-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel A Sweeney, Bonifride Tuyishimire, Neera Ahuja, John H Beigel, Tatiana Beresnev, Valeria D Cantos, Jose G Castro, Stuart H Cohen, Kaitlyn Cross, Lori E Dodd, Nathan Erdmann, Monica Fung, Varduhi Ghazaryan, Sarah L George, Kevin A Grimes, Noreen A Hynes, Kathleen G Julian, Sheetal Kandiah, Hannah Jang Kim, Corri B Levine, David A Lindholm, David C Lye, Ryan C Maves, Myoung-don Oh, Catharine Paules, Rekha R Rapaka, Willam R Short, Kay M Tomashek, Cameron R Wolfe, Andre C Kalil, Baricitinib Treatment of Coronavirus Disease 2019 Is Associated With a Reduction in Secondary Infections, Open Forum Infectious Diseases, Volume 10, Issue 5, May 2023, ofad205, https://doi.org/10.1093/ofid/ofad205
Close - Share Icon Share
Abstract
We performed a secondary analysis of the National Institutes of Health-sponsored Adaptive COVID-19 Treatment Trial (ACTT-2) randomized controlled trial and found that baricitinib was associated with a 50% reduction in secondary infections after controlling for baseline and postrandomization patient characteristics. This finding provides a novel mechanism of benefit for baricitinib and supports the safety profile of this immunomodulator for the treatment of coronavirus disease 2019.
The second stage of the National Institutes of Health-sponsored Adaptive COVID-19 Treatment Trial (ACTT-2) compared the combination of baricitinib plus remdesivir with that of placebo plus remdesivir in adult patients hospitalized for coronavirus disease 2019 (COVID-19). The primary analysis showed that baricitinib treatment was associated with a shorter patient recovery time and a lower risk of progression to invasive ventilation or death [1]. In addition, baricitinib treatment, compared with placebo, was also associated with a significantly lower rate of new secondary infections. This latter finding was unexpected, as an increased risk of secondary infections could be anticipated owing to the immunomodulatory properties of baricitinib. While baricitinib has potential antiviral activity, it is not known to have antibacterial or antifungal activity [2]. To better understand the mechanism by which baricitinib therapy improved outcomes of hospitalized patients with COVID-19, we conducted a secondary analysis of the ACTT-2 data set, aiming to evaluate whether baricitinib reduced the risk of secondary infections in patients hospitalized for COVID-19 after controlling for baseline patient characteristics.
METHODS
This analysis used data from ACTT-2, a double-blind, randomized, placebo-controlled trial evaluating baricitinib plus remdesivir combination treatment in hospitalized adults with COVID-19 [1]. Study patients received remdesivir (≤10 days) and either baricitinib (≤14 days) or placebo. The primary outcome of this secondary ACTT-2 analysis was to assess the risk of secondary infections, defined as a new infection diagnosed after trial enrollment, occurring in patients who were randomized and were treated with either baricitinib or placebo. During ACTT-2, investigators used their clinical judgment to identify all secondary infections based on clinical, laboratory, and radiologic assessment, including those caused by bacteria, viruses, and fungi, as part of the prespecified safety analysis. The investigators conducting this analysis (all of whom are nurses or physicians who participated as investigators in the ACTT-2 and were blinded to the treatment arm assignment) systematically reviewed home medications of all patients with secondary infections and further identified those patients who were immunosuppressed at baseline. An immunosuppressed patient was defined as someone prescribed ≥1 immunosuppressant agent before study enrollment.
The ACTT-2 trial protocol was approved by the institutional review board at each site (or a centralized institutional review board as applicable). Written informed consent was obtained from each patient or from the patient's legally authorized representative if the patient was unable to provide consent.
Univariate analysis was performed to determine the association between the risk of secondary infections and baseline patient characteristics that were chosen a priori: age, race, ethnicity, types and number of coexisting health conditions, treatment with immunosuppressive agents (including glucocorticoid therapy), and COVID-19 disease severity. Univariate variables with P values <.15 and variables deemed clinically relevant for the primary outcome in the univariate analyses were evaluated for inclusion into the multivariable logistic regression analysis. Kaplan-Meier methods and Fine-Gray competing risk modeling were used to analyze time-to-event data. An additional exploratory analysis was also performed whereby postrandomization patient characteristics—hospital length of stay and intubation status (ie, whether the patient was intubated before the secondary infection)—were independently added to the multivariate model.
RESULTS
Of the 1016 patients with COVID-19 enrolled in ACTT-2, a secondary infection developed in 87 (8%) after study enrollment (Supplementary Table 1). Patients who experienced secondary infections in the baricitinib treatment and placebo control arms of the study did not markedly differ in terms of baseline characteristics, including race, COVID-19 disease severity, and number of coexisting health conditions. Likewise, the anatomic sites of the secondary infections and the infectious organisms, bacterial and nonbacterial, were similar between the treatment and placebo arms (Supplementary Tables 2 and 3).
The risk for secondary infections was assessed by univariate and multivariable regression. Univariate analysis showed that baricitinib was associated with a 50% reduction in secondary infections (Table 1). Age group (P = .10) was included in the multivariate analysis, based on a predefined P value threshold (P < .15). Of note, univariate analysis did not identify participant coexisting health conditions as risk factors for secondary infections; nonetheless, we decided to perform a sensitivity analysis on the number coexisting health conditions in the multivariate model due to their known clinical importance, and the results remained unchanged (odds ratio, 0.50 [95% confidence interval .31–.79]). Accordingly, the multivariable models showed that baricitinib was associated with a 50% reduction in the odds of secondary infection (Table 1). When either hospital length of stay or postrandomization intubation status was added to the multivariate analysis, the reduction in the odds of secondary infection associated with baricitinib remained significant (odds ratio, 0.57 [95% confidence interval, .33–.79] and 0.57 [.35–.93], respectively.
Risk of Secondary Infections in Patients Hospitalized for Coronavirus Disease 2019: Univariate and Multivariate Analyses
| Parameters by Model Type . | OR (95% CI) . | P Value . |
|---|---|---|
| Univariate model | ||
| Baricitinib + remdesivir treatment arm | 0.50 (.31–.79) | .003 |
| No. of coexisting health conditions | ||
| 1 | 0.99 (.51–1.94) | .98 |
| ≥2 | 0.78 (.42–1.45) | .44 |
| Age group | ||
| 40–64 y | 1.76 (.85–3.66) | .13 |
| ≥65 y | 1.92 (.89–4.16) | .10 |
| Use of ≥1 immunosuppressant | 1.56 (.79–3.05) | .20 |
| Multivariate model | ||
| Baricitinib + remdesivir treatment arm and age group | 0.50 (.31–.79) | .003 |
| Parameters by Model Type . | OR (95% CI) . | P Value . |
|---|---|---|
| Univariate model | ||
| Baricitinib + remdesivir treatment arm | 0.50 (.31–.79) | .003 |
| No. of coexisting health conditions | ||
| 1 | 0.99 (.51–1.94) | .98 |
| ≥2 | 0.78 (.42–1.45) | .44 |
| Age group | ||
| 40–64 y | 1.76 (.85–3.66) | .13 |
| ≥65 y | 1.92 (.89–4.16) | .10 |
| Use of ≥1 immunosuppressant | 1.56 (.79–3.05) | .20 |
| Multivariate model | ||
| Baricitinib + remdesivir treatment arm and age group | 0.50 (.31–.79) | .003 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Risk of Secondary Infections in Patients Hospitalized for Coronavirus Disease 2019: Univariate and Multivariate Analyses
| Parameters by Model Type . | OR (95% CI) . | P Value . |
|---|---|---|
| Univariate model | ||
| Baricitinib + remdesivir treatment arm | 0.50 (.31–.79) | .003 |
| No. of coexisting health conditions | ||
| 1 | 0.99 (.51–1.94) | .98 |
| ≥2 | 0.78 (.42–1.45) | .44 |
| Age group | ||
| 40–64 y | 1.76 (.85–3.66) | .13 |
| ≥65 y | 1.92 (.89–4.16) | .10 |
| Use of ≥1 immunosuppressant | 1.56 (.79–3.05) | .20 |
| Multivariate model | ||
| Baricitinib + remdesivir treatment arm and age group | 0.50 (.31–.79) | .003 |
| Parameters by Model Type . | OR (95% CI) . | P Value . |
|---|---|---|
| Univariate model | ||
| Baricitinib + remdesivir treatment arm | 0.50 (.31–.79) | .003 |
| No. of coexisting health conditions | ||
| 1 | 0.99 (.51–1.94) | .98 |
| ≥2 | 0.78 (.42–1.45) | .44 |
| Age group | ||
| 40–64 y | 1.76 (.85–3.66) | .13 |
| ≥65 y | 1.92 (.89–4.16) | .10 |
| Use of ≥1 immunosuppressant | 1.56 (.79–3.05) | .20 |
| Multivariate model | ||
| Baricitinib + remdesivir treatment arm and age group | 0.50 (.31–.79) | .003 |
Abbreviations: CI, confidence interval; OR, odds ratio.
The protective effect of baricitinib against the development of secondary infections was further supported by the results of time-to-event Kaplan-Meier analysis, which also accounted for competing risks. Among all patients enrolled, baricitinib was associated with a 49% reduction in the time to the development of a secondary infection when using enrollment time as a reference (Figure 1). This reduction in the risk of secondary infections associated with baricitinib was constant over time for all treated patients.
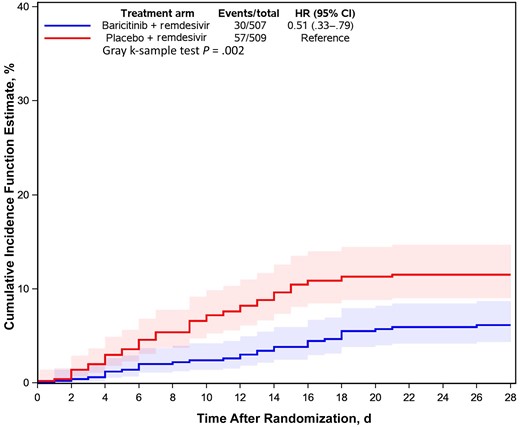
Cumulative incidence function estimate for secondary infections among patients hospitalized for coronavirus disease 2019 and treated with baricitinib or placebo. The reference point is the day of randomization. Abbreviations: CI, confidence interval; HR, hazard ratio.
DISCUSSION
The main result of this study—that baricitinib therapy for patients hospitalized due to COVID-19 was associated with a 50% reduction in secondary infections—provides needed insight into a possible mechanism by which this therapy is beneficial. There is robust clinical trial evidence supporting baricitinib as a COVID-19 treatment [1, 3–5]; however, the means by which this treatment benefits patients hospitalized for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has not been fully elucidated. The ACTT-2 investigators chose to evaluate baricitinib based on its known anticytokine effects and its potential antiviral properties [6]. Baricitinib, a Janus kinase (JAK1/JAK2) inhibitor and rheumatoid arthritis therapy, subsequently received US Food and Drug Administration approval as the only immunomodulatory drug shown in a double-blind, randomized placebo-controlled controlled trial to reduce the COVID-19–associated mortality rate [3, 4, 7, 8]. Compared with dexamethasone, another anti-inflammatory drug, baricitinib treatment for patients hospitalized for COVID-19 had similar efficacy in terms of ventilator-free survival, and importantly showed significantly fewer severe and life-threatening adverse events [9].
One possible benefit of baricitinib treatment for COVID-19, compared with other anti-inflammatory therapies, is its association with a reduction in the incidence of secondary infections. With all immunomodulatory agents, the risk of secondary infection is a concern and has been described with corticosteroids and IL-6 inhibitor therapy during the COVID-19 pandemic [10]. In this multivariate analysis of the ACTT-2 data set, we demonstrate that baricitinib treatment of patients hospitalized for COVID-19 is independently associated with a 50% reduction in secondary infections after controlling for baseline participant variables (treatment arm, and age group). Kaplan-Meier analysis further confirmed that baricitinib treatment reduced the time to development of secondary infections by 49%, the same magnitude observed in the logistic regression model.
Because the results of this study have patient safety implications, we performed an additional analysis in which 2 postrandomization variables were independently added to the multivariate model: hospital length of stay and postrandomization intubation status. Interpreting the effect of postrandomization variables in multivariable analysis is controversial. as these covariates may either be a risk factor for the study outcome (in this case, the development of a secondary infection) or may simply be a response to the treatment therapy [11]. Yet, even with the inclusion of either hospital length of stay or postenrollment intubation status in the multivariate analysis, a robust protective effect of baricitinib remained.
The finding that baricitinib treatment of COVID-19 is associated with a reduction in secondary infections has several implications. First, this result should further alleviate one of the safety concerns raised when baricitinib was initially proposed as a treatment for COVID-19. Despite the baricitinib package insert warning and prior studies in patients with rheumatoid arthritis suggesting an increased risk, trial patients with COVID-19 receiving baricitinib were not predisposed to developing a secondary infection [12, 13]. This difference in rates of infection may be due, in part, to the relatively short course of therapy (≤14 days) for patient with COVID-19 compared with the much longer duration of treatment (months to years) for patients with rheumatoid arthritis. These new results agree with the safety data from the COV-BARRIER trial and further suggest that baricitinib can be safely combined with glucocorticoids to treat COVID-19 without increasing the risk of secondary infections [3].
Second, the reduction of secondary infections may, in part, explain the beneficial effect of baricitinib for the treatment of hospitalized patients with COVID-19. Secondary infections complicating viral respiratory illnesses such as influenza are known to increase the mortality rate but may be even more consequential in patients with COVID-19 [14]. Whether baricitinib has direct antimicrobial properties beyond its purported antiviral properties has not been determined. Alternatively, one could hypothesize that the specific immunomodulatory effects of baricitinib treatment may strengthen the host immune response against secondary infections. We hope that this finding will spur basic research to answer this question.
Third, these results raise the possibility that baricitinib may represent a more “balanced” immunomodulator, one that has direct anti-inflammatory and indirect antimicrobial properties and therefore may have a potential role in treating other infectious diseases including sepsis in which both infection and a dysregulated immune response are thought to contribute to morbidity and mortality risks.
The limitations of our study are inherent to all post hoc analyses that cannot prove causal effects. Similar to those in other randomized controlled clinical trials, our study protocol did not use explicit criteria for identifying secondary infections; rather, secondary infections were reported by site investigators based on their clinical, laboratory, and radiologic assessment. Compared with other clinical trials, documentation of secondary infections was remarkably complete and included both the associated organism and the anatomical site [15]. Any outcome reporting bias would be expected to be minimized as the investigators were blinded to whether patients were receiving baricitinib or placebo during the trial. Moreover, our blinded adjudication of all secondary infections and our intentionally conservative statistical analysis strategy lend further reliability to our novel findings.
Another potential concern regarding these results is the apparent inconsistency with the findings of subsequent trials investigating the efficacy of adjuvant baricitinib to treat COVID-19, namely, the COV-BARRIER trial and ACTT-4 , neither of which showed a similar reduction in risk of secondary infections with baricitinib treatment. However, the majority of patients in both studies (>70%) received steroids, and the overall rate of secondary infections in both arms of the COV-BARRIER study (16%) was higher than that observed in ACTT-2. Thus, both the higher rates of secondary infections and the similar rates of secondary infections seen in both the treatment and control arms are easily explained by the use of steroids.
In conclusion, our secondary analysis of ACTT-2 shows that baricitinib independently reduced the rate of secondary infections by 50% in patients hospitalized for COVID-19, identifying a novel mechanism of benefit and a potential role for baricitinib as a treatment for other infectious diseases characterized by a dysregulated immune response.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. B. T and K. C. had full access to the study data and take responsibility for both the fidelity of the data and the accuracy of the analysis. Concept and design: A. C. K. Analysis and interpretation of the data: All authors. Initial draft of the manuscript: D. A. S. and A. C. K. Editing and revisions of the manuscript: All authors.
Disclaimer. The views expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, Uniformed Services University of the Health Sciences (USUHS), the Department of Defense, or any agencies under the US government.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts.





Comments