-
PDF
- Split View
-
Views
-
Cite
Cite
Alya Heirali, Shirin Moossavi, Marie Claire Arrieta, Bryan Coburn, Principles and Terminology for Host–Microbiome–Drug Interactions, Open Forum Infectious Diseases, Volume 10, Issue 5, May 2023, ofad195, https://doi.org/10.1093/ofid/ofad195
Close - Share Icon Share
Abstract
Interactions between the microbiome and medical therapies are distinct and bidirectional. The existing term “pharmacomicrobiomics” describes the effects of the microbiome on drug distribution, metabolism, efficacy, and toxicity. We propose that the term “pharmacoecology” be used to describe the effects that drugs and other medical interventions such as probiotics have on microbiome composition and function. We suggest that the terms are complementary but distinct and that both are potentially important when assessing drug safety and efficacy as well as drug–microbiome interactions. As a proof of principle, we describe the ways in which these concepts apply to antimicrobial and non-antimicrobial medications.
“Pharmacokinetics” (PK) refers to drug absorption, distribution, and metabolism. “Pharmacodynamics” (PD) refers to the biochemical, physiological, and therapeutic effects of drugs on target tissue/organ(s). The impact of host genetics on drug metabolism, safety, and efficacy is referred to as “pharmacogenomics” (PG) (Figure 1). These terms and concepts are well established in drug development, clinical research, and practice. However, the bidirectional interactions between drugs and the microbiome are not as thoroughly described, nor are they routinely incorporated into research and clinical care. We define the microbiome here as microbes and their genomic content in the human host [1]. The host genome is relatively stable; however, the microbiome is susceptible to change and may be influenced by diet, drug co-administration, environmental exposures, age, and sex (Figure 1). Indeed, numerous studies have demonstrated that drugs, including both antimicrobials and non-antimicrobials (such as antidiabetics, proton pump inhibitors, antipsychotics, and nonsteroidal anti-inflammatory drugs), can affect microbiome composition and function, both as a therapeutic target and as an off-target effect [2–4]. Likewise the microbiome may affect responses to, and toxicities of, some drugs by metabolizing drugs and their byproducts or through synergism or antagonism [5, 6]. While interactions between the microbiome and therapeutics are bidirectional—that is, drugs may induce changes to the microbiome and the microbiome may also alter drug PK/PD—these represent two directionally distinguishable concepts that can be defined independently (Supplementary Figure 1). We propose employing the existing term “pharmacomicrobiomics” to describe exclusively the effects of the microbiome on PK and PD and using “pharmacoecology” to describe the effects of therapeutic interventions on microbiome composition and function (e.g., microbial metabolic enzymes) (Figure 1).
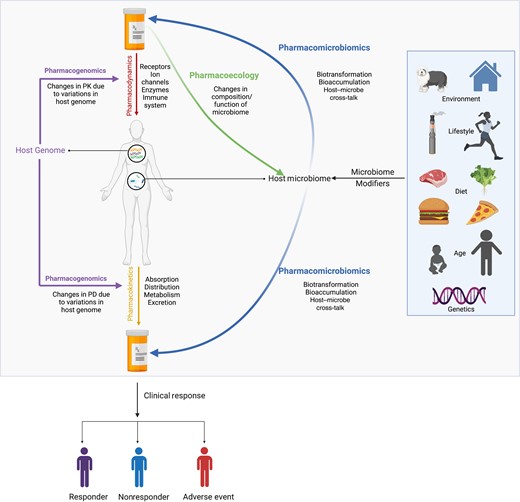
Schematic representation of directionality of terminology used to understand pharmacology and how these features collectively impact clinical response. For simplicity, gut microbiota is represented in the schematic. Figure created in Biorender. Abbreviations: PD, pharmacodynamics; PK, pharmacokinetics.
Pharmacomicrobiomics: The Effects of Microbiomes on Drug Pharmacokinetics, Pharmacodynamics, Efficacy, and Toxicity
Although Rizkallah et al. coined the term pharmacomicrobiomics in 2010 [7], examples of microbial metabolism of therapeutic drugs antedate that term by several decades [8, 9]. With the emergence of the Human Microbiome Project [10] and high-throughput sequencing technologies, we now have a better understanding of the role of the microbiome in health, disease, and drug metabolism. Rizkallah et al. described pharmacomicrobiomics as “the study of how intra- and inter-individual microbiome variations affect drug action, disposition, efficacy and toxicity, with an emphasis on the effect of microbiome variations on pharmacokinetics and pharmacodynamics of drug therapy, rather than interactions between drugs and individual microbes” [7, 11–13]. Thus, pharmacomicrobiomics represent the unidirectional effects of microbiomes on drugs, which can either be direct through biotransformation and bioaccumulation or indirect through effects on host gene expression and the immune system.
Microbes can decrease effective drug concentration by biotransformation and bioaccumulation. Biotransformation of drugs by microbes that may alter bioavailability and/or bioactivity [14–16] is a prototypic pharmacomicrobiomic effect. Likely the best known example of microbial production of drug-degrading enzymes is antimicrobial resistance by enzymes such as beta-lactamases [17]. Notably, this form of biotransformation can be from either the target organism of antimicrobial therapy or by nontarget resident microbial species [18–21]. Microbes also transform non-antimicrobial drugs, including degradation of levodopa (an agent used to treat Parkinson's disease) by specific strains of Enterococcus faecalis and E. lenta [14] and digoxin (an anti-arrhythmic cardiac glycoside), whose degradation by E. lenta strains was described almost four decades ago [15, 22]. Bioaccumulation refers to the intracellular accumulation of unmodified drugs by microbes with minimal impact on bacterial growth, which may also alter drug disposition [23]. Klunemann et al. screened 15 non-antibacterial drugs against 25 gut microbes and found 29 novel bacterial–drug interactions, 17 of which were defined as bioaccumulation events [23]. In vivo experiments using Caenorhabditis elegans showed that bioaccumulation of duloxetine (an antidepressant) by Escherichia coli attenuated response to the drug [23].
Bacteria also indirectly alter drug response through their effects on host gene expression and immune response. Germ-free mice demonstrate decreased hepatic expression of cytochrome CYP450 genes compared with mice colonized by bacteria, suggesting that bacteria play an important role in shaping the expression of the metabolic genes involved in drug metabolism [24]. Microbes can influence response to drugs through immune effects, for example by stimulating T cells through antigen presentation and biomimicry or production of immune-active microbial metabolites, mechanisms that have been implicated in response to cancer immunotherapy [25–27]. Thus, there remains potential to optimize immune system tone during the treatment of infectious, inflammatory, and neoplastic diseases via manipulation of the microbiome [28].
As these concepts illustrate, microbial modification of drug concentration or activity is in many ways parallel to that of pharmacogenomics; that is, it represents a category of unidirectional influences of the microbiome on drug activity that needs to be taken into account in pharmacological research and clinical practice.
Pharmacoecology: The Effect of Interventions on Microbiome Composition and Function
Pharmacoecology was first coined by C. Flexner to define the environmental influence on drug pharmacokinetics and response [29], but the term is no longer in wide use. We propose to adapt the term pharmacoecology to refer to the ecological impact of medications on the microbiome. Many and varied interventions, such as diet, antimicrobials, and non-antimicrobials, can alter the microbiome directly through gains and losses (or increases and decreases) in taxa or their functions, or indirectly by modifying host physiological and immune responses, which in turn affect microbial community composition and function [2, 3, 30–36]. Pharmacoecological effects are the unidirectional effects of drugs on microbiome composition and function and are thus a complementary concept to the unidirectional effects constituting pharmacomicrobiomics. Below we summarize and exemplify pharmacoecological effects and their clinical implications.
We propose that pharmacoecological effects are any gains or losses in microbial taxa or microbe-specific function due to the direct or indirect microbicidal or promicrobial activity of a drug, regardless of whether its indicated use is microbiome-targeting. Antimicrobials including antibacterials, antifungals, antivirals, and antiparasitic agents kill or suppress pathogenic organisms and may have effects on both their target (usually a disease-causing pathogen) and off-target effects on other microbiome members [37]. However, microbicidal drug effects are not restricted to drugs whose therapeutic indication is treatment of infection. In an extensive screening of 1197 compounds against 40 representative gut bacteria, 203/835 (24%) of the non-antibiotic compounds demonstrated in vitro antimicrobial activity [3]. These effects have been described in clinical studies of antidiabetic medications [38] and proton pump inhibitors [30, 31]. Conversely, promicrobials including prebiotics, probiotics, defined microbial consortia, and fecal microbiome transplantation (FMT) are intended to promote the health benefits commensals provide [39]. As such, uncovering the ecological shifts in the microbiome following treatment will allow researchers and clinicians to better understand the impact of interventions on the microbiome and potential associations with and mechanisms of clinical benefit. Pharmacoecologic assessment aims to answer the following question: How does the intervention impact microbial community composition/function?
Pharmacoecologic assessment of the microbiome is intended to complement PK/PD during investigations of new interventions. The utility of this assessment is most intuitive when investigating interventions in which a pathologically perturbed microbiome is the therapeutic target, such as treatment of recurrent Clostridioides difficile infection (rCDI) with FMT [40–42] or microbial consortia [43–45]; novel microbiome-targeting therapies can also be used for other indications in which the microbiome has been mechanistically implicated in disease or treatment response [45]. However, this assessment is also valuable when the intervention does not target the microbiome and effects on the microbiome are unintentional.
Implications: Pharmacoecology and Disease Risk, Drug Metabolism, and Clinical Outcomes
Drug-induced changes in the microbiome can increase risk of both infectious and noninfectious diseases. For example, the use of antibiotics or proton pump inhibitors (PPIs) alters risk of infection and colonization with antimicrobial-resistant organisms including C. difficile or vancomycin-resistant Enterococcus [46]. Antimicrobial exposure early in life has been associated with increased risks of allergic and respiratory diseases, likely through the impact of antimicrobials on the microbiome [47–49]. Unlike the human genome, the microbiome is not fixed, and therefore pharmacoecological changes induced by one therapy may affect the pharmacomicrobiomics of subsequent (or coincident) therapies. Understanding the pharmacoecology of treatments and how they may shape response to other therapeutics will be critical to personalization of medicine in the future. Such effects have already been observed in cancer immunotherapy and vaccine studies, in which prior antimicrobial exposure is associated with decreased responsiveness to subsequent therapy [26, 33]. Drug-induced changes in the microbiome can increase risk or harms, but also decrease risk or confer health benefits. However, data from clinical trials assessing the effects of promicrobials such as probiotics, FMTs, and microbial consortia have been demonstrated to exert taxonomic and functional changes in the microbiome with varied clinical benefit for both infectious and noninfectious diseases [41, 43, 44, 50, 51]. It is crucial to understand the long-term, on- and off-target effects of therapies on the microbiome, and assessment of these effects may help elucidate the underlying reasons for variability in therapeutic efficacy.
How to Assess Pharmacomicrobiomics and Pharmacoecology
Measures of pharmacomicrobiomics and pharmacoecology can be achieved using several methods including culturomics, targeted amplicon sequencing of the 16S (bacteria) or 18S (fungi) rRNA genes, 16S/18S quantitative polymerase chain reaction, microbial-derived targeted metabolomics, or more comprehensively using shotgun metagenomics (SGM). However, as the microbiome field is continuously evolving and there is a lack of standardized technical, analytical, and computational techniques, it is challenging to establish standardized criteria (similar to those established for PK/PD) to assess pharmacoecology. Nonetheless, pharmacoecology can be measured by assessing changes in (1) the abundance of target and nontarget taxa; (2) summary taxonomic composition metrics including alpha- and beta-diversity; (3) metagenomic compositional metrics including microbial gene/pathway abundances; (4) direct measurement of microbial metabolites; and/or (5) measures of host–microbiome interactions (such as antimicrobe immune responses). Examples of current methods available to better understand pharmacoecology along with their strengths and limitations are listed in Table 1. For pharmacomicrobiomics, we refer readers to a review written by J. Bisanz et al., which provides a guide on how to assess the effects of microbes on the absorption, distribution, metabolism, and excretion of drugs [59].
Technical Approaches Used to Measure Pharmacoecology, Strengths Limitation, and Examples From the Literature
| Technical Approaches . | PE Measure . | Strengths . | Limitations . | PE Examples . |
|---|---|---|---|---|
| Culturomics | Composition | Identify effect of drug on individual bacterial isolates. Can identify isolates at the species and strain levels [52]. | Need to culture and purify individual isolates. In vitro effect may be different than in vivo impact of drugs on microbial composition. | Decrease in Pseudomonas aeruginosa density post–inhaled aztreonam compared with placebo [53]. |
| 16S/18S amplicon sequencing | Composition | High-throughput sequencing for low cost [54]. 16S rRNA gene sequencing for bacteria. 18S internal transcribed spacer region for fungi. | Inter-study differences in variable region sequencing, primer use, extraction methods, etc., limit ability to perform meta-analyses [55]. Limited taxonomic resolution [54]. | Increase in alpha diversity post–Microbial Ecosystems Therapeutic 2 initiation in individuals with recurrent Clostridioides difficile infection [43]. |
| 16S/18S qPCR | Composition/function | Allows you to identify microbial load in sample [55]. Can identify whether certain genes are present [55]. | Amplification bias [55]. Does not allow identification of unknown species/genes [55]. | Preterm neonates randomized to receive probiotics had significantly higher load of bacteria found in probiotic compared with untreated controls [56]. |
| Metagenomics | Composition/function | High resolution [52, 54, 55]. Allows you to identify uncultured taxa [52, 54, 55]. Enables characterization of functional content [52, 54, 55]. Allows you to capture bacterial, fungal, DNA viruses, and other microbes [52, 54, 55]. | Expensive [52, 54, 55]. Data analysis computationally demanding [52, 54, 55]. | Differentially abundant microbial pathways in adolescents with obesity treated with FMT compared with placebo [50]. |
| Metatranscriptomics | Composition/function | Captures microbial transcribed RNA [54, 55]. May identify differential gene expression profiles [54, 55]. | May be difficult to assess microbial transcripts if sample is contaminated with host nucleic acids [54, 55]. Lack of standardized protocols may result in bias [54, 55]. | Study assessing impact of nonantibiotic drugs on a synthetic community of 32 human gut–derived bacteria demonstrates that antipsychotic drug chlorpromazine induces stress responses related to protein quality control [57]. |
| Metaproteomics | Composition/function | High-resolution microbial protein/peptide identification [55]. Allows you to identify novel proteins/peptides and assess for differential abundance between samples [55]. | Lack of standardized protocols, databases, and analytical methods makes it challenging to do meta-analyses [55]. | Study assessing the impact of different drugs on gut microbiome in vitro demonstrated that antibiotics, fructooligosaccharide, berberine, and diclofenac all changed the function and composition of the microbiome [58]. |
| Metabolomics | Function | Can be targeted panels or nontargeted discovery based [55]. | Can be difficult to differentiate host- vs microbiome-derived metabolites [55]. Difficult to establish standards for nontargeted approach [55]. | In a randomized controlled trial, males treated with metformin had significant increases in stool butyrate and propionate [38]. |
| Technical Approaches . | PE Measure . | Strengths . | Limitations . | PE Examples . |
|---|---|---|---|---|
| Culturomics | Composition | Identify effect of drug on individual bacterial isolates. Can identify isolates at the species and strain levels [52]. | Need to culture and purify individual isolates. In vitro effect may be different than in vivo impact of drugs on microbial composition. | Decrease in Pseudomonas aeruginosa density post–inhaled aztreonam compared with placebo [53]. |
| 16S/18S amplicon sequencing | Composition | High-throughput sequencing for low cost [54]. 16S rRNA gene sequencing for bacteria. 18S internal transcribed spacer region for fungi. | Inter-study differences in variable region sequencing, primer use, extraction methods, etc., limit ability to perform meta-analyses [55]. Limited taxonomic resolution [54]. | Increase in alpha diversity post–Microbial Ecosystems Therapeutic 2 initiation in individuals with recurrent Clostridioides difficile infection [43]. |
| 16S/18S qPCR | Composition/function | Allows you to identify microbial load in sample [55]. Can identify whether certain genes are present [55]. | Amplification bias [55]. Does not allow identification of unknown species/genes [55]. | Preterm neonates randomized to receive probiotics had significantly higher load of bacteria found in probiotic compared with untreated controls [56]. |
| Metagenomics | Composition/function | High resolution [52, 54, 55]. Allows you to identify uncultured taxa [52, 54, 55]. Enables characterization of functional content [52, 54, 55]. Allows you to capture bacterial, fungal, DNA viruses, and other microbes [52, 54, 55]. | Expensive [52, 54, 55]. Data analysis computationally demanding [52, 54, 55]. | Differentially abundant microbial pathways in adolescents with obesity treated with FMT compared with placebo [50]. |
| Metatranscriptomics | Composition/function | Captures microbial transcribed RNA [54, 55]. May identify differential gene expression profiles [54, 55]. | May be difficult to assess microbial transcripts if sample is contaminated with host nucleic acids [54, 55]. Lack of standardized protocols may result in bias [54, 55]. | Study assessing impact of nonantibiotic drugs on a synthetic community of 32 human gut–derived bacteria demonstrates that antipsychotic drug chlorpromazine induces stress responses related to protein quality control [57]. |
| Metaproteomics | Composition/function | High-resolution microbial protein/peptide identification [55]. Allows you to identify novel proteins/peptides and assess for differential abundance between samples [55]. | Lack of standardized protocols, databases, and analytical methods makes it challenging to do meta-analyses [55]. | Study assessing the impact of different drugs on gut microbiome in vitro demonstrated that antibiotics, fructooligosaccharide, berberine, and diclofenac all changed the function and composition of the microbiome [58]. |
| Metabolomics | Function | Can be targeted panels or nontargeted discovery based [55]. | Can be difficult to differentiate host- vs microbiome-derived metabolites [55]. Difficult to establish standards for nontargeted approach [55]. | In a randomized controlled trial, males treated with metformin had significant increases in stool butyrate and propionate [38]. |
Technical Approaches Used to Measure Pharmacoecology, Strengths Limitation, and Examples From the Literature
| Technical Approaches . | PE Measure . | Strengths . | Limitations . | PE Examples . |
|---|---|---|---|---|
| Culturomics | Composition | Identify effect of drug on individual bacterial isolates. Can identify isolates at the species and strain levels [52]. | Need to culture and purify individual isolates. In vitro effect may be different than in vivo impact of drugs on microbial composition. | Decrease in Pseudomonas aeruginosa density post–inhaled aztreonam compared with placebo [53]. |
| 16S/18S amplicon sequencing | Composition | High-throughput sequencing for low cost [54]. 16S rRNA gene sequencing for bacteria. 18S internal transcribed spacer region for fungi. | Inter-study differences in variable region sequencing, primer use, extraction methods, etc., limit ability to perform meta-analyses [55]. Limited taxonomic resolution [54]. | Increase in alpha diversity post–Microbial Ecosystems Therapeutic 2 initiation in individuals with recurrent Clostridioides difficile infection [43]. |
| 16S/18S qPCR | Composition/function | Allows you to identify microbial load in sample [55]. Can identify whether certain genes are present [55]. | Amplification bias [55]. Does not allow identification of unknown species/genes [55]. | Preterm neonates randomized to receive probiotics had significantly higher load of bacteria found in probiotic compared with untreated controls [56]. |
| Metagenomics | Composition/function | High resolution [52, 54, 55]. Allows you to identify uncultured taxa [52, 54, 55]. Enables characterization of functional content [52, 54, 55]. Allows you to capture bacterial, fungal, DNA viruses, and other microbes [52, 54, 55]. | Expensive [52, 54, 55]. Data analysis computationally demanding [52, 54, 55]. | Differentially abundant microbial pathways in adolescents with obesity treated with FMT compared with placebo [50]. |
| Metatranscriptomics | Composition/function | Captures microbial transcribed RNA [54, 55]. May identify differential gene expression profiles [54, 55]. | May be difficult to assess microbial transcripts if sample is contaminated with host nucleic acids [54, 55]. Lack of standardized protocols may result in bias [54, 55]. | Study assessing impact of nonantibiotic drugs on a synthetic community of 32 human gut–derived bacteria demonstrates that antipsychotic drug chlorpromazine induces stress responses related to protein quality control [57]. |
| Metaproteomics | Composition/function | High-resolution microbial protein/peptide identification [55]. Allows you to identify novel proteins/peptides and assess for differential abundance between samples [55]. | Lack of standardized protocols, databases, and analytical methods makes it challenging to do meta-analyses [55]. | Study assessing the impact of different drugs on gut microbiome in vitro demonstrated that antibiotics, fructooligosaccharide, berberine, and diclofenac all changed the function and composition of the microbiome [58]. |
| Metabolomics | Function | Can be targeted panels or nontargeted discovery based [55]. | Can be difficult to differentiate host- vs microbiome-derived metabolites [55]. Difficult to establish standards for nontargeted approach [55]. | In a randomized controlled trial, males treated with metformin had significant increases in stool butyrate and propionate [38]. |
| Technical Approaches . | PE Measure . | Strengths . | Limitations . | PE Examples . |
|---|---|---|---|---|
| Culturomics | Composition | Identify effect of drug on individual bacterial isolates. Can identify isolates at the species and strain levels [52]. | Need to culture and purify individual isolates. In vitro effect may be different than in vivo impact of drugs on microbial composition. | Decrease in Pseudomonas aeruginosa density post–inhaled aztreonam compared with placebo [53]. |
| 16S/18S amplicon sequencing | Composition | High-throughput sequencing for low cost [54]. 16S rRNA gene sequencing for bacteria. 18S internal transcribed spacer region for fungi. | Inter-study differences in variable region sequencing, primer use, extraction methods, etc., limit ability to perform meta-analyses [55]. Limited taxonomic resolution [54]. | Increase in alpha diversity post–Microbial Ecosystems Therapeutic 2 initiation in individuals with recurrent Clostridioides difficile infection [43]. |
| 16S/18S qPCR | Composition/function | Allows you to identify microbial load in sample [55]. Can identify whether certain genes are present [55]. | Amplification bias [55]. Does not allow identification of unknown species/genes [55]. | Preterm neonates randomized to receive probiotics had significantly higher load of bacteria found in probiotic compared with untreated controls [56]. |
| Metagenomics | Composition/function | High resolution [52, 54, 55]. Allows you to identify uncultured taxa [52, 54, 55]. Enables characterization of functional content [52, 54, 55]. Allows you to capture bacterial, fungal, DNA viruses, and other microbes [52, 54, 55]. | Expensive [52, 54, 55]. Data analysis computationally demanding [52, 54, 55]. | Differentially abundant microbial pathways in adolescents with obesity treated with FMT compared with placebo [50]. |
| Metatranscriptomics | Composition/function | Captures microbial transcribed RNA [54, 55]. May identify differential gene expression profiles [54, 55]. | May be difficult to assess microbial transcripts if sample is contaminated with host nucleic acids [54, 55]. Lack of standardized protocols may result in bias [54, 55]. | Study assessing impact of nonantibiotic drugs on a synthetic community of 32 human gut–derived bacteria demonstrates that antipsychotic drug chlorpromazine induces stress responses related to protein quality control [57]. |
| Metaproteomics | Composition/function | High-resolution microbial protein/peptide identification [55]. Allows you to identify novel proteins/peptides and assess for differential abundance between samples [55]. | Lack of standardized protocols, databases, and analytical methods makes it challenging to do meta-analyses [55]. | Study assessing the impact of different drugs on gut microbiome in vitro demonstrated that antibiotics, fructooligosaccharide, berberine, and diclofenac all changed the function and composition of the microbiome [58]. |
| Metabolomics | Function | Can be targeted panels or nontargeted discovery based [55]. | Can be difficult to differentiate host- vs microbiome-derived metabolites [55]. Difficult to establish standards for nontargeted approach [55]. | In a randomized controlled trial, males treated with metformin had significant increases in stool butyrate and propionate [38]. |
Additional Considerations
Several factors may alter pharmacomicrobiomics and pharmacoecology [13]. For example, age, diet, lifestyle, polypharmacy, comorbidities, and hormonal changes may result in temporal shifts in the microbiome. Similarly, spatial differences in microbial ecosystems such as differences in the physiology of the target site, pH, and oxygen gradients may further impact host–microbiome–drug interactions [13]. It is important to consider these variables when assessing pharmacomicrobiomoics and pharmacoecology. Careful study design and randomized controlled trials will help us better understand how drugs impact the microbiome and how the microbiome may impact drugs.
Our proposed use of the terms pharmacomicrobiomics and pharmacoecology attempts to clarify the terminology used to assess these distinct interactions. We suggest that the use of this framework meet the following criteria for their use:
The concepts/terms apply to drug–microbiome interactions regardless of the drug target, therapeutic indication, or host.
They are taxonomically generalizable—that is, they can be applied to any host-associated organisms (bacterial, fungal, viral, or protists).
They distinguish between the directional effects of microbiomes on drugs and drugs on microbiomes.
They are measurable with existing technologies.
As integration of drug–microbiome interactions into clinical practice progresses, we propose that the incorporation of these terms will be useful to help clarify and consolidate concepts and measures relevant to understanding the outcomes of disease treatments across a range of diseases and drug classes.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank Coburn lab members for their feedback and suggestions. We would like to thank the peer reviewers at Open Forum Infectious Diseases for taking the time to review the manuscript. We sincerely appreciate all their valuable comments and suggestions, which helped us improve the quality of the manuscript. We acknowledge and are grateful for the support of the Tomcyzk AI and Microbiome Working Group.
Financial support. A.H. was supported by the Tomcyzk AI and Microbiome Working Group and the Princess Margaret Cancer Foundation. S.M. was supported by CIHR and Killam Postdoctoral Fellowships.
Author contributions. A.H. conceived the manuscript. A.H. created the figures and table. All authors contributed to revising and editing the manuscript. M.C.A. and B.C. supervised the work.
Patient consent. This manuscript does not include factors necessitating patient consent.
References
Author notes
Potential conflicts of interest. All authors have no conflicts to report.





Comments