-
PDF
- Split View
-
Views
-
Cite
Cite
Flaminia Olearo, Veronica Zanichelli, Aimilia Exarchakou, Anna Both, Ilker Uςkay, Martin Aepfelbacher, Holger Rohde, The Impact of Antimicrobial Therapy Duration in the Treatment of Prosthetic Joint Infections Depending on Surgical Strategies: A Systematic Review and Meta-analysis, Open Forum Infectious Diseases, Volume 10, Issue 5, May 2023, ofad246, https://doi.org/10.1093/ofid/ofad246
Close - Share Icon Share
Abstract
The aim of this systematic review was to address the question if short antibiotic treatment (SAT; at least 4 but <12 weeks) versus long antibiotic treatment (LAT) affects outcomes in prosthetic joint infections (PJIs). Database research (Medline, Embase, Web of Science, Scopus, Cochrane) retrieved 3740 articles, of which 10 studies were included in the analysis. Compared to LAT, 11% lower odds of treatment failure in the SAT group were found, although the difference was not statistically significant (pooled odds ratio, 0.89 [95% confidence interval, .53–1.50]). No difference in treatment failure was found between SAT and LAT once stratified by type of surgery, studies conducted in the United States versus Europe, study design, and follow-up. There is still no conclusive evidence that antibiotic treatment of PJIs for 12 weeks or longer is associated with better outcomes, irrespective of the type of surgical procedure. Most recent, high-quality studies tend to favor longer antibiotic courses, making them preferable in most situations.
Infections are an important cause of prosthetic joint failure, associated with high morbidity that has a significant impact on patients’ quality of life [1]. In 2015, the prevalence of prosthetic joint–associated infections (PJIs) varied from 0.79% to 1.24% for hip arthroplasty and from 0.88% to 1.28% for knee prosthesis [2]; with the aging of the population and the increasing prevalence of obesity worldwide, these numbers will likely increase in the near future [3]. Management of PJI is complex and requires a combination of antibiotic therapy and 1 of 3 surgical options: debridement, antibiotics, and implant retention (DAIR); arthroplasty exchange (1- or 2-stage exchange arthroplasty [SEA]); or permanent resection of the prosthesis including amputation. DAIR is usually reserved to early infections (<3 months after surgery), whereas SEA is the preferred approach to treat delayed (3–24 months after surgery) or late (>24 months after surgery) infections [4]. Finally, arthrodesis and amputation are reserved to a limited number of complicated cases, for example, in case of critical soft tissue conditions.
Despite the evident importance for clinical management, the optimal duration of systemic antibiotic therapy after DAIR or SEA remains a matter of debate. In case of DAIR, due to implant retention, experts usually recommend longer treatments (at least 3 months for PJI of the hip and 6 months for PJI of the knee) [5–7]. However, in many centers shorter antibiotic therapies for PJI are used with positive experiences [8]. For SEA, different antibiotic therapy durations are suggested depending on the surgical approach chosen (ie, 1- or 2-stage exchange). In particular, longer durations are usually encouraged in case of 1-stage exchange if the infection is caused by Staphylococcus aureus (2–6 weeks intravenous followed by 3 months of oral treatment), whereas for other pathogens the recommendations are similar in case of 1- or 2-stage exchange (4–6 weeks of intravenous or highly bioavailable oral antibiotic therapy) [5]. However, in the majority of settings, treatment duration is often based on expert opinion rather than on international guidelines, with the tendency to privilege longer treatments (including sometimes lifelong treatments) [9]. As a matter of fact, despite the insufficient evidence to assess the therapeutic efficacy of long antibiotic therapy, in particular chronic antibiotic suppression [10], for PJI after surgical treatment, there is an increasing body of literature showing the negative impact of longer courses of antibiotics, often associated with side effects (15.4% of the cases, whereas the mean rate of adverse effects leading to discontinuation of suppressive antibiotic therapy is 4.3% [8]), development of antibiotic resistance, and increased costs for the healthcare system [8, 10, 11]. Furthermore, the success of therapy depends on the compliance of patients, which is usually low in the community, in particular for prolonged antibiotic therapy [12]. Consequently, in recent years, several randomized controlled trials (RCTs) have been conducted to prove the efficacy of shorter versus longer courses of antibiotic therapy for the treatment of many entities from the spectrum of infectious diseases [13].
So far, 3 systematic reviews (1 with meta-analysis) have been conducted on the topic of duration of antibiotic therapy in PJI [14–16]. In 2019, the meta-analysis by Yen et al showed no significant difference in terms of treatment failure between short and long courses of antibiotics (pooled relative risk, 0.87 [95% confidence interval {CI}, .62–1.22]) [14]. However, 4 studies published after 2018 (including a recent RCT [17]) were not included in this analysis. Additionally, previously published systematic reviews had few data to allow a comparison on the antibiotic treatment duration between DAIR and SEA.
Therefore, as new evidence has become available in the past few years, we decided to perform a new systematic review and meta-analysis to explore whether these new findings could bring more clarity to this clinically highly relevant question. In extension to previous meta-analyses, we also decided to describe the impact of total treatment duration depending on the surgical procedure used (DAIR vs SEA). The duration of intravenous versus oral antibiotic therapy or the use of local antibiotics was not the focus of this study.
METHODS
Selection Criteria, Search Strategy, Screening Process, and Data Extraction
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18] were used for this systematic review.
The primary objective was to assess if a shorter duration of antibiotic therapy (SAT; defined as ≥4 but <12 weeks of total antibiotics) after PJI surgery is associated with a similar risk of treatment failure compared to longer antibiotic treatment courses (LAT; excluding long-term suppressive therapy). A second objective was to stratify results by type of surgical procedure (DAIR vs SEA), year of publication, duration of follow-up, geographical location of the study, and study design. To be eligible, studies had to clearly report the criteria used to diagnose the PJI (ie, presence of intra-articular pus and at least 1 positive microbiological culture from intraoperative tissue specimens) or the criteria used by either the Musculoskeletal Infection Society [7], the Infectious Diseases Society of America [5], or the European Bone and Joint Infection Society [19]. Studies restricted to patients with native arthritis or osteomyelitis (ie, no prosthetic material) and studies addressing PJI caused by specific pathogens (Mycobacterium tuberculosis, Brucella spp, Borrelia burgdorferi, and Neisseria gonorrhoeae) were excluded.
Eligible studies were any study including adult patients (≥18 years of age) surgically treated for a PJI (any joint) and receiving antibiotic treatment, provided that the duration of antibiotic treatment and the outcome at the end of treatment were clearly reported by study authors. Over the years, studies applied different definitions to indicate short or long treatment durations, and in particular some older studies used the term “long treatment” even when total treatment duration was <12 weeks. Therefore for the purpose of our analysis, all studies were reviewed and included according to our SAT (≥4 weeks but <12 weeks) or LAT (excluding suppression therapy) group definitions.
With the exception of reviews and case reports, all study designs were eligible. Only studies published in English, Italian, German, and French were included.
To identify appropriate studies, a search strategy was used that combined key terms for “antibiotics,” “prosthetic joint infection,” and “duration of treatment.” Medline, Embase, Web of Science, Scopus, and Cochrane were searched from their inception up to 30 June 2022. Details for each literature database query are provided in the Supplementary material. All titles and abstracts were screened by 1 reviewer (F. O.) through DistillerSR software using an algorithm based on the inclusion and exclusion criteria previously mentioned. Full texts were then independently screened by 2 reviewers (F. O. and V. Z.). Any disagreement on inclusion or exclusion of studies was resolved through discussion with a third author (A. E.).
Risk of Bias Assessment
A quality assessment of included studies has been performed; for nonrandomized trials, we used the Newcastle-Ottawa Quality Assessment Scale tool [20] and for randomized trials the Jadad Score tool [21]. The assessment was performed by 1 author (F. O.) and double-checked independently by a second author (V. Z.).
Meta-analysis
For the purposes of this meta-analysis, recurrence of the infection, microbiological failure, clinical failure, necessity to retreat, and death were considered proxies of treatment failure and their presence or absence was used to calculate the odds of treatment failure in the SAT and LAT group for each study.
Eligible studies identified from database searches were used to perform a meta-analysis in which the odds ratio (OR) of treatment failure (binary outcome) was compared between SAT and LAT groups (head-to-head). Unadjusted ORs were calculated for each study using the data reported in each publication (adjusted estimates of the effect of duration were inconsistently reported across studies and were therefore not used). The pooled OR for treatment failure was calculated using a random-effects model because of the level of between-study heterogeneity (eg, different study populations, type of surgical procedure, antibiotic treatment, definitions of short/long treatment duration) leading to fluctuations in the magnitude of the effect.
Heterogeneity was assessed by the I2 value, with 25%, 50%, and 75% the cutoff points used for low, medium, and high heterogeneity, respectively, as illustrated by Higgins et al [22]. A sensitivity analysis was performed to further investigate the reasons for the heterogeneity between studies (ie, to assess whether any study in particular contributed to the high heterogeneity). This was done by repeating the meta-analysis excluding each study 1 at a time to check whether any improvement in the heterogeneity (ie, I2 <50%) could be seen [23].
Finally, we did a meta-regression to investigate if pooled effect estimates varied between subgroups of studies (DAIR vs SEA, geographic location, study design, duration of follow-up, and year of publication).
Publication bias was assessed graphically by funnel plot and by performing an Egger test for small-study effects.
All analyses were conducted in Stata software (version 16; StataCorp, College Station, Texas).
Because this project is a systematic review and meta-analysis of previously published data only, it did not require ethics approval. This systematic review was not registered.
RESULTS
Study Selection
The search strategy identified 3740 articles in published peer-reviewed journals (Figure 1). These included 1291 citations from Medline, 251 from Scopus, 578 from Web of Science, 1587 from Embase, and 33 from Cochrane Library. In total, 907 duplicates were identified and removed, leaving 2833 study titles and abstracts to be reviewed, of which 2794 (98.6%) were deemed irrelevant. Thirty-nine published manuscripts were reviewed in full and 10 studies were included in the qualitative synthesis and meta-analysis.
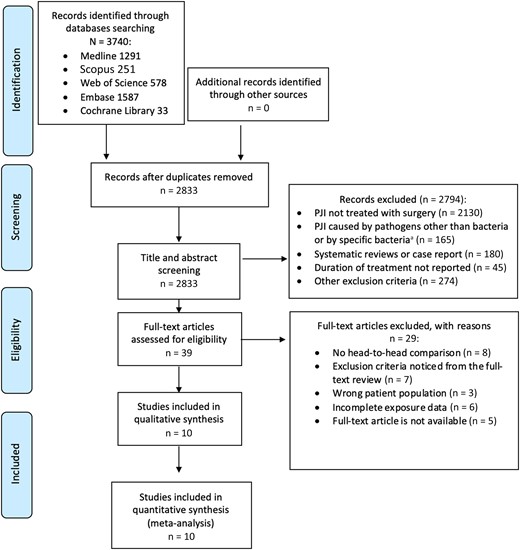
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. aBrucella spp, Mycobacterium tuberculosis, Borrelia burgdorferi, and Neisseria gonorrhoeae. Abbreviation: PJI, prosthetic joint–associated infection.
Study Characteristics
The 10 included studies were published between 2007 and 2022. Study characteristics are presented in Table 1. Details on age distribution, infected joint and causative microorganisms, antibiotic regimens, differences in clinical characteristics between the 2 groups (SAT vs LAT), criteria used to diagnose PJIs, and duration of follow-up for each study included are available in the Supplementary Table 1. Overall, 6 of 10 were cohort studies and 4 of 10 were RCTs. Four studies were conducted in the United States and 6 in Europe.
Characteristics of the Studies Included in the Systematic Review and Meta-analysis
| First Author and Publication Year . | Country . | Sample Size, No. . | Study Design . | Definition of the Outcome . | Type of Surgery . | Duration of antibiotic Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|
| Kelly 2022 [24] | USA | 211 | RCoh, single-center | Primary outcome: presence of resistant organisms in any subsequent infection in the same joint; secondary outcome: time to recurrence of PJI | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for ≥2 wk after 2nd OP | Failure: 24 (15%) LAT, 11 (21%) SAT, P = .35 |
| Yang 2020 [25] | USA | 142 | RCT, multicenter | Failure due to reinfection | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for 3 mo after 2nd OP | Failure: 20 (28.6%) SAT, 9 (12.5%) LAT, P = .012 |
| Lora-Tamayo 2016 [26] | Spain | 63 | RCT, multicenter | Cure rate defined as clinical signs of infection resolved and progressive decrease in CRP levels | DAIR | SAT: 8 wk LAT: 6 mo (knee), 3 mo (hip) | Cure (ITT analysisa): LAT 19 (57.6%), SAT 22 (73.3%) Difference (LAT – SAT): −15.7% (95% CI, −39.2% to 7.8%) |
| Bernard 2021 [17] | France | 404 | RCT, multicenter | Persistent infection within 2 y after the end of antibiotic therapy | DAIR, n = 167b; 1-stage exchange, n = 150c; 2-stage exchange, n = 87d | SAT: 6 wk LAT: 12 wk | Failure (SAT vs LAT): unadjusted RD, 8.7 (95% CI, 1.8–15.6); adjusted RD, 9.0 (95% CI, 2.3–15.7) |
| Benkabouche 2019 [27] | Switzerland | 38 | RCT, single-center | Remission of infection defined as presence of ≥2 positive cultures + systemic signs of infection at the operative site | 2-stage exchange | SAT: 4 wk (±3 d); LAT: 6 wk (±3 d) | Recurrence of clinical infection: 1 (7.1%) patient in SAT, 3 (15%) in LAT, P = .64 |
| Chaussade 2017 [8] | France and Switzerland | 87 | RCoh, multicenter | Remission 1 y after DAIR: (1) No signs of infection after 1 y of follow-up; (2) no need to continue antibiotic therapy | DAIR | SAT: 6 wk AB LAT: 12 wk AB | Remission (SAT vs LAT): unadjusted OR, 0.87 (95% CI, .35–2.16), P = .76; adjusted OR, 0.76 (95% CI, .27–2.10), P = .6 |
| Puhto 2012 [28] | Finland | 55e | RCoh, historical control, single-center | Treatment failure defined as (1) patient referred for 2-stage exchange surgery at any time during follow-up, (2) patient had symptoms or signs of infection after the end of antibiotic treatment or (3) suppressive antibiotic treatment | DAIR | SAT: 8 wk AB LAT: 12 wk | Failure: 16 (46%) in SAT vs 10 (50%) in LAT, P = .76 |
| El Helou 2011 [29] | USA | 208 | RCoh, single-center | Treatment failure defined as: (1) recurrence of PJI at any time after 2nd OP; (2) death caused by PJI at any time after reimplantation surgery; (3) clinical failure defined as clinical, laboratory, or radiographic findings suggestive of PJI during follow-up | 2-stage exchange | SAT: 5 wk AB LAT: 7 wk AB | Failure (Cox proportional hazards model adjusted using a propensity score): adjusted HR (LAT vs SAT), 1.4 (95% CI, .7–2.7), P = .31 |
| Bernard 2010 [30] | Switzerland | 144 | PCoh, single-center | Cure defined as no local signs of infection after a minimum follow-up of 2 y post-OP | 1-stage exchange, n = 10; 2-stage exchange, n = 57; DAIR, n = 60; Girdlestone, n = 17 | SAT: 6 wk AB LAT: 12 wk AB | Cure: Univariate OR for SAT, 3.8 (95% CI, 1.5–9.6); multivariate OR for SAT, 2.7 (95% CI, .9–8.3) |
| Mittal 2007 [31] | USA | 37 | RCoh, multicenter | Failure of 2-stage reimplantation, defined as need for an arthrodesis or amputation or reinfection with the same organism | 2-stage exchange | SAT: ≤6 wk LAT: >6 wk | Failure: n = 3 (25%) SAT, n = 6 (37.5%) LAT, P = .7 |
| First Author and Publication Year . | Country . | Sample Size, No. . | Study Design . | Definition of the Outcome . | Type of Surgery . | Duration of antibiotic Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|
| Kelly 2022 [24] | USA | 211 | RCoh, single-center | Primary outcome: presence of resistant organisms in any subsequent infection in the same joint; secondary outcome: time to recurrence of PJI | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for ≥2 wk after 2nd OP | Failure: 24 (15%) LAT, 11 (21%) SAT, P = .35 |
| Yang 2020 [25] | USA | 142 | RCT, multicenter | Failure due to reinfection | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for 3 mo after 2nd OP | Failure: 20 (28.6%) SAT, 9 (12.5%) LAT, P = .012 |
| Lora-Tamayo 2016 [26] | Spain | 63 | RCT, multicenter | Cure rate defined as clinical signs of infection resolved and progressive decrease in CRP levels | DAIR | SAT: 8 wk LAT: 6 mo (knee), 3 mo (hip) | Cure (ITT analysisa): LAT 19 (57.6%), SAT 22 (73.3%) Difference (LAT – SAT): −15.7% (95% CI, −39.2% to 7.8%) |
| Bernard 2021 [17] | France | 404 | RCT, multicenter | Persistent infection within 2 y after the end of antibiotic therapy | DAIR, n = 167b; 1-stage exchange, n = 150c; 2-stage exchange, n = 87d | SAT: 6 wk LAT: 12 wk | Failure (SAT vs LAT): unadjusted RD, 8.7 (95% CI, 1.8–15.6); adjusted RD, 9.0 (95% CI, 2.3–15.7) |
| Benkabouche 2019 [27] | Switzerland | 38 | RCT, single-center | Remission of infection defined as presence of ≥2 positive cultures + systemic signs of infection at the operative site | 2-stage exchange | SAT: 4 wk (±3 d); LAT: 6 wk (±3 d) | Recurrence of clinical infection: 1 (7.1%) patient in SAT, 3 (15%) in LAT, P = .64 |
| Chaussade 2017 [8] | France and Switzerland | 87 | RCoh, multicenter | Remission 1 y after DAIR: (1) No signs of infection after 1 y of follow-up; (2) no need to continue antibiotic therapy | DAIR | SAT: 6 wk AB LAT: 12 wk AB | Remission (SAT vs LAT): unadjusted OR, 0.87 (95% CI, .35–2.16), P = .76; adjusted OR, 0.76 (95% CI, .27–2.10), P = .6 |
| Puhto 2012 [28] | Finland | 55e | RCoh, historical control, single-center | Treatment failure defined as (1) patient referred for 2-stage exchange surgery at any time during follow-up, (2) patient had symptoms or signs of infection after the end of antibiotic treatment or (3) suppressive antibiotic treatment | DAIR | SAT: 8 wk AB LAT: 12 wk | Failure: 16 (46%) in SAT vs 10 (50%) in LAT, P = .76 |
| El Helou 2011 [29] | USA | 208 | RCoh, single-center | Treatment failure defined as: (1) recurrence of PJI at any time after 2nd OP; (2) death caused by PJI at any time after reimplantation surgery; (3) clinical failure defined as clinical, laboratory, or radiographic findings suggestive of PJI during follow-up | 2-stage exchange | SAT: 5 wk AB LAT: 7 wk AB | Failure (Cox proportional hazards model adjusted using a propensity score): adjusted HR (LAT vs SAT), 1.4 (95% CI, .7–2.7), P = .31 |
| Bernard 2010 [30] | Switzerland | 144 | PCoh, single-center | Cure defined as no local signs of infection after a minimum follow-up of 2 y post-OP | 1-stage exchange, n = 10; 2-stage exchange, n = 57; DAIR, n = 60; Girdlestone, n = 17 | SAT: 6 wk AB LAT: 12 wk AB | Cure: Univariate OR for SAT, 3.8 (95% CI, 1.5–9.6); multivariate OR for SAT, 2.7 (95% CI, .9–8.3) |
| Mittal 2007 [31] | USA | 37 | RCoh, multicenter | Failure of 2-stage reimplantation, defined as need for an arthrodesis or amputation or reinfection with the same organism | 2-stage exchange | SAT: ≤6 wk LAT: >6 wk | Failure: n = 3 (25%) SAT, n = 6 (37.5%) LAT, P = .7 |
Abbreviations: AB, antibiotics; CI, confidence interval; CRP, C-reactive protein; DAIR, debridement, antibiotics, and implant retention; HR, hazard ratio; ITT, intention to treat; LAT, long antibiotic treatment group; OP, operation; OR, odds ratio; PCoh, prospective cohort study; PJI, prosthetic joint–associated infection; RCoh, retrospective cohort study; RCT, randomized controlled trial; RD, risk difference; SAT, short antibiotic treatment group; USA, United States.
Results in the per-protocol analysis were as follows: 19 patients cured in the long treatment group (95%) and 22 in the short treatment group (91.5%). The difference (% long – short) was +3.3% (−11.7% to 18.3%).
82 short treatment group, 85 long treatment group.
77 short treatment group, 73 long treatment group.
44 short treatment group and 43 long treatment group.
Only total hip arthroplasties were included in this systematic review because the total knee arthroplasty cases did not respect the inclusion criteria.
Characteristics of the Studies Included in the Systematic Review and Meta-analysis
| First Author and Publication Year . | Country . | Sample Size, No. . | Study Design . | Definition of the Outcome . | Type of Surgery . | Duration of antibiotic Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|
| Kelly 2022 [24] | USA | 211 | RCoh, single-center | Primary outcome: presence of resistant organisms in any subsequent infection in the same joint; secondary outcome: time to recurrence of PJI | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for ≥2 wk after 2nd OP | Failure: 24 (15%) LAT, 11 (21%) SAT, P = .35 |
| Yang 2020 [25] | USA | 142 | RCT, multicenter | Failure due to reinfection | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for 3 mo after 2nd OP | Failure: 20 (28.6%) SAT, 9 (12.5%) LAT, P = .012 |
| Lora-Tamayo 2016 [26] | Spain | 63 | RCT, multicenter | Cure rate defined as clinical signs of infection resolved and progressive decrease in CRP levels | DAIR | SAT: 8 wk LAT: 6 mo (knee), 3 mo (hip) | Cure (ITT analysisa): LAT 19 (57.6%), SAT 22 (73.3%) Difference (LAT – SAT): −15.7% (95% CI, −39.2% to 7.8%) |
| Bernard 2021 [17] | France | 404 | RCT, multicenter | Persistent infection within 2 y after the end of antibiotic therapy | DAIR, n = 167b; 1-stage exchange, n = 150c; 2-stage exchange, n = 87d | SAT: 6 wk LAT: 12 wk | Failure (SAT vs LAT): unadjusted RD, 8.7 (95% CI, 1.8–15.6); adjusted RD, 9.0 (95% CI, 2.3–15.7) |
| Benkabouche 2019 [27] | Switzerland | 38 | RCT, single-center | Remission of infection defined as presence of ≥2 positive cultures + systemic signs of infection at the operative site | 2-stage exchange | SAT: 4 wk (±3 d); LAT: 6 wk (±3 d) | Recurrence of clinical infection: 1 (7.1%) patient in SAT, 3 (15%) in LAT, P = .64 |
| Chaussade 2017 [8] | France and Switzerland | 87 | RCoh, multicenter | Remission 1 y after DAIR: (1) No signs of infection after 1 y of follow-up; (2) no need to continue antibiotic therapy | DAIR | SAT: 6 wk AB LAT: 12 wk AB | Remission (SAT vs LAT): unadjusted OR, 0.87 (95% CI, .35–2.16), P = .76; adjusted OR, 0.76 (95% CI, .27–2.10), P = .6 |
| Puhto 2012 [28] | Finland | 55e | RCoh, historical control, single-center | Treatment failure defined as (1) patient referred for 2-stage exchange surgery at any time during follow-up, (2) patient had symptoms or signs of infection after the end of antibiotic treatment or (3) suppressive antibiotic treatment | DAIR | SAT: 8 wk AB LAT: 12 wk | Failure: 16 (46%) in SAT vs 10 (50%) in LAT, P = .76 |
| El Helou 2011 [29] | USA | 208 | RCoh, single-center | Treatment failure defined as: (1) recurrence of PJI at any time after 2nd OP; (2) death caused by PJI at any time after reimplantation surgery; (3) clinical failure defined as clinical, laboratory, or radiographic findings suggestive of PJI during follow-up | 2-stage exchange | SAT: 5 wk AB LAT: 7 wk AB | Failure (Cox proportional hazards model adjusted using a propensity score): adjusted HR (LAT vs SAT), 1.4 (95% CI, .7–2.7), P = .31 |
| Bernard 2010 [30] | Switzerland | 144 | PCoh, single-center | Cure defined as no local signs of infection after a minimum follow-up of 2 y post-OP | 1-stage exchange, n = 10; 2-stage exchange, n = 57; DAIR, n = 60; Girdlestone, n = 17 | SAT: 6 wk AB LAT: 12 wk AB | Cure: Univariate OR for SAT, 3.8 (95% CI, 1.5–9.6); multivariate OR for SAT, 2.7 (95% CI, .9–8.3) |
| Mittal 2007 [31] | USA | 37 | RCoh, multicenter | Failure of 2-stage reimplantation, defined as need for an arthrodesis or amputation or reinfection with the same organism | 2-stage exchange | SAT: ≤6 wk LAT: >6 wk | Failure: n = 3 (25%) SAT, n = 6 (37.5%) LAT, P = .7 |
| First Author and Publication Year . | Country . | Sample Size, No. . | Study Design . | Definition of the Outcome . | Type of Surgery . | Duration of antibiotic Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|
| Kelly 2022 [24] | USA | 211 | RCoh, single-center | Primary outcome: presence of resistant organisms in any subsequent infection in the same joint; secondary outcome: time to recurrence of PJI | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for ≥2 wk after 2nd OP | Failure: 24 (15%) LAT, 11 (21%) SAT, P = .35 |
| Yang 2020 [25] | USA | 142 | RCT, multicenter | Failure due to reinfection | 2-stage exchange | SAT: 6 wk after 1st OP, no treatment after 2nd OP LAT: 6 wk after 1st OP plus oral AB for 3 mo after 2nd OP | Failure: 20 (28.6%) SAT, 9 (12.5%) LAT, P = .012 |
| Lora-Tamayo 2016 [26] | Spain | 63 | RCT, multicenter | Cure rate defined as clinical signs of infection resolved and progressive decrease in CRP levels | DAIR | SAT: 8 wk LAT: 6 mo (knee), 3 mo (hip) | Cure (ITT analysisa): LAT 19 (57.6%), SAT 22 (73.3%) Difference (LAT – SAT): −15.7% (95% CI, −39.2% to 7.8%) |
| Bernard 2021 [17] | France | 404 | RCT, multicenter | Persistent infection within 2 y after the end of antibiotic therapy | DAIR, n = 167b; 1-stage exchange, n = 150c; 2-stage exchange, n = 87d | SAT: 6 wk LAT: 12 wk | Failure (SAT vs LAT): unadjusted RD, 8.7 (95% CI, 1.8–15.6); adjusted RD, 9.0 (95% CI, 2.3–15.7) |
| Benkabouche 2019 [27] | Switzerland | 38 | RCT, single-center | Remission of infection defined as presence of ≥2 positive cultures + systemic signs of infection at the operative site | 2-stage exchange | SAT: 4 wk (±3 d); LAT: 6 wk (±3 d) | Recurrence of clinical infection: 1 (7.1%) patient in SAT, 3 (15%) in LAT, P = .64 |
| Chaussade 2017 [8] | France and Switzerland | 87 | RCoh, multicenter | Remission 1 y after DAIR: (1) No signs of infection after 1 y of follow-up; (2) no need to continue antibiotic therapy | DAIR | SAT: 6 wk AB LAT: 12 wk AB | Remission (SAT vs LAT): unadjusted OR, 0.87 (95% CI, .35–2.16), P = .76; adjusted OR, 0.76 (95% CI, .27–2.10), P = .6 |
| Puhto 2012 [28] | Finland | 55e | RCoh, historical control, single-center | Treatment failure defined as (1) patient referred for 2-stage exchange surgery at any time during follow-up, (2) patient had symptoms or signs of infection after the end of antibiotic treatment or (3) suppressive antibiotic treatment | DAIR | SAT: 8 wk AB LAT: 12 wk | Failure: 16 (46%) in SAT vs 10 (50%) in LAT, P = .76 |
| El Helou 2011 [29] | USA | 208 | RCoh, single-center | Treatment failure defined as: (1) recurrence of PJI at any time after 2nd OP; (2) death caused by PJI at any time after reimplantation surgery; (3) clinical failure defined as clinical, laboratory, or radiographic findings suggestive of PJI during follow-up | 2-stage exchange | SAT: 5 wk AB LAT: 7 wk AB | Failure (Cox proportional hazards model adjusted using a propensity score): adjusted HR (LAT vs SAT), 1.4 (95% CI, .7–2.7), P = .31 |
| Bernard 2010 [30] | Switzerland | 144 | PCoh, single-center | Cure defined as no local signs of infection after a minimum follow-up of 2 y post-OP | 1-stage exchange, n = 10; 2-stage exchange, n = 57; DAIR, n = 60; Girdlestone, n = 17 | SAT: 6 wk AB LAT: 12 wk AB | Cure: Univariate OR for SAT, 3.8 (95% CI, 1.5–9.6); multivariate OR for SAT, 2.7 (95% CI, .9–8.3) |
| Mittal 2007 [31] | USA | 37 | RCoh, multicenter | Failure of 2-stage reimplantation, defined as need for an arthrodesis or amputation or reinfection with the same organism | 2-stage exchange | SAT: ≤6 wk LAT: >6 wk | Failure: n = 3 (25%) SAT, n = 6 (37.5%) LAT, P = .7 |
Abbreviations: AB, antibiotics; CI, confidence interval; CRP, C-reactive protein; DAIR, debridement, antibiotics, and implant retention; HR, hazard ratio; ITT, intention to treat; LAT, long antibiotic treatment group; OP, operation; OR, odds ratio; PCoh, prospective cohort study; PJI, prosthetic joint–associated infection; RCoh, retrospective cohort study; RCT, randomized controlled trial; RD, risk difference; SAT, short antibiotic treatment group; USA, United States.
Results in the per-protocol analysis were as follows: 19 patients cured in the long treatment group (95%) and 22 in the short treatment group (91.5%). The difference (% long – short) was +3.3% (−11.7% to 18.3%).
82 short treatment group, 85 long treatment group.
77 short treatment group, 73 long treatment group.
44 short treatment group and 43 long treatment group.
Only total hip arthroplasties were included in this systematic review because the total knee arthroplasty cases did not respect the inclusion criteria.
The sample size per study ranged from 37 to 404 participants, resulting in a total of 1389 patients included in this systematic review. Overall, 748 hip, 638 knee, 2 shoulder, and 1 elbow surgical procedures were included. In total, 604 patients were allocated to the SAT and 759 to the LAT group (this group stratification accounts only for the patients in intention-to-treat analysis for the 2021 study of Bernard et al [17]). Seventy-seven knee arthroplasties described in the study by Puhto et al [28] were excluded from the meta-analysis because SAT was defined as >12 weeks (ie, in conflict with our predefined categorization criteria). The most common procedure was 2-stage arthroplasty exchange (n = 780 [56.2%]), followed by DAIR (n = 432 [31.1%]), 1-stage exchange (n = 160 [11.5%]), and Girdlestone procedure (n = 17 [1.2%]).
PJIs were usually diagnosed based on the Musculoskeletal Infection Society criteria [7] (see more information below in the Quality Assessment section). Follow-up lasted for at least 1 year except in 1 study [28]. Prevalence of the different causative microorganisms was often reported, but the association between pathogen and outcome was not assessed in most studies [8, 26, 29, 30]; for example, Chaussade et al [8] calculated the strength of the association between specific causative pathogens (coagulase-negative staphylococci and methicillin-resistant S aureus) and remission of the infection. Antibiotic regimens varied largely between studies but they were not always reported, with very few studies evaluating the association between antibiotic regimen and outcome [8, 29, 30].
Of notice, we decided to include the study by El Helou et al [29], even though 12% (8/82) and 9% (11/126) of patients with PJI received long-term oral antibiotic suppressive therapy. We considered that to be a small portion of the total sample population and we therefore made the decision to include the study.
Quality Assessment
Six of the 10 studies included were observational cohort studies and the risk of bias was assessed using the Newcastle-Ottawa quality assessment tool (Supplementary Table 2). Most studies (n = 5) were considered to have a low risk of bias [8, 28–31], and only 1 was considered to be at moderate risk [24]. The overall score ranged from 6 to 9 (maximum score is 9). In 4 cohort studies, the estimate of the association between treatment duration and failure was adjusted for at least sex and age.
The quality of the 4 RCTs was assessed using the Jadad score tool (Supplementary Table 3). Scores ranged from 2 to 3 (maximum score is 5); therefore, all RCTs were considered to be at moderate risk of bias. None of the 4 RCTs was blinded.
Meta-analysis
The median duration of antibiotic treatment was 6 weeks (range, 4–8 weeks) in the SAT group and 12 weeks (range, 6–24 weeks) in the LAT group. Figure 2 shows the meta-analysis comparing the effects on treatment failure of SAT versus LAT. An 11% lower risk of treatment failure in the SAT group was estimated compared to the LAT group (pooled OR, 0.89 [95% CI, .53–1.50]), even though the CI encompassed the point of no difference between the 2 treatment groups (ie, no statistically significant difference).
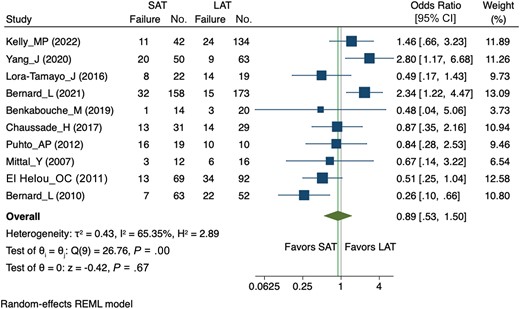
Forest plot of the random-effects meta-analysis comparing the effect of short versus long courses of antibiotics on treatment failure. For each trial, the square area is proportional to the weight of the study. The diamond represents the overall random-effects summary odds ratio (OR). The confidence interval is given by the width of the diamond. The unbroken vertical line is the null value (OR = 1, meaning no effect). Abbreviations: CI, confidence interval; LAT, long antibiotic treatment group; REML, Restricted Maximum Likelihood; SAT, short antibiotic treatment group.
When the analysis was stratified by type of surgery (Figure 3A), no statically significant difference was detected, although the pooled OR tended to favor SAT in patients managed with DAIR (OR, 0.76 [95% CI, .32–1.80]) and LAT in those undergoing SEA (OR, 1.19 [95% CI, .62–2.31]). A meta-regression was performed and did not show any difference between the DAIR versus SEA groups (coefficient = 0.42; P = 0.45). Of notice, there was a high heterogeneity in the DAIR subgroup (I2 = 60.89%; P = .01), probably because of the discordant results between the 2 studies by Bernard et al [17, 30].
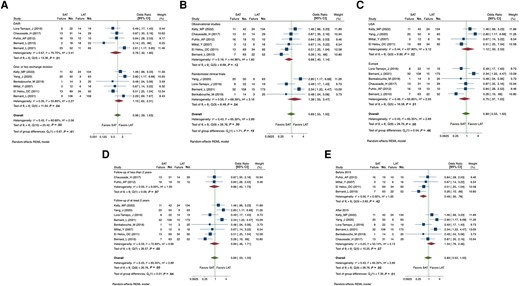
Forest plot of the random-effects meta-analysis comparing the effect of short versus long courses of antibiotics on treatment failure stratified by type of surgery (debridement, antibiotics, and implant retention vs 1- or 2-stage exchange, A), geographic location (United States vs Europe, B), study design (observational vs randomized controlled trial, C), duration of follow-up (<2 vs ≥2 years, D), and year of publication (before vs after 2015, E). Abbreviations: CI, confidence interval; DAIR, debridement, antibiotics, and implant retention; LAT, long antibiotic treatment group; REML, Restricted Maximum Likelihood; SAT, short antibiotic treatment group; USA, United States.
Other subgroup analyses were conducted, in particular taking into account the study setting (United States vs Europe; Figure 3B), the study design (observational vs RCTs; Figure 3C), and the duration of follow-up (<2 years vs ≥2 years, Figure 3D), but no statistically significant difference between subgroups was detected (Table 2). A cumulative meta-analysis by year of publication was also performed (Supplementary Figure 1) and showed a trend toward fewer treatment failures with LAT in the most recent studies. When comparing studies published before and after 2015, the probability of treatment failure with SAT was 2.8 times higher in trials conducted after 2015 than in those conducted before 2015 (P = .04) (Table 2). The year 2015 was chosen as the cutoff because it fell in between the oldest and most recent publication years (2007 and 2022, respectively).
Meta-regression of Subgroup Analyses Results of the Effects of Short Versus Long Antibiotic Treatment on Treatment Failure in Prosthetic Joint–Associated Infection
| Variable . | OR (95% CI) . | Coefficient . | Meta-regression P Value . | I2 . | No. of Studies Included in the Subanalysis . |
|---|---|---|---|---|---|
| Type of operation | |||||
| DAIR | 1 | … | … | 5 | |
| 1- or 2-stage exchange | 1.52 (.45–5.18) | 0.42 | .45 | 64.01% | 6a |
| Geographic location | |||||
| United States | 1 | … | … | 4 | |
| Europe | 0.67 (.2–2.4) | −0.37 | .788 | 69.68% | 6 |
| Study design | |||||
| Observational | 1 | … | … | 6 | |
| RCT | 2.2 (.7–6.6) | 0.77 | .16 | 53.4% | 4 |
| Follow-up | |||||
| <2 y | 1 | … | … | 2 | |
| ≥2 y | 1.04 (.22–5.2) | 0.05 | .95 | 69.89% | 8 |
| Year of publication | |||||
| Before 2015 | 1 | … | … | 4 | |
| After 2015 | 2.8 (1.1–7.3) | 1.03 | .04 | 38.83% | 6 |
| Variable . | OR (95% CI) . | Coefficient . | Meta-regression P Value . | I2 . | No. of Studies Included in the Subanalysis . |
|---|---|---|---|---|---|
| Type of operation | |||||
| DAIR | 1 | … | … | 5 | |
| 1- or 2-stage exchange | 1.52 (.45–5.18) | 0.42 | .45 | 64.01% | 6a |
| Geographic location | |||||
| United States | 1 | … | … | 4 | |
| Europe | 0.67 (.2–2.4) | −0.37 | .788 | 69.68% | 6 |
| Study design | |||||
| Observational | 1 | … | … | 6 | |
| RCT | 2.2 (.7–6.6) | 0.77 | .16 | 53.4% | 4 |
| Follow-up | |||||
| <2 y | 1 | … | … | 2 | |
| ≥2 y | 1.04 (.22–5.2) | 0.05 | .95 | 69.89% | 8 |
| Year of publication | |||||
| Before 2015 | 1 | … | … | 4 | |
| After 2015 | 2.8 (1.1–7.3) | 1.03 | .04 | 38.83% | 6 |
Abbreviations: CI, confidence interval; DAIR, debridement, antibiotics, and implant retention; OR, odds ratio; RCT, randomized controlled trial.
Data from Bernard et al [17] were available for both type of surgeries.
Meta-regression of Subgroup Analyses Results of the Effects of Short Versus Long Antibiotic Treatment on Treatment Failure in Prosthetic Joint–Associated Infection
| Variable . | OR (95% CI) . | Coefficient . | Meta-regression P Value . | I2 . | No. of Studies Included in the Subanalysis . |
|---|---|---|---|---|---|
| Type of operation | |||||
| DAIR | 1 | … | … | 5 | |
| 1- or 2-stage exchange | 1.52 (.45–5.18) | 0.42 | .45 | 64.01% | 6a |
| Geographic location | |||||
| United States | 1 | … | … | 4 | |
| Europe | 0.67 (.2–2.4) | −0.37 | .788 | 69.68% | 6 |
| Study design | |||||
| Observational | 1 | … | … | 6 | |
| RCT | 2.2 (.7–6.6) | 0.77 | .16 | 53.4% | 4 |
| Follow-up | |||||
| <2 y | 1 | … | … | 2 | |
| ≥2 y | 1.04 (.22–5.2) | 0.05 | .95 | 69.89% | 8 |
| Year of publication | |||||
| Before 2015 | 1 | … | … | 4 | |
| After 2015 | 2.8 (1.1–7.3) | 1.03 | .04 | 38.83% | 6 |
| Variable . | OR (95% CI) . | Coefficient . | Meta-regression P Value . | I2 . | No. of Studies Included in the Subanalysis . |
|---|---|---|---|---|---|
| Type of operation | |||||
| DAIR | 1 | … | … | 5 | |
| 1- or 2-stage exchange | 1.52 (.45–5.18) | 0.42 | .45 | 64.01% | 6a |
| Geographic location | |||||
| United States | 1 | … | … | 4 | |
| Europe | 0.67 (.2–2.4) | −0.37 | .788 | 69.68% | 6 |
| Study design | |||||
| Observational | 1 | … | … | 6 | |
| RCT | 2.2 (.7–6.6) | 0.77 | .16 | 53.4% | 4 |
| Follow-up | |||||
| <2 y | 1 | … | … | 2 | |
| ≥2 y | 1.04 (.22–5.2) | 0.05 | .95 | 69.89% | 8 |
| Year of publication | |||||
| Before 2015 | 1 | … | … | 4 | |
| After 2015 | 2.8 (1.1–7.3) | 1.03 | .04 | 38.83% | 6 |
Abbreviations: CI, confidence interval; DAIR, debridement, antibiotics, and implant retention; OR, odds ratio; RCT, randomized controlled trial.
Data from Bernard et al [17] were available for both type of surgeries.
A sensitivity analysis, performed to investigate the source of heterogeneity, failed to identify any specific study as a major contributor of the high heterogeneity (ie, the removal of no study in particular reduced the level of heterogeneity—I2 below the commonly used cutoff of 50%) (Supplementary Table 4). For this reason, no study was removed from the meta-analysis.
The risk of publication bias was evaluated with a funnel plot (Supplementary Figure 2), which did not show any evidence of publication bias; this was confirmed with the Egger test (P = .332).
DISCUSSION
Optimal duration of antibiotic treatment is among the most controversial topics in PJI therapy. Here, not only is the debate about the total duration of therapy still open, but it also remains unclear whether different surgical strategies (DAIR vs 1-stage vs 2-stage exchange arthroplasty) require differentiated, specific antibiotic treatment approaches. This review aims to generate scientific evidence on whether currently available clinical trials, when aggregated, can help resolve this controversy.
Compared to a previous meta-analysis [14] where no restriction in the definition of SAT and LAT was applied, we selected studies according to a strict definition of treatment duration (SAT: at least 4 but <12 weeks, and all studies including lifelong treatment in the LAT group were excluded). However, for the LAT group definition, we decided not to use a strict cutoff of ≥12 weeks to account for the fact that the definition of LAT duration has changed over the years. However, even when excluding all the studies that used a LAT definition of <12 weeks [24, 27, 29, 31], the overall results did not change (OR, 0.96 [95% CI, .46–2.02]; see Supplementary Figure 3), so again no difference in the outcome between SAT and LAT was found.
In the meta-analysis, we found that the odds of treatment failure were 11% lower with shorter treatments, irrespective of which surgical approach was applied (DAIR or SEA), even though the strength of the association was not statistically significant (pooled OR, 0.89 [95% CI, .53–1.50]). This result is in line with a previous meta-analysis by Yen et al published in 2019 [14]. Therefore, despite having included 2 additional recent large RCTs that concluded for better clinical outcomes with longer treatments [17, 25], the conclusions did not change.
This systematic review also investigated the question of the importance of different antibiotic treatment durations according to the type of surgical procedure. In particular, we looked at DAIR and 1- or 2-stage exchange strategies. Our meta-analysis found a tendency toward better outcomes with shorter antibiotic treatments after DAIR than with 1- or 2-stage exchange strategies (the OR for treatment failure with SAT comparing SEA vs DAIR was 1.52 [95% CI, .45–5.18]). Importantly, this is in contrast with the latest RCT in the field [17] that found better outcomes for LAT in DAIR (risk difference for DAIR [short vs long], 16.2% [95% CI, 2.9%–29.5%], larger than the overall risk difference of 8.7% [95% CI, 1.8%–15.6%]). It is worth mentioning that this RCT is the only study included in this meta-analysis that investigated both surgical procedures, DAIR and SEA (data on the SEA group of the 2010 Bernard et al study [30] were not available for the subanalysis), so only in this study have the 2 surgical approaches been compared under similar conditions (ie, antibiotic regimen, surgical experience of the surgeons, pathogens involved). On the other hand, another possible explanation for the better clinical outcomes with SAT in patients with a surgical indication for DAIR is that usually this procedure is indicated in case of early infection, stable implant, good soft tissue, growth of a pathogen susceptible to antibiotic agents with activity against biofilm, or negative cultures [4]. These are all conditions that with timely surgical treatment and appropriate antibiotics will promptly achieve clinical success. On the other hand, subacute and chronic PJI are characterized by the presence of mature bacterial biofilms, a phenotype that is often associated with bacterial recalcitrance, which may therefore in some cases benefit from prolonged antibiotic exposure [32].
Overall, this meta-analysis indicates that observational trials show a tendency in favor of SAT, while RCTs favor LAT, even though neither subgroup analysis produced overall statistically significant results. Importantly, in observational studies the choice of treatment duration was mostly left to the treating physician [8, 29, 31]. The differences between observational and interventional studies suggest (in observational studies) the possible introduction of bias due to specific patients’ risk factors, which influence physicians’ prescription practices (ie, initiating a shorter treatment course for patients perceived as less likely to fail). Of note, physicians’ perceived risk factors may not be recorded in every study. Unrecorded variables that may have an influence on the treatment choice in observational studies include the functional status of the patient, orthopedic complexity of the PJI (implant loosening, bone loss, primary or secondary prosthesis, periprosthetic fracture), availability of a microbiological diagnosis at the time of surgery, and which antibiotic treatment options were available. Indeed, researchers undertaking observational studies in this field should, whenever possible, make sure to report patients’ general functional scores, microbiology, and the complexity of the PJI (eg, by using the joint-specific BACH score) [33].
Of notice, we have also found that in observational studies published before 2015 (compared to those published after), there was a 52% lower risk of treatment failure with shorter antibiotic treatments. The reasons for this phenomenon are unclear; however, we can speculate that, given the progressive aging of the population and the increase in the number of comorbidities present at the time of joint replacement, a longer-term antibiotic treatment may be favored.
This study has several limitations. Although we focused on antibiotic duration for the treatment of PJI, eligible studies were highly heterogeneous. This was due in part to the different definitions of “short” and “long” durations, but also to the variety of causative pathogens, antibiotic regimens, kind of joints, different PJI definitions, and different outcomes in the included studies. It should be mentioned that in most of the included studies [24, 26, 27, 29, 31], a clarification of how they treated survivor bias and early termination of antibiotic treatment was either not mentioned or only partially commented.
However, our search strategy was comprehensive; several databases were searched and, compared to the previous meta-analysis, 4 additional randomized trials were included and all included studies were considered to be at low or moderate risk of bias.
Furthermore, the heterogeneity of microbiological data, local antibiotics, duration of intravenous versus oral systemic antibiotic therapy, and different choices of antibiotic agent use prevented us from conducting subgroup analyses by causative pathogen, specific antibiotics, and administration route of antibiotics. Of notice, we did not take into consideration the exchange of modular components during the DAIR procedures, because this was not reported in the included studies, although it could have had an impact on the risk of treatment failure [34].
In conclusion, this is currently the most recent and largest systematic review and meta-analysis on the impact of duration of antibiotic therapy for PJI. We found no significant difference in the risk of treatment failure when shorter or longer antibiotic regimens were used. However, this evidence does not yet suffice to make a clear recommendation on the optimal treatment duration in PJI. While our results point toward no difference in the risk of treatment failure between SAT and LAT in both DAIR and SAE, the tendency of RCTs to favor LAT is raising some concerns with the use of shorter treatments. Similarly, those studies published most recently showed a clear benefit of LAT. For these reasons, until this trend in favor of shorter antibiotic treatment duration is supported by new, high-quality studies (such as RCTs), we suggest to use a longer antibiotic treatment duration (eg, ≥12 weeks). In the future, we will hopefully have further high-quality RCTs stratified by type of surgical approach, kind of joints, causative pathogen, and antibiotic treatment options to obtain evidence to inform best practices for the antibiotic treatment of PJI [35].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. F. O. and H. R. conceived of the presented idea. F. O. conducted the research literature with the support of V. Z. and A. E. All titles and abstracts were screened by F. O., and full texts were independently screened by F. O. and V. Z. Data extraction from selected studies was performed by F. O., and V. Z. and A. E. verified the analytical methods. F. O. wrote the manuscript, supervised by H. R. All authors discussed and contributed to the final manuscript.
Patient consent. The study was submitted to the London School of Hygiene and Tropical Medicine MSc Research Ethics Committee (reference number 26970). Because this project is a systematic review and meta-analysis of previously published data, it did not require ethics approval.
References
Author notes
Potential conflicts of interest. The authors: No reported conflicts of interest.





Comments