-
PDF
- Split View
-
Views
-
Cite
Cite
Ashley Lew, Ashley Tippett, Luis W Salazar, Laila Hussaini, Chris Choi, Khalel De Castro, Elizabeth G Taylor, Olivia Reese, Humerazehra Momin, Caroline R Ciric, Amrita Banerjee, Amy E Keane, Laura A Puzniak, Robin Hubler, Srinivas Valluri, Timothy L Wiemken, Benjamin Lopman, Nadine Rouphael, Satoshi Kamidani, Evan J Anderson, Christina A Rostad, 2364. Predictive Ability of mRNA COVID-19 Vaccines Against COVID-19 Disease Severity, Open Forum Infectious Diseases, Volume 10, Issue Supplement_2, December 2023, ofad500.1985, https://doi.org/10.1093/ofid/ofad500.1985
Close - Share Icon Share
Abstract
Prior studies demonstrated the vaccine effectiveness and safety of mRNA COVID-19 vaccines, but additional data is needed regarding the effects of timing and number of doses on disease severity. This study determined predicted protection against severe COVID-19 by number of vaccine doses.
We enrolled adults hospitalized with acute respiratory infection (ARI) and/or related diagnoses at two Emory University hospitals from May 2021 – Aug 2022. This analysis included COVID-19 positive patients among unvaccinated and 2 or 3 doses of an mRNA vaccine. Vaccinations ≤ 14 days prior to admission were excluded. Medical and social histories were obtained from interviews, medical records, and the state vaccine registry. We used stepwise logistic regression to determine dose-specific odds ratios (OR) against severe outcomes (pneumonia (PNA), length of hospital stay (LOS) ≥ 4 days, ICU admission, mechanical ventilation, and death). Analysis was performed using SAS v9.4 software.
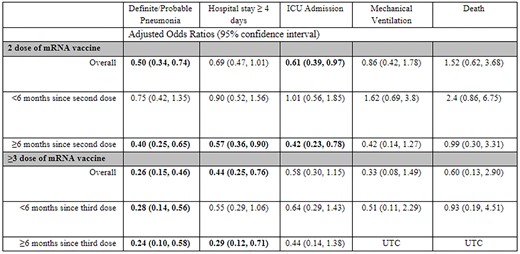
Adjusted odds ratios of mRNA SARS-CoV-2 vaccination by dose number and time since administration against severe disease outcomes in those SARS-CoV-2 positive. OR adjusted for race, age and employment. Unable to calculate (UTC) due to having no patients with the outcome during specified vaccination timeframe and dose count.
Among COVID-19 positive adults hospitalized with ARIs, both two and three doses of a COVID-19 mRNA vaccine predicted protection against severe COVID-19 outcomes, with durability lasting more than 6 months. Future studies should explore the role of additional boosters and vaccine regimens in the prevention of severe COVID-19.
Laura A. Puzniak, PhD. MPH, Pfizer, Inc.: Employee|Pfizer, Inc.: Stocks/Bonds Robin Hubler, MS, Pfizer, Inc.: Employee|Pfizer, Inc.: Stocks/Bonds Srinivas Valluri, PhD, Pfizer Inc: Pfizer Employee and hold Pfizer stocks/options|Pfizer Inc: Ownership Interest|Pfizer Inc: Stocks/Bonds Timothy L. Wiemken, PhD, Pfizer Inc: Employee|Pfizer Inc: Stocks/Bonds Benjamin Lopman, PhD, Epidemiological Research and Methods, LLC: Advisor/Consultant|Hillevax, Inc: Advisor/Consultant Nadine Rouphael, MD, Icon, EMMES, Sanofi, Seqirus, Moderna: Advisor/Consultant Satoshi Kamidani, MD, CDC: Grant/Research Support|Emergent BioSolutions: Grant/Research Support|NIH: Grant/Research Support|Pfizer Inc: Grant/Research Support Evan J. Anderson, MD, GSK: Advisor/Consultant|GSK: Grant/Research Support|Janssen: Advisor/Consultant|Janssen: Grant/Research Support|Kentucky Bioprocessing, Inc.: Safety Monitoring Board|Moderna: Advisor/Consultant|Moderna: Grant/Research Support|Moderna: Currently an employee|Moderna: Stocks/Bonds|Pfizer: Advisor/Consultant|Pfizer: Grant/Research Support|Sanofi Pasteur: Advisor/Consultant|Sanofi Pasteur: Grant/Research Support|Sanofi Pasteur: Safety Monitoring Board|WCG/ACI Clinical: Data Adjudication Board Christina A. Rostad, MD, BioFire Inc.: Grant/Research Support|GlaxoSmithKline Biologicals: Grant/Research Support|Janssen: Grant/Research Support|MedImmune LLC: Grant/Research Support|Meissa Vaccines, Inc.: RSV vaccine technology|Merck & Co., Inc.: Grant/Research Support|Micron Technology, Inc.: Grant/Research Support|Moderna, Inc.: Grant/Research Support|Novavax: Grant/Research Support|PaxVax: Grant/Research Support|Pfizer, Inc.: Grant/Research Support|Regeneron: Grant/Research Support|Sanofi Pasteur: Grant/Research Support
Author notes
Session: 234. COVID-19: Vaccines
Saturday, October 14, 2023: 12:15 PM
- medical records
- kidney failure, chronic
- adult
- biological products
- centers for disease control and prevention (u.s.)
- comorbidity
- consultants
- disclosure
- employment
- hematological diseases
- hospitals, university
- immunocompromised host
- intensive care unit
- length of stay
- ownership
- pneumonia
- respiratory syncytial virus vaccines
- respiratory tract infections
- rna, messenger
- safety
- software
- vaccination
- vaccines
- unemployment
- disability
- mechanical ventilation
- autologous chondrocyte implantation
- prevention
- severity of illness
- employee
- higher education
- mrna vaccines
- sars-cov-2
- covid-19
- covid-19 vaccines
- vaccine efficacy





Comments