-
PDF
- Split View
-
Views
-
Cite
Cite
Annabelle M de St. Maurice, Yasmeen Z Qwaider, Tess Stopczynski, John V Williams, Marian G Michaels, Leila C Sahni, Julie A Boom, Andrew J Spieker, Eileen J Klein, Janet A Englund, Mary A Staat, Elizabeth P Schlaudecker, Rangaraj Selvarangan, Jennifer E Schuster, Yingtao Zhou, Heidi L Moline, Peter G Szilagyi, Natasha B Halasa, Geoffrey A Weinberg, 1104. Characteristics and Seasonality of Children Hospitalized with Parainfluenza Virus Infections (PIV), including PIV-4, New Vaccine Surveillance Network, 2016–2020, Open Forum Infectious Diseases, Volume 10, Issue Supplement_2, December 2023, ofad500.077, https://doi.org/10.1093/ofid/ofad500.077
Close - Share Icon Share
Abstract
Human parainfluenza viruses (PIV) are a leading cause of pediatric acute respiratory infection (ARI), yet limited data exist on PIV-associated hospitalizations, particularly from serotype PIV-4, a serotype reported to cause severe disease. We describe the characteristics and seasonality of PIV-associated ARI hospitalizations, focusing on PIV-4.
During 12/01/2016-03/31/2020, we enrolled children hospitalized with fever or respiratory symptoms at the 7 children’s hospitals of the CDC’s New Vaccine Surveillance Network. Molecular testing for rhino/enterovirus (RV/EV), respiratory syncytial virus (RSV), influenza virus, PIV (serotypes 1-4), human metapneumovirus, and adenovirus were performed on mid-turbinate nasal or throat specimens collected from enrolled children. Parent interviews and chart abstractions were performed.
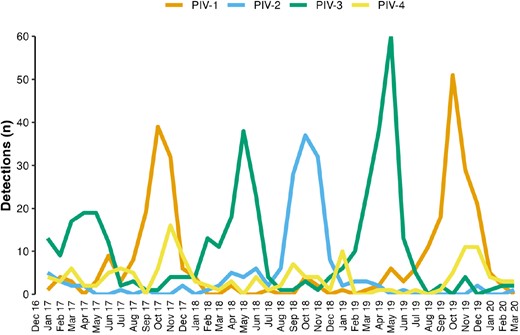
Parainfluenza virus (PIV) detections from hospitalized children by month and PIV serotype, New Vaccine Surveillance Network, 2016–2020
PIV infections were detected in 6% of ARI hospitalizations and occurred throughout the year, with seasonal variation in serotype dominance. Half of children with PIV-related hospitalizations were healthy (without underlying medical conditions). Severe disease (requirement for ICU care) was common in all PIV-associated hospitalizations. Research on PIV vaccines is warranted, with consideration to include PIV-4.
John V. Williams, MD, Merck: Grant/Research Support|Quidel: Board Member Marian G. Michaels, MD, MPH, Merck: Grant/Research Support|Viracor: Grant/Research Support Janet A. Englund, MD, Ark Biopharma: Advisor/Consultant|AstraZeneca: Advisor/Consultant|AstraZeneca: Grant/Research Support|GlaxoSmithKline: Grant/Research Support|Meissa Vaccines: Advisor/Consultant|Merck: Grant/Research Support|Moderna: Advisor/Consultant|Moderna: Grant/Research Support|Pfizer: Advisor/Consultant|Pfizer: Grant/Research Support|Sanofi Pasteur: Advisor/Consultant Mary A. Staat, MD, MPH, CDC: Grant/Research Support|Cepheid: Grant/Research Support|Merck: Grant/Research Support|NIH: Grant/Research Support|Pfizer: Grant/Research Support|Up-To-Date: Honoraria Elizabeth P. Schlaudecker, MD, MPH, Pfizer: Grant/Research Support|Sanofi Pasteur: Advisor/Consultant Rangaraj Selvarangan, BVSc, PhD, D(ABMM), FIDSA, FAAM, Abbott: Honoraria|Altona Diagnostics: Grant/Research Support|Baebies Inc: Advisor/Consultant|BioMerieux: Advisor/Consultant|BioMerieux: Grant/Research Support|Bio-Rad: Grant/Research Support|Cepheid: Grant/Research Support|GSK: Advisor/Consultant|Hologic: Grant/Research Support|Lab Simply: Advisor/Consultant|Luminex: Grant/Research Support Natasha B. Halasa, MD, MPH, Merck: Grant/Research Support|Quidell: Grant/Research Support|Quidell: donation of kits|Sanofi: Grant/Research Support|Sanofi: vaccine support Geoffrey A. Weinberg, MD, Merck & Co: Honoraria
Author notes
Session: 123. Respiratory Infections
Friday, October 13, 2023: 10:30 AM







Comments