-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Conway, Annabelle Stevens, Carolyn Murray, Bianca Prain, Cherie Power, Anna McNulty, Nigel Carrington, Heng Lu, Melanie Kingsland, Colette McGrath, Phillip Read, Mitchell Starr, Beth Catlett, Philip Cunningham, Jason Grebely, Hepatitis C Treatment Uptake Following Dried Blood Spot Testing for Hepatitis C RNA in New South Wales, Australia: The NSW DBS Pilot Study, Open Forum Infectious Diseases, Volume 10, Issue 11, November 2023, ofad517, https://doi.org/10.1093/ofid/ofad517
Close - Share Icon Share
Abstract
Dried blood spot (DBS) testing for hepatitis C virus (HCV) RNA provides a sampling option that avoids venepuncture and can be carried out in a nonclinical setting. Large-scale evaluations are needed to understand how DBS testing can reduce HCV burden. This study estimated prevalence of, and factors associated with, HCV RNA and treatment initiation among people enrolled in a state-wide pilot of people testing in the NSW DBS Pilot in New South Wales, Australia.
People at risk of HIV/HCV could participate via (1) self-registration online with a DBS collection kit delivered and returned by conventional postal service; or (2) assisted DBS sample collection at a community site or prison. Logistic regression was used to identify factors associated with detectable HCV RNA and treatment initiation within 6 months of testing.
Between September 2017 and December 2020, 5960 people were tested for HCV (76% men, 35% Aboriginal and/or Torres Strait Islander, 55% recently injected drugs): 21% online self-registration, 34% assisted registration in the community, 45% assisted registration in prison. Fifteen percent had detectable HCV RNA (878/5960). Overall, 44% (n = 386/878) of people with current HCV initiated treatment within 6 months (13% online self-registration, 27% assisted registration in the community, 61% assisted registration in prison). Testing in prison compared with the community (adjusted odds ratio [aOR], 4.28; 95% CI, 3.04–6.03) was associated with increased odds of treatment initiation. Being a woman compared with a man (aOR, 0.68; 95% CI, 0.47–0.97) was associated with reduced treatment initiation.
The NSW DBS Pilot demonstrates the feasibility of using DBS to promote HCV testing and treatment in community and prison settings.
The World Health Organization's Global Health Sector Strategy on Viral Hepatitis 2016–2021 presented targets to reach the elimination of hepatitis C (HCV) as a major public health threat by 2030 [1]. In Australia, the National HCV Strategy 2018–2022 laid out priority areas for action, including implementing approaches to maximize the number of people with HCV who are diagnosed [2]. HCV and the behaviors associated with its transmission, such as injecting drug use, are stigmatized, which can be a barrier to testing and treatment initiation [3]. Despite direct-acting antiviral (DAA) therapy being available in Australia since 2016, inequities in treatment uptake persist [4, 5]. Simplified testing and treatment pathways can mitigate structural stigma to advance progress toward HCV elimination.
Standard of care HCV testing and treatment often involve multiple visits to different providers, which is burdensome on the patient and risks increasing gaps in the cascade of care [6]. Offering testing and treatment in settings that are regularly used by people at risk of HCV is convenient and can improve treatment outcomes [7]. Among people who inject drugs, venepuncture can be painful and arduous [8] and has been shown to be less acceptable than fingerprick sampling [9, 10]. Using dried blood spot (DBS) samples can simplify HCV testing pathways and is associated with improved testing [11, 12] and linkage to care [12]. Studies have demonstrated high sensitivity and specificity in using DBS testing to detect HCV RNA [13]. DBS sample collection can be performed by a lay person with minimal training, and samples can be easily transported without specialized storage, making it convenient to use in low-resource settings [14]. Although there have been some evaluations of DBS testing for HCV [15], the majority of studies have been limited to the United Kingdom, and there have been few data on the characteristics of people receiving testing, which are needed to inform population-level scale-up of DBS testing. In an environment where multiple testing modalities are available, it is important to understand the role that DBS testing can play in improving health equity.
The New South Wales (NSW) DBS Pilot was launched in November 2016 with the primary objective of increasing access to HIV testing for priority populations and was expanded to offer HCV RNA testing from September 2017. This analysis evaluated factors associated with detectable HCV RNA and factors associated with HCV treatment initiation postenrollment among people receiving testing for HCV RNA in the NSW DBS Pilot, a study evaluating scale-up of DBS testing for HCV RNA in NSW, Australia.
METHODS
Study Population and Design
The NSW DBS Pilot is a state-wide observational cohort study evaluating the scale-up of HCV and HIV testing from DBS sample collection. Participants were recruited via 3 pathways (online self-registration for at-home sample collection, assisted registration at community sites, or assisted registration in prison) for HIV and/or HCV testing. Participants were recruited across New South Wales, online, at 36 community sites (providing some or all of the following services: drug treatment, needle and syringe provision, sexual health), and at 21 prisons. The NSW DBS Pilot is ongoing, and this analysis reports data from people receiving testing between September 2017 and December 2020 and people initiating treatment until June 30, 2021.
The NSW DBS Pilot recruited people who provided informed consent and were aged ≥16 years. The NSW DBS Pilot began in November 2016, offering HIV testing. From September 2017, HCV testing inclusion criteria were identifying as Aboriginal or Torres Strait Islander or having a history of injecting drug use. From June 2019 to December 2020 (end of analysis period), inclusion criteria for HCV testing were expanded to people born in Asia or Africa and people who had ever been incarcerated. Participants who met the inclusion criteria for HCV testing were eligible for HIV testing. People who met the inclusion criteria for HIV testing (gay and other men who have sex with men [MSM], people from Sub-Saharan Africa and Southeast Asia, and people with current/previous sexual partners from Sub-Saharan Africa and Southeast Asia) were not automatically eligible for HCV testing.
Patient Consent
All participants provided informed consent via an online Participant Information Sheet and Consent Form. The initial study protocol and all subsequent amendments were approved by St Vincent's Hospital (Sydney) Human Research Ethics Committee (2019/ETH09614 HREC/15/SVH/400), and additional ethics approval was received from the Aboriginal Health and Medical Research Council Human Research Ethics Committee, NSW Corrective Services Ethics Committee, and Local Health District governance.
DBS Testing
DBS collection kits were distributed to sites and study participants. They contained a test card, a lancet, alcohol swabs, band-aids, cotton balls, a foil envelope, and a reply-paid envelope. Procedures for DBS sample collection and testing (elution, spot size, punching protocols, validation, testing algorithm) have been described elsewhere [16, 17]. The tests used in the NSW DBS Pilot were Murex HIV-1.2.0 antibody enzyme-linked immunosorbent assay (Diasorin, Macquarie Park, Australia) and New Lav-Blot-1 (Bio-Rad, Gladesville, Australia) for HIV testing, and Aptima HCV Quant Dx assay (Hologic, Macquarie Park, Australia) for HCV RNA testing. Once a DBS sample was collected and dried, it was mailed back to the NSW State Reference Laboratory for HIV (St Vincent's Hospital, Sydney, Australia). Depending on the request, the card was tested for HIV and/or hepatitis C. The algorithm for testing was based on current evidence on DBS testing [13, 16, 18]. All reactive (HIV antibody) and detectable (HCV RNA) results required a confirmatory test via venepuncture for diagnosis.
Procedures
For online registration, participants completed an online survey, and if eligible, they received a testing kit via post for sample collection at home. Kits contained a link to an online instructional video (https://www.health.nsw.gov.au/dbstest), and from December 2017, kits included a visual aid to facilitate sample collection. Participants returned the sample to the DBS group laboratory situated at St Vincent's Centre for Applied Medical Research (St Vincent's Hospital, Sydney, Australia) for testing, and a result was returned to the person via SMS or phone. People with a reactive (HIV antibody) or detectable (HCV RNA) result received an SMS asking them to call the state-wide Sexual Health Infolink, where a nurse offered post-test counseling and supported linkage to confirmatory testing and care. People with a nonreactive (HIV antibody) or undetectable (HCV RNA) result received an SMS notifying them of the result. The Sexual Health Infolink submitted a standardized online case report form for each participant with detectable HCV RNA including data on HCV treatment initiation and loss to follow-up at 6 months.
For assisted registration in the community or in prison, participants were assisted to complete the online survey, and, according to their eligibility, a DBS sample for HIV or HCV testing was collected. The sample was returned to the laboratory, and participants were informed of their results by SMS, phone, or in-person at the participating site. Depending on the site, linkage to care post-diagnosis was the responsibility of the state-wide Sexual Health Infolink or clinical staff at the site level. The Sexual Health Infolink or clinic site coordinators submitted a standardized case report form for each participant with detectable HCV RNA including data on HCV treatment initiation and loss to follow-up at 6 months. For participants in prison, the case report form was completed by Justice Health, which only reported on treatment initiations in the prison setting.
All participants completed an online survey of baseline data, including demographics, behavioral risk, and HIV and HCV testing history. There was no compensation or financial incentive offered as part of the study, but some sites implemented this as part of local initiatives.
Where a participant tested more than once during the study period, a unique identifier was used to extract the most recent detectable test result or most recent undetectable result for each participant, and only these episodes were included in the analysis. Demographic and behavioral characteristics were reported from the enrollment survey. HCV RNA test results were reported from the laboratory database. Treatment initiation was collected from the standardized case report forms completed by sites and the state-wide Sexual Health Infolink. All databases were linked using a medical record number unique to each testing episode.
Study Outcomes
The primary study outcome was detectable HCV RNA. The secondary study outcome, treatment uptake within 6 months of testing, was defined as HCV treatment prescribed within 6 months of the registration date. Observation time for treatment initiation commenced on the date of NSW DBS pilot enrollment and ended on the date of HCV treatment initiation.
Exposures
Demographic and behavioral factors hypothesized to be associated with detectable HCV RNA and treatment initiation were determined from the literature and included (i) testing setting (online self-registration, assisted registration in the community, assisted registration in prison), (ii) gender (male, female, other [including nonbinary and transgender]), (iii) age at survey (5 categories: ≤25, 25–34, 35–44, 45–54, >55), (iv) Aboriginal and/or Torres Strait Islander, (v) major city postcode, (vi) born outside of Australia (no, yes [Asia/Africa], yes [other]), (vii) speaks English at home, (viii) recently injected drugs (no, yes, prefer not to say). Due to changes in the survey from the beginning of 2019, the definition of recent drug injection changed from in the last 12 months (pre-2019) to in the last month (2019 onwards).
Statistical Analyses
Logistic regression models were used to estimate crude and adjusted odds ratios (aORs) and corresponding 95% confidence intervals to evaluate factors associated with detectable HCV RNA and HCV treatment initiation. Variables with a P value <.10 in the unadjusted logistic regression models were retained in adjusted models if no collinearity was identified.
For all analyses, statistically significant differences were assessed at a .05 level; P values were 2-sided. All analyses were performed using Stata, version 14.0 (StataCorp, College Station, TX, USA).
RESULTS
Sample Characteristics
Overall, 6600 HCV RNA tests were performed during the study period, and 5960 people were tested. Considering the most recent test, among the 5960 people tested (Figure 1), the most common registration pathway was assisted registration in prison (55%, n = 3275), then assisted registration in the community (40%, n = 2357), and lastly online self-registration (6%, n = 328). The proportion of tests performed in each pathway changed per quarter (Figure 2). The median age of people tested (range, interquartile range) was 38 (16–91, 29–46) years, a quarter were women (24%), 35% identified as Aboriginal or Torres Strait Islander, 85% were born in Australia, 93% spoke English at home, and 55% had recently injected drugs (Table 1).

Flowchart of people tested for HCV RNA in the NSW DBS Study, September 2017–December 2020. Abbreviation: HCV, hepatitis C virus.
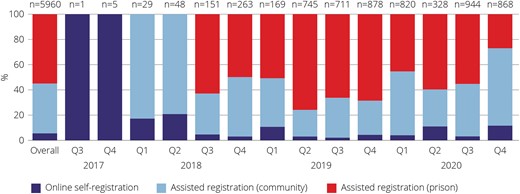
People tested for HCV RNA in each pathway per quarter in the NSW DBS Pilot, September 2017–December 2020 (n = 5960). Abbreviation: HCV, hepatitis C virus.
Characteristics of People Tested for HCV RNA in the NSW DBS Study, September 2017–December 2020 (n = 5960)
| Variables . | People Tested for HCV . | |
|---|---|---|
| No. . | %a . | |
| Overall | 5960 | … |
| Registration type | ||
| Online | 328 | 6 |
| Assisted (community) | 2357 | 40 |
| Assisted (prison) | 3275 | 55 |
| Test performed | ||
| HIV + HCV | 5472 | 92 |
| HCV only | 488 | 8 |
| Gender | ||
| Men | 4517 | 76 |
| Women | 1390 | 23 |
| Other | 53 | 1 |
| Age | ||
| ≤25 y | 749 | 13 |
| 25–34 y | 1768 | 30 |
| 35–44 y | 1755 | 29 |
| 45–54 y | 1162 | 19 |
| >55 y | 526 | 9 |
| Aboriginal and/or Torres Strait Islander | ||
| No | 3857 | 65 |
| Yes | 2103 | 35 |
| Major city postcode (n = 2685, excluding prisons) | ||
| No | 586 | 22 |
| Yes | 2099 | 78 |
| Men who have sex with men | ||
| No | 5526 | 93 |
| Yes | 434 | 7 |
| Born outside of Australia | ||
| No | 5084 | 85 |
| Yes, Asia or Africa | 347 | 6 |
| Yes, other | 529 | 9 |
| Speaks English at home | ||
| No | 439 | 7 |
| Yes | 5521 | 93 |
| Recently injected drugs | ||
| No | 2443 | 41 |
| Yes | 3292 | 55 |
| Prefer not to say | 225 | 4 |
| Variables . | People Tested for HCV . | |
|---|---|---|
| No. . | %a . | |
| Overall | 5960 | … |
| Registration type | ||
| Online | 328 | 6 |
| Assisted (community) | 2357 | 40 |
| Assisted (prison) | 3275 | 55 |
| Test performed | ||
| HIV + HCV | 5472 | 92 |
| HCV only | 488 | 8 |
| Gender | ||
| Men | 4517 | 76 |
| Women | 1390 | 23 |
| Other | 53 | 1 |
| Age | ||
| ≤25 y | 749 | 13 |
| 25–34 y | 1768 | 30 |
| 35–44 y | 1755 | 29 |
| 45–54 y | 1162 | 19 |
| >55 y | 526 | 9 |
| Aboriginal and/or Torres Strait Islander | ||
| No | 3857 | 65 |
| Yes | 2103 | 35 |
| Major city postcode (n = 2685, excluding prisons) | ||
| No | 586 | 22 |
| Yes | 2099 | 78 |
| Men who have sex with men | ||
| No | 5526 | 93 |
| Yes | 434 | 7 |
| Born outside of Australia | ||
| No | 5084 | 85 |
| Yes, Asia or Africa | 347 | 6 |
| Yes, other | 529 | 9 |
| Speaks English at home | ||
| No | 439 | 7 |
| Yes | 5521 | 93 |
| Recently injected drugs | ||
| No | 2443 | 41 |
| Yes | 3292 | 55 |
| Prefer not to say | 225 | 4 |
Abbreviation: HCV: hepatitis C virus.
aColumn percentage.
Characteristics of People Tested for HCV RNA in the NSW DBS Study, September 2017–December 2020 (n = 5960)
| Variables . | People Tested for HCV . | |
|---|---|---|
| No. . | %a . | |
| Overall | 5960 | … |
| Registration type | ||
| Online | 328 | 6 |
| Assisted (community) | 2357 | 40 |
| Assisted (prison) | 3275 | 55 |
| Test performed | ||
| HIV + HCV | 5472 | 92 |
| HCV only | 488 | 8 |
| Gender | ||
| Men | 4517 | 76 |
| Women | 1390 | 23 |
| Other | 53 | 1 |
| Age | ||
| ≤25 y | 749 | 13 |
| 25–34 y | 1768 | 30 |
| 35–44 y | 1755 | 29 |
| 45–54 y | 1162 | 19 |
| >55 y | 526 | 9 |
| Aboriginal and/or Torres Strait Islander | ||
| No | 3857 | 65 |
| Yes | 2103 | 35 |
| Major city postcode (n = 2685, excluding prisons) | ||
| No | 586 | 22 |
| Yes | 2099 | 78 |
| Men who have sex with men | ||
| No | 5526 | 93 |
| Yes | 434 | 7 |
| Born outside of Australia | ||
| No | 5084 | 85 |
| Yes, Asia or Africa | 347 | 6 |
| Yes, other | 529 | 9 |
| Speaks English at home | ||
| No | 439 | 7 |
| Yes | 5521 | 93 |
| Recently injected drugs | ||
| No | 2443 | 41 |
| Yes | 3292 | 55 |
| Prefer not to say | 225 | 4 |
| Variables . | People Tested for HCV . | |
|---|---|---|
| No. . | %a . | |
| Overall | 5960 | … |
| Registration type | ||
| Online | 328 | 6 |
| Assisted (community) | 2357 | 40 |
| Assisted (prison) | 3275 | 55 |
| Test performed | ||
| HIV + HCV | 5472 | 92 |
| HCV only | 488 | 8 |
| Gender | ||
| Men | 4517 | 76 |
| Women | 1390 | 23 |
| Other | 53 | 1 |
| Age | ||
| ≤25 y | 749 | 13 |
| 25–34 y | 1768 | 30 |
| 35–44 y | 1755 | 29 |
| 45–54 y | 1162 | 19 |
| >55 y | 526 | 9 |
| Aboriginal and/or Torres Strait Islander | ||
| No | 3857 | 65 |
| Yes | 2103 | 35 |
| Major city postcode (n = 2685, excluding prisons) | ||
| No | 586 | 22 |
| Yes | 2099 | 78 |
| Men who have sex with men | ||
| No | 5526 | 93 |
| Yes | 434 | 7 |
| Born outside of Australia | ||
| No | 5084 | 85 |
| Yes, Asia or Africa | 347 | 6 |
| Yes, other | 529 | 9 |
| Speaks English at home | ||
| No | 439 | 7 |
| Yes | 5521 | 93 |
| Recently injected drugs | ||
| No | 2443 | 41 |
| Yes | 3292 | 55 |
| Prefer not to say | 225 | 4 |
Abbreviation: HCV: hepatitis C virus.
aColumn percentage.
The registration pathways tested different populations. Online self-registration tested a higher proportion of men who have sex with men (55%, vs 7% in the community and 3% in prison; P < .001) and people born outside of Australia (48%, vs 15% in the community and 11% in prison; P < .001). Assisted registration in the community tested a higher proportion of women (35%, vs 18% in online self-registration and 16% in prison; P < .001) and people who recently injected drugs (68%, vs 26% in online self-registration and 49% in prison; P < .001). Assisted registration in prisons tested a higher proportion of Aboriginal and/or Torres Strait Islander people (41%, vs 16% in online self-registration and 30% in the community; P < .001) (Supplementary Table 1).
Detectable HCV RNA
Of those tested for HCV (n = 5960), 15% (n = 878) had detectable HCV RNA. The proportion with detectable HCV RNA was lower in online self-registration (5%) than assisted registration in the community (17%) and assisted registration in prison (14%; P < .001). The proportion with detectable HCV RNA was highest in Aboriginal and/or Torres Strait Islander people (17% vs 14% in non-Aboriginal and Torres Strait Islander people; P = .003), people born in Australia (16% vs 5% in people born in Asia or Africa; P < .001), and people who recently injected drugs (20% vs 8% in people who did not recently inject drugs; P < .001).
In the adjusted logistic regression model, detectable HCV RNA was lower in people participating via online self-registration compared with assisted registration in the community (aOR, 0.38; 95% CI, 0.22–0.65). Older age groups were associated with lower odds of detectable HCV RNA (being aged 25–34, vs <25: aOR, 0.75; 95% CI, 0.58–0.97; or >55, vs <25: aOR, 0.62; 95% CI, 0.43–0.90). Identifying as Aboriginal and/or Torres Strait Islander (aOR, 1.20; 95% CI, 1.03–1.40), speaking English at home (aOR, 1.46; 95% CI, 1.01–2.12), and recently injecting drugs (aOR, 2.78; 95% CI, 2.33–3.32) were associated with higher odds of detectable HCV RNA (Table 2).
Factors Associated With Detectable HCV RNA Among People Tested for HCV RNA in the NSW DBS Study, September 2017–December 2020 (n = 5960)
| Variables . | Detectable HCV RNA . | Unadjusted Odds Ratio . | Adjusted Odds Ratio . | |
|---|---|---|---|---|
| No. . | %a . | (95% CI) . | (95% CI) . | |
| Overall | 878 | 15 | … | … |
| Registration type | ||||
| Online | 15 | 5 | 0.23 (0.13–0.39) | 0.38 (0.22–0.65) |
| Assisted (community) | 409 | 17 | 1 | 1 |
| Assisted (prison) | 454 | 14 | 0.77 (0.66–0.89) | 0.94 (0.79–1.10) |
| Test performed | ||||
| HIV + HCV | 811 | 15 | … | … |
| HCV only | 67 | 14 | … | … |
| Gender | ||||
| Men | 664 | 15 | 1 | … |
| Women | 202 | 15 | 0.99 (0.83–1.17) | … |
| Other | 12 | 23 | 1.70 (0.89–3.25) | … |
| Age | ||||
| ≤25 y | 110 | 15 | 1 | 1 |
| 25–34 y | 213 | 12 | 0.80 (0.62–1.02) | 0.75 (0.58–0.97) |
| 35–44 y | 284 | 16 | 1.12 (0.88–1.42) | 0.96 (0.75–1.24) |
| 45–54 y | 220 | 19 | 1.36 (1.06–1.74) | 1.13 (0.87–1.48) |
| >55 y | 51 | 10 | 0.62 (0.44–0.89) | 0.62 (0.43–0.90) |
| Aboriginal and/or Torres Strait Islander | ||||
| No | 529 | 14 | 1 | 1 |
| Yes | 349 | 17 | 1.25 (1.08–1.45) | 1.20 (1.03–1.40) |
| Major city postcode (n = 424, excluding prisons) | ||||
| Yes | 322 | 15 | 1 | … |
| No | 102 | 17 | 1.16 (0.91–1.48) | … |
| Men who have sex with men | ||||
| No | 844 | 15 | 2.12 (1.48–3.03) | … |
| Yes | 34 | 8 | 1 | … |
| Born outside of Australia | ||||
| No | 813 | 16 | 1 | … |
| Yes, Asia or Africa | 16 | 5 | 0.25 (0.15–0.42) | … |
| Yes, other | 49 | 9 | 0.54 (0.40–0.73) | … |
| Speaks English at home | ||||
| No | 34 | 8 | 1 | 1 |
| Yes | 844 | 15 | 2.15 (1.50–3.07) | 1.46 (1.01–2.12) |
| Recently injected drugs | ||||
| No | 188 | 8 | 1 | 1 |
| Yes | 674 | 20 | 3.09 (2.60–3.66) | 2.78 (2.33–3.32) |
| Prefer not to say | 16 | 7 | 0.92 (0.54–1.56) | 0.90 (0.53–1.53) |
| Variables . | Detectable HCV RNA . | Unadjusted Odds Ratio . | Adjusted Odds Ratio . | |
|---|---|---|---|---|
| No. . | %a . | (95% CI) . | (95% CI) . | |
| Overall | 878 | 15 | … | … |
| Registration type | ||||
| Online | 15 | 5 | 0.23 (0.13–0.39) | 0.38 (0.22–0.65) |
| Assisted (community) | 409 | 17 | 1 | 1 |
| Assisted (prison) | 454 | 14 | 0.77 (0.66–0.89) | 0.94 (0.79–1.10) |
| Test performed | ||||
| HIV + HCV | 811 | 15 | … | … |
| HCV only | 67 | 14 | … | … |
| Gender | ||||
| Men | 664 | 15 | 1 | … |
| Women | 202 | 15 | 0.99 (0.83–1.17) | … |
| Other | 12 | 23 | 1.70 (0.89–3.25) | … |
| Age | ||||
| ≤25 y | 110 | 15 | 1 | 1 |
| 25–34 y | 213 | 12 | 0.80 (0.62–1.02) | 0.75 (0.58–0.97) |
| 35–44 y | 284 | 16 | 1.12 (0.88–1.42) | 0.96 (0.75–1.24) |
| 45–54 y | 220 | 19 | 1.36 (1.06–1.74) | 1.13 (0.87–1.48) |
| >55 y | 51 | 10 | 0.62 (0.44–0.89) | 0.62 (0.43–0.90) |
| Aboriginal and/or Torres Strait Islander | ||||
| No | 529 | 14 | 1 | 1 |
| Yes | 349 | 17 | 1.25 (1.08–1.45) | 1.20 (1.03–1.40) |
| Major city postcode (n = 424, excluding prisons) | ||||
| Yes | 322 | 15 | 1 | … |
| No | 102 | 17 | 1.16 (0.91–1.48) | … |
| Men who have sex with men | ||||
| No | 844 | 15 | 2.12 (1.48–3.03) | … |
| Yes | 34 | 8 | 1 | … |
| Born outside of Australia | ||||
| No | 813 | 16 | 1 | … |
| Yes, Asia or Africa | 16 | 5 | 0.25 (0.15–0.42) | … |
| Yes, other | 49 | 9 | 0.54 (0.40–0.73) | … |
| Speaks English at home | ||||
| No | 34 | 8 | 1 | 1 |
| Yes | 844 | 15 | 2.15 (1.50–3.07) | 1.46 (1.01–2.12) |
| Recently injected drugs | ||||
| No | 188 | 8 | 1 | 1 |
| Yes | 674 | 20 | 3.09 (2.60–3.66) | 2.78 (2.33–3.32) |
| Prefer not to say | 16 | 7 | 0.92 (0.54–1.56) | 0.90 (0.53–1.53) |
aProportion numerator in first column, denominator in Table 1.
Factors Associated With Detectable HCV RNA Among People Tested for HCV RNA in the NSW DBS Study, September 2017–December 2020 (n = 5960)
| Variables . | Detectable HCV RNA . | Unadjusted Odds Ratio . | Adjusted Odds Ratio . | |
|---|---|---|---|---|
| No. . | %a . | (95% CI) . | (95% CI) . | |
| Overall | 878 | 15 | … | … |
| Registration type | ||||
| Online | 15 | 5 | 0.23 (0.13–0.39) | 0.38 (0.22–0.65) |
| Assisted (community) | 409 | 17 | 1 | 1 |
| Assisted (prison) | 454 | 14 | 0.77 (0.66–0.89) | 0.94 (0.79–1.10) |
| Test performed | ||||
| HIV + HCV | 811 | 15 | … | … |
| HCV only | 67 | 14 | … | … |
| Gender | ||||
| Men | 664 | 15 | 1 | … |
| Women | 202 | 15 | 0.99 (0.83–1.17) | … |
| Other | 12 | 23 | 1.70 (0.89–3.25) | … |
| Age | ||||
| ≤25 y | 110 | 15 | 1 | 1 |
| 25–34 y | 213 | 12 | 0.80 (0.62–1.02) | 0.75 (0.58–0.97) |
| 35–44 y | 284 | 16 | 1.12 (0.88–1.42) | 0.96 (0.75–1.24) |
| 45–54 y | 220 | 19 | 1.36 (1.06–1.74) | 1.13 (0.87–1.48) |
| >55 y | 51 | 10 | 0.62 (0.44–0.89) | 0.62 (0.43–0.90) |
| Aboriginal and/or Torres Strait Islander | ||||
| No | 529 | 14 | 1 | 1 |
| Yes | 349 | 17 | 1.25 (1.08–1.45) | 1.20 (1.03–1.40) |
| Major city postcode (n = 424, excluding prisons) | ||||
| Yes | 322 | 15 | 1 | … |
| No | 102 | 17 | 1.16 (0.91–1.48) | … |
| Men who have sex with men | ||||
| No | 844 | 15 | 2.12 (1.48–3.03) | … |
| Yes | 34 | 8 | 1 | … |
| Born outside of Australia | ||||
| No | 813 | 16 | 1 | … |
| Yes, Asia or Africa | 16 | 5 | 0.25 (0.15–0.42) | … |
| Yes, other | 49 | 9 | 0.54 (0.40–0.73) | … |
| Speaks English at home | ||||
| No | 34 | 8 | 1 | 1 |
| Yes | 844 | 15 | 2.15 (1.50–3.07) | 1.46 (1.01–2.12) |
| Recently injected drugs | ||||
| No | 188 | 8 | 1 | 1 |
| Yes | 674 | 20 | 3.09 (2.60–3.66) | 2.78 (2.33–3.32) |
| Prefer not to say | 16 | 7 | 0.92 (0.54–1.56) | 0.90 (0.53–1.53) |
| Variables . | Detectable HCV RNA . | Unadjusted Odds Ratio . | Adjusted Odds Ratio . | |
|---|---|---|---|---|
| No. . | %a . | (95% CI) . | (95% CI) . | |
| Overall | 878 | 15 | … | … |
| Registration type | ||||
| Online | 15 | 5 | 0.23 (0.13–0.39) | 0.38 (0.22–0.65) |
| Assisted (community) | 409 | 17 | 1 | 1 |
| Assisted (prison) | 454 | 14 | 0.77 (0.66–0.89) | 0.94 (0.79–1.10) |
| Test performed | ||||
| HIV + HCV | 811 | 15 | … | … |
| HCV only | 67 | 14 | … | … |
| Gender | ||||
| Men | 664 | 15 | 1 | … |
| Women | 202 | 15 | 0.99 (0.83–1.17) | … |
| Other | 12 | 23 | 1.70 (0.89–3.25) | … |
| Age | ||||
| ≤25 y | 110 | 15 | 1 | 1 |
| 25–34 y | 213 | 12 | 0.80 (0.62–1.02) | 0.75 (0.58–0.97) |
| 35–44 y | 284 | 16 | 1.12 (0.88–1.42) | 0.96 (0.75–1.24) |
| 45–54 y | 220 | 19 | 1.36 (1.06–1.74) | 1.13 (0.87–1.48) |
| >55 y | 51 | 10 | 0.62 (0.44–0.89) | 0.62 (0.43–0.90) |
| Aboriginal and/or Torres Strait Islander | ||||
| No | 529 | 14 | 1 | 1 |
| Yes | 349 | 17 | 1.25 (1.08–1.45) | 1.20 (1.03–1.40) |
| Major city postcode (n = 424, excluding prisons) | ||||
| Yes | 322 | 15 | 1 | … |
| No | 102 | 17 | 1.16 (0.91–1.48) | … |
| Men who have sex with men | ||||
| No | 844 | 15 | 2.12 (1.48–3.03) | … |
| Yes | 34 | 8 | 1 | … |
| Born outside of Australia | ||||
| No | 813 | 16 | 1 | … |
| Yes, Asia or Africa | 16 | 5 | 0.25 (0.15–0.42) | … |
| Yes, other | 49 | 9 | 0.54 (0.40–0.73) | … |
| Speaks English at home | ||||
| No | 34 | 8 | 1 | 1 |
| Yes | 844 | 15 | 2.15 (1.50–3.07) | 1.46 (1.01–2.12) |
| Recently injected drugs | ||||
| No | 188 | 8 | 1 | 1 |
| Yes | 674 | 20 | 3.09 (2.60–3.66) | 2.78 (2.33–3.32) |
| Prefer not to say | 16 | 7 | 0.92 (0.54–1.56) | 0.90 (0.53–1.53) |
aProportion numerator in first column, denominator in Table 1.
Among people with detectable HCV RNA (n = 878), 61% (536/878) had a confirmatory laboratory HCV RNA test performed within 6 months of DBS testing (Figure 1). Of those with detectable HCV RNA, 89% (n = 784) had detectable and quantifiable HCV RNA and 11% (n = 94) had detectable but not quantifiable HCV RNA. Among those with detectable and quantifiable HCV RNA (n = 784), 61% (n = 476) had a confirmatory HCV RNA test and 95% (n = 452) had confirmed detectable HCV RNA. Among those with detectable and not quantifiable HCV RNA (n = 94), 64% (n = 60) had a confirmatory HCV RNA test and 55% (n = 33) had confirmed detectable HCV RNA.
Treatment Initiation
Among those with a detectable HCV RNA DBS result, 44% (386/878) initiated treatment within 6 months of testing. In the online self-registration pathway, 13% (2/15) initiated treatment within 6 months. Due to the low number of people diagnosed via online self-registration, this pathway was excluded from the treatment initiation analysis, leaving 863 people with detectable HCV RNA, of whom 44% (n = 384) initiated treatment within 6 months.
Treatment initiation was higher in assisted registration in prison compared with the community (61% vs 26%; P < .001), men compared with women (48% vs 32%; P < .001), and people aged <25 compared with >55 (62% vs 20%; P < .001) (Table 3). In the adjusted logistic regression model, treatment initiation was higher with assisted registration in prison compared with the community (aOR, 4.28; 95% CI, 3.04–6.03) and lower among women compared with men (aOR, 0.68; 95% CI, 0.47–0.97).
Factors Associated With Treatment Uptake Among People Tested for HCV RNA in Prison and Community in the NSW DBS Study, September 2017–December 2020 (n = 863)
| Variables . | Detectable HCV RNA, No. . | Initiated Treatment Within 6 Months . | Unadjusted Odds Ratio (95% CI) . | Adjusted Odds Ratio (95% CI) . | |
|---|---|---|---|---|---|
| No. . | % . | ||||
| Overall | 863 | 384 | 44 | … | … |
| Registration type | |||||
| Assisted (community) | 409 | 107 | 26 | 1 | 1 |
| Assisted (prison) | 454 | 277 | 61 | 4.42 (3.30–5.90) | 4.28 (3.04–6.03) |
| Gender | |||||
| Men | 652 | 316 | 48 | 1 | 1 |
| Women | 199 | 64 | 32 | 0.50 (0.36–0.70) | 0.68 (0.47–0.97) |
| Other | 12 | 4 | 33 | 0.53 (0.16–1.78) | 0.92 (0.27–3.19) |
| Age | |||||
| ≤25 y | 110 | 68 | 62 | 1 | 1 |
| 25–34 y | 211 | 106 | 50 | 0.62 (0.39–1.00) | 0.67 (0.41–1.09) |
| 35–44 y | 277 | 114 | 41 | 0.43 (0.27–0.68) | 0.78 (0.48–1.27) |
| 45–54 y | 215 | 86 | 40 | 0.41 (0.26–0.66) | 1.02 (0.60–1.74) |
| >55 y | 50 | 10 | 20 | 0.15 (0.07–0.34) | 0.43 (0.18–1.01) |
| Aboriginal and/or Torres Strait Islander | |||||
| No | 519 | 220 | 42 | 1 | … |
| Yes | 344 | 164 | 48 | 1.24 (0.94–1.63) | … |
| Born outside of Australia | |||||
| No | 798 | 356 | 45 | 1 | … |
| Yes, Asia or Africa | 16 | 7 | 44 | 0.97 (0.36–2.62) | … |
| Yes, other | 49 | 21 | 43 | 0.93 (0.52–1.67) | … |
| Speaks English at home | |||||
| No | 34 | 18 | 53 | 1 | … |
| Yes | 829 | 366 | 44 | 0.70 (0.35–1.40) | … |
| Recently injected drugs | |||||
| No | 185 | 84 | 45 | 1 | … |
| Yes | 662 | 288 | 44 | 0.93 (0.67–1.28) | … |
| Prefer not to say | 16 | 12 | 75 | 3.61 (1.12–11.60) | … |
| Variables . | Detectable HCV RNA, No. . | Initiated Treatment Within 6 Months . | Unadjusted Odds Ratio (95% CI) . | Adjusted Odds Ratio (95% CI) . | |
|---|---|---|---|---|---|
| No. . | % . | ||||
| Overall | 863 | 384 | 44 | … | … |
| Registration type | |||||
| Assisted (community) | 409 | 107 | 26 | 1 | 1 |
| Assisted (prison) | 454 | 277 | 61 | 4.42 (3.30–5.90) | 4.28 (3.04–6.03) |
| Gender | |||||
| Men | 652 | 316 | 48 | 1 | 1 |
| Women | 199 | 64 | 32 | 0.50 (0.36–0.70) | 0.68 (0.47–0.97) |
| Other | 12 | 4 | 33 | 0.53 (0.16–1.78) | 0.92 (0.27–3.19) |
| Age | |||||
| ≤25 y | 110 | 68 | 62 | 1 | 1 |
| 25–34 y | 211 | 106 | 50 | 0.62 (0.39–1.00) | 0.67 (0.41–1.09) |
| 35–44 y | 277 | 114 | 41 | 0.43 (0.27–0.68) | 0.78 (0.48–1.27) |
| 45–54 y | 215 | 86 | 40 | 0.41 (0.26–0.66) | 1.02 (0.60–1.74) |
| >55 y | 50 | 10 | 20 | 0.15 (0.07–0.34) | 0.43 (0.18–1.01) |
| Aboriginal and/or Torres Strait Islander | |||||
| No | 519 | 220 | 42 | 1 | … |
| Yes | 344 | 164 | 48 | 1.24 (0.94–1.63) | … |
| Born outside of Australia | |||||
| No | 798 | 356 | 45 | 1 | … |
| Yes, Asia or Africa | 16 | 7 | 44 | 0.97 (0.36–2.62) | … |
| Yes, other | 49 | 21 | 43 | 0.93 (0.52–1.67) | … |
| Speaks English at home | |||||
| No | 34 | 18 | 53 | 1 | … |
| Yes | 829 | 366 | 44 | 0.70 (0.35–1.40) | … |
| Recently injected drugs | |||||
| No | 185 | 84 | 45 | 1 | … |
| Yes | 662 | 288 | 44 | 0.93 (0.67–1.28) | … |
| Prefer not to say | 16 | 12 | 75 | 3.61 (1.12–11.60) | … |
Abbreviation: HCV, hepatitis C virus.
Factors Associated With Treatment Uptake Among People Tested for HCV RNA in Prison and Community in the NSW DBS Study, September 2017–December 2020 (n = 863)
| Variables . | Detectable HCV RNA, No. . | Initiated Treatment Within 6 Months . | Unadjusted Odds Ratio (95% CI) . | Adjusted Odds Ratio (95% CI) . | |
|---|---|---|---|---|---|
| No. . | % . | ||||
| Overall | 863 | 384 | 44 | … | … |
| Registration type | |||||
| Assisted (community) | 409 | 107 | 26 | 1 | 1 |
| Assisted (prison) | 454 | 277 | 61 | 4.42 (3.30–5.90) | 4.28 (3.04–6.03) |
| Gender | |||||
| Men | 652 | 316 | 48 | 1 | 1 |
| Women | 199 | 64 | 32 | 0.50 (0.36–0.70) | 0.68 (0.47–0.97) |
| Other | 12 | 4 | 33 | 0.53 (0.16–1.78) | 0.92 (0.27–3.19) |
| Age | |||||
| ≤25 y | 110 | 68 | 62 | 1 | 1 |
| 25–34 y | 211 | 106 | 50 | 0.62 (0.39–1.00) | 0.67 (0.41–1.09) |
| 35–44 y | 277 | 114 | 41 | 0.43 (0.27–0.68) | 0.78 (0.48–1.27) |
| 45–54 y | 215 | 86 | 40 | 0.41 (0.26–0.66) | 1.02 (0.60–1.74) |
| >55 y | 50 | 10 | 20 | 0.15 (0.07–0.34) | 0.43 (0.18–1.01) |
| Aboriginal and/or Torres Strait Islander | |||||
| No | 519 | 220 | 42 | 1 | … |
| Yes | 344 | 164 | 48 | 1.24 (0.94–1.63) | … |
| Born outside of Australia | |||||
| No | 798 | 356 | 45 | 1 | … |
| Yes, Asia or Africa | 16 | 7 | 44 | 0.97 (0.36–2.62) | … |
| Yes, other | 49 | 21 | 43 | 0.93 (0.52–1.67) | … |
| Speaks English at home | |||||
| No | 34 | 18 | 53 | 1 | … |
| Yes | 829 | 366 | 44 | 0.70 (0.35–1.40) | … |
| Recently injected drugs | |||||
| No | 185 | 84 | 45 | 1 | … |
| Yes | 662 | 288 | 44 | 0.93 (0.67–1.28) | … |
| Prefer not to say | 16 | 12 | 75 | 3.61 (1.12–11.60) | … |
| Variables . | Detectable HCV RNA, No. . | Initiated Treatment Within 6 Months . | Unadjusted Odds Ratio (95% CI) . | Adjusted Odds Ratio (95% CI) . | |
|---|---|---|---|---|---|
| No. . | % . | ||||
| Overall | 863 | 384 | 44 | … | … |
| Registration type | |||||
| Assisted (community) | 409 | 107 | 26 | 1 | 1 |
| Assisted (prison) | 454 | 277 | 61 | 4.42 (3.30–5.90) | 4.28 (3.04–6.03) |
| Gender | |||||
| Men | 652 | 316 | 48 | 1 | 1 |
| Women | 199 | 64 | 32 | 0.50 (0.36–0.70) | 0.68 (0.47–0.97) |
| Other | 12 | 4 | 33 | 0.53 (0.16–1.78) | 0.92 (0.27–3.19) |
| Age | |||||
| ≤25 y | 110 | 68 | 62 | 1 | 1 |
| 25–34 y | 211 | 106 | 50 | 0.62 (0.39–1.00) | 0.67 (0.41–1.09) |
| 35–44 y | 277 | 114 | 41 | 0.43 (0.27–0.68) | 0.78 (0.48–1.27) |
| 45–54 y | 215 | 86 | 40 | 0.41 (0.26–0.66) | 1.02 (0.60–1.74) |
| >55 y | 50 | 10 | 20 | 0.15 (0.07–0.34) | 0.43 (0.18–1.01) |
| Aboriginal and/or Torres Strait Islander | |||||
| No | 519 | 220 | 42 | 1 | … |
| Yes | 344 | 164 | 48 | 1.24 (0.94–1.63) | … |
| Born outside of Australia | |||||
| No | 798 | 356 | 45 | 1 | … |
| Yes, Asia or Africa | 16 | 7 | 44 | 0.97 (0.36–2.62) | … |
| Yes, other | 49 | 21 | 43 | 0.93 (0.52–1.67) | … |
| Speaks English at home | |||||
| No | 34 | 18 | 53 | 1 | … |
| Yes | 829 | 366 | 44 | 0.70 (0.35–1.40) | … |
| Recently injected drugs | |||||
| No | 185 | 84 | 45 | 1 | … |
| Yes | 662 | 288 | 44 | 0.93 (0.67–1.28) | … |
| Prefer not to say | 16 | 12 | 75 | 3.61 (1.12–11.60) | … |
Abbreviation: HCV, hepatitis C virus.
DISCUSSION
This study evaluated HCV testing and treatment following the implementation of a large state-wide program to scale up DBS testing. Higher HCV treatment uptake following DBS testing was observed in prison compared with assisted collection in community and online self-registration. These data demonstrate the utility of DBS to improve the reach of HCV testing outside of traditional health care settings, while emphasizing the need for improved pathways to care in community sites. The World Health Organization recommends DBS for sampling outside of health care settings, so the findings of this study are important to guide clinical practice and policy (eg, national plans) for implementing HCV testing programs and advance progress toward HCV elimination.
Detectable HCV RNA was 15% overall, similar to national studies in needle syringe programs (16% in 2021) [19] and drug treatment clinics (17% in 2019–2021) in Australia [4]. Among people who had recently injected drugs, 20% had detectable HCV RNA, likely reflecting the data being collected across 2017–2020 and consistent with data from older studies of people who inject drugs [4, 19]. The proportion of MSM with detectable HCV RNA was 8%, higher than a meta-analysis from 2000 to 2019, which estimated pooled HCV prevalence in men who have sex with men in Australia at 2.8% [20]. HIV prevalence among MSM in the NSW DBS Pilot was relatively low (0.6%), so the higher HCV prevalence is likely due to the high proportion of recent injecting (54%) among MSM who tested for HCV in the study. The criteria for HCV testing in the NSW DBS Pilot (history of injecting drug use, being Aboriginal or Torres Strait Islander, or ever being incarcerated) are important to target testing to people most at risk of having current HCV infection.
Varied HCV treatment uptake in this study emphasizes the need to improve linkage to care and treatment initiation, particularly in the community. In the community, 26% initiated treatment at 6 months postenrollment, which was comparable to 27% in standard of care among people with recent drug dependence in New South Wales [21]. Studies that offered point-of-care HCV RNA testing but required confirmatory testing before treatment initiation produced similar treatment uptake (23%–49%) among people who inject drugs [22, 23]. In Australia, the regulatory requirement to have a confirmatory test via venepuncture following a DBS result of detectable HCV RNA increases the potential for loss to follow-up. Given the limits of the current regulations, there are alternatives to DBS sampling. Microvette collection tubes (many of which already have regulatory approval for sample collection) can be used to collect capillary whole blood by fingerstick, which can then be transported to central laboratories for serological (HCV antibody) and molecular (HCV RNA) tests. One study in Myanmar has demonstrated the feasibility of this strategy using Xpert HCV Viral Load Fingerstick testing [24], and a similar strategy is being implemented in the United Kingdom [25].
The highest treatment uptake was observed in prison (61%). Treatment uptake was higher than comparable studies (21% in an English study using DBS in prison [26] and >26% in an Australian study using DBS in prison [27]). In the current study, DBS sample collection was often performed in high-intensity testing campaigns, collecting samples from large numbers of people in recreational areas instead of bringing people individually to a clinical space. This model of testing requires reduced staff time compared with standard of care (which required people to be taken individually to a clinical space for venepuncture), and treatment initiation was facilitated by streamlined care pathways. Gaining regulatory approval to use DBS for HCV diagnosis would remove the requirement for confirmatory testing and could further increase treatment initiation in high-intensity testing campaign models in prison by reducing the number of visits and the time between testing and diagnosis.
Treatment uptake was lower among women in the NSW DBS Pilot, consistent with a population-based study of DAA treatment uptake among women in the same state in Australia [28]. In the current study, the higher proportion of men in the prison pathway and higher treatment uptake in the prison pathway resulted in higher treatment uptake among men overall. With women being more likely to test in the community, it is important that pathways to care are strengthened across all settings. Incorporating a gender lens into the design of HCV interventions can help avoid the entrenchment of gender inequities [29] and ensure that women are supported to access HCV treatment no matter where they are tested.
This study has several limitations. Changes to the survey during the study period mean that at different time points, recent injecting was reported as in the last 12 months or in the last month. A recent study of people who inject drugs in Australia reported that 75% of people injecting in the last year had injected in the last month [4], which indicates that these groups may be similar. Processes for recruitment were not standardized across treatment pathways or across sites in the same pathway; for example, some community sites offered financial incentives to participate, but the timing and size of incentives were not reported to the study. Processes to facilitate linkage to care were not standardized across sites. This may have led to differences in testing and treatment uptake across different sites. Some sites did not have on-site venepuncture and had to refer patients off-site for confirmatory testing and treatment, possibly impacting HCV treatment uptake. Given the sensitivity and specificity of DBS testing, it is possible that some people with low HCV RNA levels that were not detectable with DBS testing would have been detectable with confirmatory testing. People who participated via the online self-registration pathway were contacted to self-report treatment initiation, which may have impacted reporting. For people diagnosed with HCV in prison who initiated treatment in the community, treatment uptake was not reported.
This study has important implications for the development of local, national, and international testing strategies. DBS testing is an important testing modality to be offered as part of a suite of testing options, especially in resource-limited settings such as prison and outreach. In prisons, DBS can be used for high-intensity testing campaigns where samples are collected from large numbers of people on the wings, without requiring individuals to attend a clinical space and reducing the burden on staff to accompany individuals to testing. Other studies have identified interventions that improve DAA treatment uptake, for example, low-threshold care (flexible appointment scheduling and a supportive harm reduction framework) being provided in needle syringe programs [30] and assignment of a care coordinator [31]. Qualitative research is needed to better understand patient and provider barriers and facilitators for DBS testing in the community to simplify pathways to care, develop effective implementation strategies to support providers, and improve treatment uptake. This study will support an ongoing parallel study to inform an economic analysis to estimate the HCV prevalence at which each alternative testing strategy (antibody testing, DBS RNA testing, RNA point-of-care testing) is most cost-effective. The aforementioned sample collection method in microvette tubes is an alternative to DBS that could be implemented with a similar model and could be integrated into clinical care in Australia's current regulatory environment. Although not feasible for all settings, on-site point-of-care HCV RNA testing is a promising intervention that is being scaled up in Australia [32]. Studies have found high treatment uptake in needle syringe programs [33] and prison [27] following on-site point-of-care HCV RNA testing compared with standard of care [34]. Further investigation is needed to understand when and where each strategy is appropriate.
Overall, 15% of people tested in this study had detectable HCV RNA, and 26% initiated treatment, with higher treatment uptake in prison (61%). Our findings demonstrate that DBS could be an important strategy to improve the reach of HCV testing and improve treatment uptake in resource-limited settings such as prison. This study informs the development of large-scale DBS programs globally, providing one strategy to advance progress to HCV elimination by 2030. Further work is needed to compare the cost-effectiveness of different HCV testing modalities to inform practice and policy. DBS is an important option as part of a range of testing modalities to strengthen local, national, and international HCV elimination strategies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We would like to thank all the staff at sites that participated in the delivery of the study, particularly the principal investigators: Alexandra Wade (Lismore Liver Clinic), Alison Nikitas (Murrumbidgee HARP Unit, Southern NSW LHD HARP Unit), Annie Malcolm (Drug Health Services), Dr. Catriona Ooi (The Beaches Clinic), Dr. Craig Rodgers (Rankin Court Treatment Centre), Dr. David Smith (Lismore Sexual Health Service), Dr. Eva Jackson (NBM south Court Primary Care [NSP]), Dr. Katherine Brown (ISHDACS), Dr. Mark Montebello (NSLHD Drug and Alcohol Service), Dr. Phillip Read (Kirketon Road Centre), Dr. Tony Gill (Southern NSW LHD Drug and Alcohol Service), Dr. Winston Kardell (NBM DACS/OTP), Gary Keogh (Drug Health Services), Jo Lenton (Far Western Primary Health Care), Karen Gilham (HNE DACS [Northern]), Sarah Smith (iCHAT community hepatitis service), Carolyn Stubley (WHOS [We Help Ourselves]), Dr. James Blogg (Sydney Drug Health Service), Dr. Miriam Levy (South Western Sydney Gastro and Liver Service), Dr. Thao Lam (Western Sydney Drug Health Service), Karl Johnson (ACON Health Ltd), Kim Grant (Western NSW HARP), Marian Bloomfield CNC (Justice Health & Forensic Mental Health Network), Phinn Borg (The Gender Centre), Sandhya Goundar (Liverpool Sexual Health), Shih Chi Kao (Sydney LHD HARP Unit), and Sinead Sheils (Gastro and Liver [AW Morrow]). Finally, we wish to thank everyone who agreed to participate in the pilot.
Funding. The work was supported by the NSW Ministry of Health. Staff at the NSW Ministry of Health had an active role in the design, conduct, and analysis of the study, interpretation of the data, and writing of the report. Some staff members were included as study co-authors (A.S., C.M., B.P., C.P., N.C.). J.G. receives the (Australian) National Health and Medical Research Council Investigator Grant (1176131). The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government.
References
Author notes
Equal contribution.
Potential conflicts of interest. J.G. is a consultant/advisor for and has received research grants from AbbVie, Abbott, bioLytical, Camurus, Cepheid, Gilead Sciences, Hologic, Indivior, and Roche. P.R. has received research funding and honoraria for talks from Gilead Sciences. All other authors report no potential conflicts.





Comments