-
PDF
- Split View
-
Views
-
Cite
Cite
Tatyana Kushner, Custon T Nyabanga, Scott J Cotler, Ohad Etzion, Harel Dahari, Modeling-Based Response-Guided Hepatitis C Treatment During Pregnancy and Postpartum, Open Forum Infectious Diseases, Volume 10, Issue 2, February 2023, ofad027, https://doi.org/10.1093/ofid/ofad027
Close - Share Icon Share
Abstract
Treating hepatitis C virus (HCV) in pregnancy would address HCV during prenatal care and potentially reduce the risk of vertical transmission. Response-guided therapy could provide a means to individualize and the reduce duration of HCV treatment during pregnancy. Data from a 27-year-old woman indicated that, pretreatment, HCV was stable and that it dropped in a biphasic manner during sofosbuvir/velpatasvir therapy, reaching target not detected at time of delivery—16 days post–initiation of therapy. Mathematical modeling of measured HCV at days 0, 7, and 14 predicted that cure could have been achieved after 7 weeks of sofosbuvir/velpatasvir, reducing the duration of therapy by 5 weeks.
Hepatitis C virus (HCV) treatment with direct-acting agents (DAAs) in pregnancy offers the opportunity to provide maternal cure, decrease the risk of vertical transmission, and possibly decrease the risk of HCV-associated pregnancy complications. Active HCV in pregnancy is associated with adverse pregnancy outcomes, most notably intrahepatic cholestasis of pregnancy (ICP), which in turn can lead to poor fetal outcomes and preterm delivery [1]. Current expert guidelines by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America state that HCV “treatment can be considered during pregnancy on an individual basis after a patient-physician discussion about the potential risks and benefits” [2]. Prior animal data and limited human data have provided preliminary evidence of the safety of DAA exposure in pregnancy [3–5]. However, the small amount of information regarding the safety of DAAs for chronic hepatitis C (CHC) treatment during pregnancy and the paucity of clinical experience with their use have limited implementation of DAA therapy during pregnancy in clinical practice.
In nonpregnant CHC patients, HCV is stable pretreatment and drops in a biphasic manner while the patient is on DAA therapy [6–9]. The rapid first-phase decline occurs within 12–48 hours of treatment initiation and is then followed by a slower second phase of several days to weeks in which viral decline continues at a constant rate. However, early HCV kinetics during pregnancy have not been characterized, precluding the development of a response-guided treatment (RGT) approach.
Mathematical modeling can reproduce the biphasic viral decline on DAA therapy, and therefore can predict time to cure (TTC), the time point at which the cure boundary is reached, that is, when the model curve (Figure 1, solid line) and cure boundary (Figure 1, lower dashed line) meet. Indeed, several retrospective studies published by our group have shown that mathematical modeling of early viral kinetics predicts TTC of <12 weeks in the majority of individuals treated with sofosbuvir-based as well as other DAA regimens [6–9]. Notably, we recently reported a proof-of-concept study showing that real-time (ie, on-treatment viral load measurement on days 0, 2, 7, 14, and 28) mathematical modeling–based RGT with DAA for CHC infection can be utilized for shortening DAA duration without compromising treatment efficacy [10]. Very recently, we showed, retrospectively, that measuring HCV on days 0, 7, and 14 of therapy (ie, without days 2 and 28) can identify patients for shortening therapy duration [11]. Whether this modeling-based RGT during pregnancy has the potential to reduce DAA has not been evaluated.
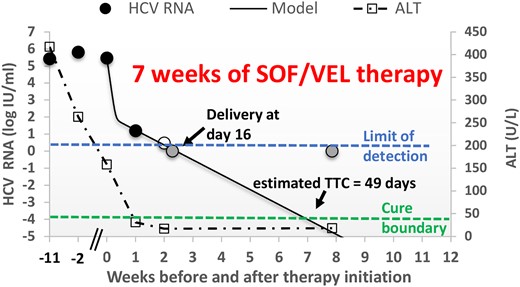
Plot of HCV RNA viral load (circles) and ALT levels (squares) over time before and after SOF/VEL therapy initiation. Filled circles, observed HCV viral load above the limit of quantification; empty circle, <15 IU/mL; gray circles, target not detected (upper horizontal dashed line); solid line, mathematical modeling (Eq. 1); lower horizontal dashed line, cure boundary. Abbreviations: ALT, alanine transaminase; HCV, hepatitis C virus; SOF/VEL, sofosbuvir/velpatasvir; TTC, time to cure.
In this study, we report the first observation of early HCV kinetics in a pregnant woman with CHC, which can guide RGT for HCV. Data from 0, 7, and 14 days post–treatment initiation with sofosbuvir/velpatasvir during pregnancy were analyzed and modeled to predict TTC.
METHODS
Patient
A 23-year-old woman with a history of prior injection drug use (last use 2 years prior) and pregnant at 20 weeks’ gestation was referred to our Women's Liver Clinic for evaluation of an HCV antibody (Ab)–positive result on her routine prenatal screening. She denied prior knowledge of her positive HCV status. She was found to have HCV genotype 1a, with serum HCV RNA of 929 IU/mL, which subsequently peaked at 1834 IU/mL at 30 weeks’ gestation. Her liver enzymes peaked at ALT 381 U/L and AST 171 U/L. She was also noted to be hepatitis B virus (HBV) core antibody positive with hepatitis B surface antigen–negative and hepatitis B surface antibody–negative and –undetectable serum HBV DNA. She was hepatitis A nonimmune. Her abdominal ultrasound revealed mild hepatomegaly and cholelithiasis, with normal spleen size. She did not have evidence of advanced fibrosis; her platelet count was 271, and her FIB-4 score was 0.99. She was then enrolled in a dedicated HCV case management program, and timing of HCV treatment was discussed with her. The patient opted to defer treatment until after delivery. Her course was complicated by ICP, with total bile acids of 18 µmol/L, and she was started on ursodiol treatment. Her ICP diagnosis prompted a decision to proceed with delivery at 37 weeks’ gestation. After delivery, the patient had a scheduled follow-up for discussion of HCV treatment initiation. She missed multiple appointments and unfortunately was lost to follow-up.
Four years later, at age 27, the patient re-presented for routine care for a subsequent pregnancy and again was found to be HCV Ab positive on routine screening. She was referred for evaluation and presented to our Liver Clinic at 23 weeks’ gestation. There was low suspicion for advanced fibrosis with a platelet count of 235 and an FIB-4 score of 1.16. She wanted to pursue HCV treatment during pregnancy, expressing hope for decreased risk of vertical transmission and more confidence in taking medication during pregnancy.
Mathematical Modeling
Parameter Estimations
We assumed the target cell (ie, hepatocytes) level remained constant during therapy at pretreatment level T0 = 1 × 107. The initial infected cell level is represented by the steady state pretreatment level of I0 = βV0T0/δ, where V0 = pretreatment measured viral load. The viral production rate constant was set to P = cV0/I0. As previously done in a proof-of-concept study [10], we assume no pharmacological delay of DAA effect in reducing viral load from pretreatment levels and set the infection parameter β to 2 × 10−7 mL/virion/d. As HCV measurements were lacking during the first 24–48 hours post–initiation of DAA, the viral clearance (parameter c) was fixed to 6 days−1, as recently done [11]. The remaining parameters ε and δ were estimated by fitting the model with the observed data using Berkeley Madonna (version 8.3).
Time to Cure
The time to cure (TTC) was defined as the time to reach <1 HCV particle in the entire extracellular body fluid, which was estimated based on body weight [10]. In particular, a value of 1 virus copy in 15 L of extracellular body fluid volume, that is, V = 7 × 10−5 IU/mL, was used as the threshold for cure. The standard HCV model (Eq. 1) was fit to the measured HCV RNA kinetic at days 0, 7, and 14 of DAA therapy in order to predict TTC.
RESULTS
Upon presentation at 23 weeks’ gestation, the patient's HCV RNA was 265 000 IU/mL (and later peaked at 640 000 IU/mL at 31 weeks’ gestation). Her initial liver tests showed ALT 417 U/L and AST 206 U/L. Her pregnancy was again complicated by ICP, with severe pruritus and serum bile acid levels peaking at 183 µmoL/L at 25 weeks’ gestation. Oral ursodiol at 15 mg/kg was initiated, and pruritus partially improved. She started sofosbuvir/velpatasvir at 34 weeks of gestation. Due to ICP with high bile acid levels, she had a planned induction of labor at 37 weeks’ gestation and had a successful uneventful vaginal delivery. Serum HCV RNA virus and ALT levels were monitored pre- and postpartum while she continued DAA therapy. She had a rapid response to treatment with near-undetectable levels noted by day 7 (Figure 1). Her HCV RNA level was undetectable at week 8 of her 12-week treatment course. The patient and newborn were doing well at the time of last follow-up, with a plan for testing of the infant at 18 months of age for HCV Ab to determine if there is evidence of vertical transmission.
HCV remained stable pretreatment in the pregnant woman and followed a biphasic decline while the patient was on sofosbuvir/velpatasvir therapy (Figure 1). Fitting the mathematical model with the current patient's HCV measurements on days 0, 7, and 14 predicted that sofosbuvir/velpatasvir efficacy in blocking viral production was ε = 0.9996 and HCV-infected cell loss/death was δ = 0.287 days−1 and that the TTC was at ∼7 weeks of sofosbuvir/velpatasvir therapy (Figure 1).
DISCUSSION
Treatment of HCV during pregnancy has been called “the last frontier” of HCV treatment and remains an active area of investigation [13]. Ongoing trials include a multicenter phase 4 study evaluating HCV treatment with sofosbuvir/velpatasvir during the second and third trimesters of pregnancy as well as a phase I trial evaluating the pharmacokinetics of sofosbuvir/velpatasvir in pregnancy. When women with HCV were surveyed on their preferences for HCV treatment in pregnancy, the majority answered that they would be interested in HCV treatment during pregnancy if it decreased risk of vertical transmission, but many expressed hesitancy due to desire for more pregnancy safety data [14]. As data emerge from ongoing clinical trials and the recently developed HCV Pregnancy registry [15], more patients and providers may consider treatment during pregnancy, and RGT would potentially offer an individualized approach that decreases exposure to medication during pregnancy (Figure 2).
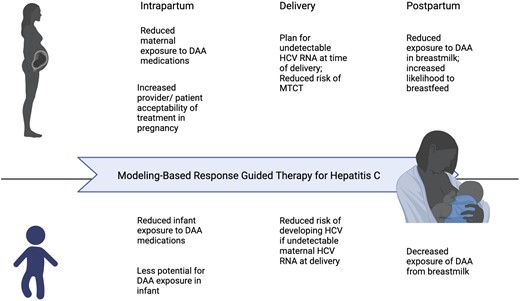
Potential benefits to modeling-based RGT for hepatitis C during pregnancy and postpartum. Abbreviations: DAA, direct-acting antiviral; MTCT, mother-to-child transmission; RGT, response-guided treatment.
At the time of delivery, HCV was not detected in the patient (Figure 1), dramatically reducing the probability of HCV transmission to the newborn compared with the average pretreatment viral load of 433 000 IU/mL [16, 17]. Theoretically, reaching the cure boundary with DAA therapy before delivery, defined as <1 virus particle in the entire extracellular body fluid by modeling [10], should also prevent vertical transmission. In cases in which HCV cure is not achieved at the time of delivery but postpartum, the potential benefit of using RGT would be to allow for a shorter time of DAA therapy to decrease exposure of DAA to the newborn from breastmilk (Figure 2).
The current study provides a response to the World Health Organization call for research into predictive factors for identifying patients with HCV who could be successfully treated with a shorter duration of DAA therapy [18]. Recent RGT approaches to shorten DAA therapy in nonpregnant CHC patients based on randomization to an arbitrary treatment duration [19] or a day 2 of treatment viral load cutoff have failed [20]. In contrast, our individualized RGT approach in nonpregnant CHC successfully used mathematical modeling to individualize and reduce DAA treatment duration in a small, prospective proof-of-concept study [10]. While results from the proof-of-concept study provide evidence to support the utility of real-time mathematical modeling for optimizing DAA therapy duration, implementation of this RGT in clinical practice requires validation in a larger clinical trial and evaluation in specific populations of interest such as during pregnancy. Even after validation, the RGT approach may not be feasible/cost-effective in some countries or settings where the infrastructure for frequent HCV RNA testing is not available or in those where the cost of additional HCV RNA measurements on therapy may exceed the price of discounted DAA treatment. Thus, expertise in RGT and predictive modeling as well as an infrastructure that allows for more frequent earlier HCV RNA testing will be needed to scale up this approach, in addition to careful cost–benefit analysis that will facilitate the implementation of RGT in specialized populations such as pregnant women who may benefit from it the most. Given the recent national White House plan [21] for HCV elimination, prioritizing this approach could be both cost-saving and beneficial to patients.
In order to implement RGT particularly in the pregnancy context, larger studies will be needed to determine whether timing of treatment (ie, second or third trimester and/or postpartum treatment initiation) impacts early viral kinetics and TTC. Viral kinetics on DAAs will also need to be compared between pregnant and nonpregnant individuals to assess for differences. These clinical and theoretical results support the further evaluation of HCV kinetics and a modeling-based RGT approach during pregnancy and postpartum; these have the potential to increase the number of people who benefit from DAA treatment while decreasing cost and exposure to HCV medication.
Acknowledgments
Financial support. This study was supported by National Institutes of Health (NIH) grants R01AI158666, R01AI078881, and R01GM121600.
Patient consent. The patient's written consent was obtained. The design of the work conforms to the standards applied in the United States.
References
Author notes
Potential conflicts of interest. T.K.: advisory for Gilead, AbbVie, Bausch. O.E.: speaker for Gilead, AbbVie. C.T.N., S.J.C., and H.D.: nothing to disclose.





Comments