 cells/uL (logrank p < 0.001).
Exposure to vGCV prophylaxis in SOT recipients is associated with high rates of hematologic toxicities, although downstream clinical consequences of these toxicities are relatively rare.
Sarah P. Hammond, MD, F2G: Advisor/Consultant|F2G: Grant/Research Support|GSK: Grant/Research Support|Pfizer: Advisor/Consultant|Scynexis: Grant/Research Support|Seres therapeutics: Advisor/Consultant Sophia Koo, MD, SM, Aerium Therapeutics: Advisor/Consultant|GSK: Grant/Research Support|Merck: Grant/Research Support|Scynexis: Grant/Research Support
Author notes
Session: 245. Transplant and Immunocompromised Host Infections
Saturday, October 14, 2023: 12:15 PM
- anemia
- mycophenolate mofetil
- body mass index procedure
- mutation
- renal function
- lung
- granulocyte colony-stimulating factor
- hemoglobin
- adult
- consultants
- disclosure
- recombinant granulocyte colony stimulating factor
- leukocytes
- leukopenia
- opportunistic infections
- rejection (psychology)
- thrombocytopenia
- tissue transplants
- trimethoprim-sulfamethoxazole combination
- infections
- cytomegalovirus
- heart
- kidney
- transplantation
- transplanted organ
- valganciclovir
- uterine fibroids
- myelosuppression
- transplant recipients
- toxic effect
- hematotoxicity
- drug holiday
- adverse event
- prevention






Comments