-
PDF
- Split View
-
Views
-
Cite
Cite
Jacob Perkins, Tyler Re, Sherry Ong, Zhongzheng Niu, Xiaozhong Wen, Meta-Analysis on Associations of Timing of Maternal Smoking Cessation Before and During Pregnancy With Childhood Overweight and Obesity, Nicotine & Tobacco Research, Volume 25, Issue 4, April 2023, Pages 605–615, https://doi.org/10.1093/ntr/ntac213
Close - Share Icon Share
Abstract
There is a lack of comprehensive review on associations of maternal smoking cessation (versus nonsmokers) with childhood overweight and obesity.
We conducted a systematic review and meta-analysis of existing evidence in this field. Within PubMed, EMBASE, and CENTRAL databases, we identified and screened 1147 abstracts. We reviewed full-texts and extracted related information from 10 eligible articles. We pooled odds ratios for overweight/obesity and mean differences in BMI z-scores by maternal smoking status around pregnancy.
Among 10 eligible studies, 71 393 children were included from ages 2 to 18 years. Compared to children of nonsmokers, the pooled unadjusted odds ratio (OR) for overweight was 1.36 (95% Confidence Interval CI: 1.14, 1.62) in children of quitters and 1.44 (1.27, 1.64) in children of continued smokers. The pooled unadjusted OR for obesity was 1.65 (1.17, 2.32) in children of quitters and 1.94 (1.38, 2.73) in children of continued smokers. The pooled unadjusted mean difference in BMI z-score was 0.51 (0.41, 0.61) in children of quitters and 0.64 (0.58, 0.70) in children of continued smokers. The pooled unadjusted OR for overweight in children of mothers quitting before pregnancy was 1.46 (1.15, 1.85), during the first trimester was 1.52 (1.27, 1.82), and during pregnancy (mixed timing, mostly first trimester) was 0.97 (0.79, 1.20).
The risk of offspring overweight and obesity was moderately higher for quitters during pregnancy compared to nonsmokers, although it might not be as high as continued smokers.
Maternal smoking during pregnancy is an established risk factor of childhood overweight and obesity. Based on our systematic review, intervention to help mothers quit smoking has the potential to reduce the risk of childhood overweight and obesity in offspring related to prenatal tobacco exposure. Quitting before pregnancy is ideal, but quitting in early pregnancy is still helpful for reducing risk.
Introduction
Obesity has been shown to be associated with one of the leading causes of death, cardiovascular disease.1 In the United States, the rate of adult and childhood obesity has progressed over time. As of 2015, in less than two decades, the prevalence of childhood obesity has increased from 13.9% to 18.5%.2 Oftentimes, overweight or obesity are associated with lack of physical activity and poor dietary choices during childhood, as well as genetic factors.3 However, maternal behaviors and lifestyles, such as smoking, are also linked to childhood overweight/obesity.4
About 23% of US women reported smoking before pregnancy.5 While maternal smoking during pregnancy is associated with fetal growth restrictions and low birth weight,6,7 it can also result in rapid infant weight gain and childhood overweight/obesity.8 Two meta-analyses have summarized the association between maternal smoking during pregnancy and childhood overweight/obesity.9,10 For example, Rayfield et al. concluded that children of smokers are at a higher risk of childhood overweight (odds ratio [OR], 1.37 (95% Confidence Interval [CI]: 1.28, 0.97) and obesity (OR, 1.55 [1.40, 1.73]) compared to nonsmokers. Although the biological mechanisms are unclear, fetal exposure to nicotine in tobacco is linked to a decrease in adipose metabolism and appetite dysregulation through the sympathetic nervous system, which are likely to carry on into later life.11,12
Pregnancy can provide an opportunity for women to quit smoking and 54% of US women who smoke before pregnancy quit during pregnancy.13 Compared to maternal smoking, less research has been done on the potential influences of maternal smoking cessation on childhood overweight/obesity. In addition, there is a substantial inconsistency in direction, magnitude, and statistical significance for this association across existing studies. For instance, the OR for overweight among children of quitters was 0.95 (0.76, 1.19) in one study,14 but 2.17 (1.15, 1.68) in another study.4 To the best of our knowledge, there was only one meta-analysis focusing on how smoking cessation can also impact childhood growth.15 This individual participant data meta-analysis reported increased risk of childhood overweight (OR 1.17 [1.02, 1.35]) in mothers who quit smoking in the first trimester compared to nonsmoking mothers. But this review was limited by only including the regions of North America and Europe, despite that multiple other studies were conducted in other regions where the prevalence of smoking and childhood obesity was different. Secondly, this review only used the outcome of overweight without considering other important outcomes such as obesity and BMI z-score. Thirdly, the only timing of quitting that was studied was the first trimester, which could not address the potentially differential effects of quitting at different periods around pregnancy such as before pregnancy and after the first trimester of pregnancy.
The timing of maternal smoking cessation also plays a role in reducing smoking-related harms. For example, the risk of low birth weight was the highest among continued smokers (OR, 2.46 [2.28, 2.67]), followed by second trimester cessation (OR 2.00 [1.60, 2.67]) and by first trimester cessation (OR 1.26 [1.05, 1.52]), compared to nonsmokers.16 However, whether and how the timing of maternal smoking cessation during pregnancy may specifically affect the risk of childhood overweight/obesity or general child growth are not well understood. Our own research has shown that a late timing of maternal smoking cessation can result in rapid body mass index (BMI) gain from birth to 12 months of age: <0.47 if smoking cessation occurred between 15 and 27 weeks of pregnancy, while 0.65 to 3.16 if smoking cessation occurred between 28 weeks to 36 weeks.17 These data suggests that timing of smoking cessation during pregnancy may also play a role in the development of childhood overweight/obesity, since rapid infant weight gain is an established risk factor for childhood obesity.18 In addition, other potential health problems may arise from infant accelerated growth such as cardiovascular disease and diabetes.19 This further places emphasis on the increased risk among children of women who quit later during pregnancy compared to those who quit earlier during pregnancy.
The objective of this study is to perform a systematic review and meta-analysis to 1) summarize the extent to which maternal smoking cessation (versus nonsmokers) was associated with childhood overweight/obesity (primary aim), and 2) explore the potential influence of timing of cessation, i.e., before pregnancy and trimesters of pregnancy (secondary aim).
Methods
Guidelines
We registered this review with PROSPERO International prospective register of systematic reviews (ID: CRD42018115932). We used the systemic review guidelines of PRISMA as recommended in the Prospero resources. This included following the PRISMA20 checklist and flow diagram for systemic reviews (Supplementary Figure 1).
Search Strategy
A systematic review of studies was conducted through the following databases: PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). The search goal was to find all previous publications on the association between maternal smoking cessation before or during pregnancy and childhood overweight/obesity. The key search terms included “pregnancy” (pregnancy, maternal, mother, pregnant, perinatal, prenatal, and prenatal exposure delayed effects), “obesity/overweight” (obesity, overweight, body mass index, weight, height, percentile, fatness, adiposity, and ponderal), “offspring” (childhood, offspring, infant, children, adolescent, and teen), and “smoking cessation” (smoking cessation, cease smoking, quit smoking, stop smoking, continued smoking, and smoked throughout). We searched all articles published on or before December 20, 2020 without language restrictions. A total of 1554 articles were initially identified through database searching. We used a de-duplication method within the Endnote software to remove duplicate articles found in different databases,21 resulting in a total of 1147 abstracts. Then, each abstract was reviewed by two authors independently and any disagreements were further evaluated by a third reviewer. Article eligibility was determined based on the title, abstract, and if necessary, full text (Supplementary Figure 1).
Search Criteria
Each full text article had to fit specific criteria for the following categories: (1) participation must include children from the age ranging from 0 to 18 years old, (2) mothers who quit before or during pregnancy, (3) a control group as mothers who did not smoke during pregnancy (nonsmokers), and (4) the weight status outcome measurements (eg, overweight, obesity, or BMI/BMI z-score).
Data Extraction and Validation
Authors (JP, TR, and SO) evaluated full text articles individually and extracted the following information: study name/design, study population, timing of quitting (before pregnancy, first trimester, and quitting during pregnancy), birth year, child age and sex, number of participants by smoking status (nonsmoking, continued smoking, and quitting smoking), number of overweight/obese children, the outcome measurements, associations (eg, mean difference, unadjusted and adjusted OR or relative risk), and confounders adjusted for (Table 1, Supplementary Table 1).
| Author . | Study population . | N . | Timing of quitting smoking . | Controlled factors . | Birth year . | Child age (years) . | Outcomes measurements . |
|---|---|---|---|---|---|---|---|
| Durmas et al.29 | Generation R Study | 5 342 | First trimester | Age, sex, parental ethnicity and education, parental height and weight, and breastfeeding. | 2002–2006 | 4 | BMI: overweight 1.1–2.3 SD and obesity >2.3 SD for age and sex* |
| Durmas et al.30 | Generation R Study | 5 243 | First trimester | Age at visit, sex and height, maternal covariates, and infant covariates. | 2002–2006 | 6 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
| <5 cigs/day | 593 | ||||||
| ≥5 cigs/day | 732 | ||||||
| Boys | 2 610 | ||||||
| Girls | 2 633 | ||||||
| Fasting et al.18 | Prevention of Allergy among Children in Trondheim (PACT) | 624 | Quitting during pregnancy | Maternal/paternal education, age, birth weight, and child sex. | 2000–2002 | 4 | BMI: overweight >17.55 (males), >17.28 (females), obesity >19.29 (males), >19.15 (females)* |
| Grzeskowiak et al.32 | Women’s and Children’s Health Network of South Australia | 7 658 | First trimester | Maternal age, socioeconomic status, race, parity, diabetes, and maternal BMI. | 2000–2005 | 4.8 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex** |
| 1–9 cigs/day | 522 | ||||||
| 10–19 cigs/day | 574 | ||||||
| ≥20 cigs/day | 202 | ||||||
| Harris et al.14 | Nurses Health Study II | 35 178 | Quitting during pregnancy | Maternal, paternal, and nurse characteristics plus self-reported body size during childhood. | 1989 | 18 | BMI: overweight 25-29, obese ≥30** |
| 1–14 cigs/day | 4 893 | ||||||
| 15–24 cigs/day | 2 486 | ||||||
| ≥ 25 cigs/day | 422 | ||||||
| Mendez et al.4 | – | 482 | First trimester | Child’s age, sex, breastfeeding duration, and maternal age, height, overweight or obesity prior to pregnancy, education, parity, and current maternal smoking. | 1997–1998 | 6.7 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex*** |
| Oken et al.26 | Project Viva | 514 | Paternal BMI; maternal pre-pregnancy BMI, gestational weight gain, education, income, race/ethnicity, parity, fetal growth, and gestation length. | 1999–2002 | 3 | BMI: overweight ≥85th percentile for age and sex** | |
| T0 | 161 | Before pregnancy | |||||
| T1 | 71 | First trimester | |||||
| Suzuki et al.31 | Project Koshu | 2 613 | Maternal BMI before pregnancy, maternal age at pregnancy, and BMI of child at birth. | 1991–2006 | 3 | BMI: overweight >17.89 (males), >17.56 (females), obesity >19.57 (males), >19.36 (females)* | |
| Boys—T0 | 60 | Before pregnancy | |||||
| Boys—T1 | 144 | First trimester | |||||
| Girls—T0 | 71 | Before pregnancy | |||||
| Girls—T1 | 168 | First trimester | |||||
| Toschke et al.27 | – | 8 765 | Before pregnancy | Breastfeeding, parental education, low birth weight, and prematurity. | 1997 | 5–7 | BMI: overweight ≥90th and obesity ≥97th percentile for age and sex |
| Toschke et al.28 | – | 4 974 | First trimester | Breastfeeding, educational level, parental obesity, watching televisions, playing electronic games occasionally, physical activity, and high infant weight gain. | 2001–2002 | 5–7 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
| Author . | Study population . | N . | Timing of quitting smoking . | Controlled factors . | Birth year . | Child age (years) . | Outcomes measurements . |
|---|---|---|---|---|---|---|---|
| Durmas et al.29 | Generation R Study | 5 342 | First trimester | Age, sex, parental ethnicity and education, parental height and weight, and breastfeeding. | 2002–2006 | 4 | BMI: overweight 1.1–2.3 SD and obesity >2.3 SD for age and sex* |
| Durmas et al.30 | Generation R Study | 5 243 | First trimester | Age at visit, sex and height, maternal covariates, and infant covariates. | 2002–2006 | 6 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
| <5 cigs/day | 593 | ||||||
| ≥5 cigs/day | 732 | ||||||
| Boys | 2 610 | ||||||
| Girls | 2 633 | ||||||
| Fasting et al.18 | Prevention of Allergy among Children in Trondheim (PACT) | 624 | Quitting during pregnancy | Maternal/paternal education, age, birth weight, and child sex. | 2000–2002 | 4 | BMI: overweight >17.55 (males), >17.28 (females), obesity >19.29 (males), >19.15 (females)* |
| Grzeskowiak et al.32 | Women’s and Children’s Health Network of South Australia | 7 658 | First trimester | Maternal age, socioeconomic status, race, parity, diabetes, and maternal BMI. | 2000–2005 | 4.8 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex** |
| 1–9 cigs/day | 522 | ||||||
| 10–19 cigs/day | 574 | ||||||
| ≥20 cigs/day | 202 | ||||||
| Harris et al.14 | Nurses Health Study II | 35 178 | Quitting during pregnancy | Maternal, paternal, and nurse characteristics plus self-reported body size during childhood. | 1989 | 18 | BMI: overweight 25-29, obese ≥30** |
| 1–14 cigs/day | 4 893 | ||||||
| 15–24 cigs/day | 2 486 | ||||||
| ≥ 25 cigs/day | 422 | ||||||
| Mendez et al.4 | – | 482 | First trimester | Child’s age, sex, breastfeeding duration, and maternal age, height, overweight or obesity prior to pregnancy, education, parity, and current maternal smoking. | 1997–1998 | 6.7 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex*** |
| Oken et al.26 | Project Viva | 514 | Paternal BMI; maternal pre-pregnancy BMI, gestational weight gain, education, income, race/ethnicity, parity, fetal growth, and gestation length. | 1999–2002 | 3 | BMI: overweight ≥85th percentile for age and sex** | |
| T0 | 161 | Before pregnancy | |||||
| T1 | 71 | First trimester | |||||
| Suzuki et al.31 | Project Koshu | 2 613 | Maternal BMI before pregnancy, maternal age at pregnancy, and BMI of child at birth. | 1991–2006 | 3 | BMI: overweight >17.89 (males), >17.56 (females), obesity >19.57 (males), >19.36 (females)* | |
| Boys—T0 | 60 | Before pregnancy | |||||
| Boys—T1 | 144 | First trimester | |||||
| Girls—T0 | 71 | Before pregnancy | |||||
| Girls—T1 | 168 | First trimester | |||||
| Toschke et al.27 | – | 8 765 | Before pregnancy | Breastfeeding, parental education, low birth weight, and prematurity. | 1997 | 5–7 | BMI: overweight ≥90th and obesity ≥97th percentile for age and sex |
| Toschke et al.28 | – | 4 974 | First trimester | Breastfeeding, educational level, parental obesity, watching televisions, playing electronic games occasionally, physical activity, and high infant weight gain. | 2001–2002 | 5–7 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
*International Obesity Task Force by Cole et al. Establish a standard definition for child overweight and obesity worldwide: international survey. BMJ (2000).
**Overweight and obesity percentiles established by the Centers for Disease Control and Prevention.
***Overweight and obesity percentiles established by the World Health Organization.
BMI, body mass index; SD, standard deviation; cigs, cigarettes; T0, before pregnancy; T1, first trimester.
| Author . | Study population . | N . | Timing of quitting smoking . | Controlled factors . | Birth year . | Child age (years) . | Outcomes measurements . |
|---|---|---|---|---|---|---|---|
| Durmas et al.29 | Generation R Study | 5 342 | First trimester | Age, sex, parental ethnicity and education, parental height and weight, and breastfeeding. | 2002–2006 | 4 | BMI: overweight 1.1–2.3 SD and obesity >2.3 SD for age and sex* |
| Durmas et al.30 | Generation R Study | 5 243 | First trimester | Age at visit, sex and height, maternal covariates, and infant covariates. | 2002–2006 | 6 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
| <5 cigs/day | 593 | ||||||
| ≥5 cigs/day | 732 | ||||||
| Boys | 2 610 | ||||||
| Girls | 2 633 | ||||||
| Fasting et al.18 | Prevention of Allergy among Children in Trondheim (PACT) | 624 | Quitting during pregnancy | Maternal/paternal education, age, birth weight, and child sex. | 2000–2002 | 4 | BMI: overweight >17.55 (males), >17.28 (females), obesity >19.29 (males), >19.15 (females)* |
| Grzeskowiak et al.32 | Women’s and Children’s Health Network of South Australia | 7 658 | First trimester | Maternal age, socioeconomic status, race, parity, diabetes, and maternal BMI. | 2000–2005 | 4.8 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex** |
| 1–9 cigs/day | 522 | ||||||
| 10–19 cigs/day | 574 | ||||||
| ≥20 cigs/day | 202 | ||||||
| Harris et al.14 | Nurses Health Study II | 35 178 | Quitting during pregnancy | Maternal, paternal, and nurse characteristics plus self-reported body size during childhood. | 1989 | 18 | BMI: overweight 25-29, obese ≥30** |
| 1–14 cigs/day | 4 893 | ||||||
| 15–24 cigs/day | 2 486 | ||||||
| ≥ 25 cigs/day | 422 | ||||||
| Mendez et al.4 | – | 482 | First trimester | Child’s age, sex, breastfeeding duration, and maternal age, height, overweight or obesity prior to pregnancy, education, parity, and current maternal smoking. | 1997–1998 | 6.7 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex*** |
| Oken et al.26 | Project Viva | 514 | Paternal BMI; maternal pre-pregnancy BMI, gestational weight gain, education, income, race/ethnicity, parity, fetal growth, and gestation length. | 1999–2002 | 3 | BMI: overweight ≥85th percentile for age and sex** | |
| T0 | 161 | Before pregnancy | |||||
| T1 | 71 | First trimester | |||||
| Suzuki et al.31 | Project Koshu | 2 613 | Maternal BMI before pregnancy, maternal age at pregnancy, and BMI of child at birth. | 1991–2006 | 3 | BMI: overweight >17.89 (males), >17.56 (females), obesity >19.57 (males), >19.36 (females)* | |
| Boys—T0 | 60 | Before pregnancy | |||||
| Boys—T1 | 144 | First trimester | |||||
| Girls—T0 | 71 | Before pregnancy | |||||
| Girls—T1 | 168 | First trimester | |||||
| Toschke et al.27 | – | 8 765 | Before pregnancy | Breastfeeding, parental education, low birth weight, and prematurity. | 1997 | 5–7 | BMI: overweight ≥90th and obesity ≥97th percentile for age and sex |
| Toschke et al.28 | – | 4 974 | First trimester | Breastfeeding, educational level, parental obesity, watching televisions, playing electronic games occasionally, physical activity, and high infant weight gain. | 2001–2002 | 5–7 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
| Author . | Study population . | N . | Timing of quitting smoking . | Controlled factors . | Birth year . | Child age (years) . | Outcomes measurements . |
|---|---|---|---|---|---|---|---|
| Durmas et al.29 | Generation R Study | 5 342 | First trimester | Age, sex, parental ethnicity and education, parental height and weight, and breastfeeding. | 2002–2006 | 4 | BMI: overweight 1.1–2.3 SD and obesity >2.3 SD for age and sex* |
| Durmas et al.30 | Generation R Study | 5 243 | First trimester | Age at visit, sex and height, maternal covariates, and infant covariates. | 2002–2006 | 6 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
| <5 cigs/day | 593 | ||||||
| ≥5 cigs/day | 732 | ||||||
| Boys | 2 610 | ||||||
| Girls | 2 633 | ||||||
| Fasting et al.18 | Prevention of Allergy among Children in Trondheim (PACT) | 624 | Quitting during pregnancy | Maternal/paternal education, age, birth weight, and child sex. | 2000–2002 | 4 | BMI: overweight >17.55 (males), >17.28 (females), obesity >19.29 (males), >19.15 (females)* |
| Grzeskowiak et al.32 | Women’s and Children’s Health Network of South Australia | 7 658 | First trimester | Maternal age, socioeconomic status, race, parity, diabetes, and maternal BMI. | 2000–2005 | 4.8 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex** |
| 1–9 cigs/day | 522 | ||||||
| 10–19 cigs/day | 574 | ||||||
| ≥20 cigs/day | 202 | ||||||
| Harris et al.14 | Nurses Health Study II | 35 178 | Quitting during pregnancy | Maternal, paternal, and nurse characteristics plus self-reported body size during childhood. | 1989 | 18 | BMI: overweight 25-29, obese ≥30** |
| 1–14 cigs/day | 4 893 | ||||||
| 15–24 cigs/day | 2 486 | ||||||
| ≥ 25 cigs/day | 422 | ||||||
| Mendez et al.4 | – | 482 | First trimester | Child’s age, sex, breastfeeding duration, and maternal age, height, overweight or obesity prior to pregnancy, education, parity, and current maternal smoking. | 1997–1998 | 6.7 | BMI: overweight ≥85th and obesity ≥95th percentile for age and sex*** |
| Oken et al.26 | Project Viva | 514 | Paternal BMI; maternal pre-pregnancy BMI, gestational weight gain, education, income, race/ethnicity, parity, fetal growth, and gestation length. | 1999–2002 | 3 | BMI: overweight ≥85th percentile for age and sex** | |
| T0 | 161 | Before pregnancy | |||||
| T1 | 71 | First trimester | |||||
| Suzuki et al.31 | Project Koshu | 2 613 | Maternal BMI before pregnancy, maternal age at pregnancy, and BMI of child at birth. | 1991–2006 | 3 | BMI: overweight >17.89 (males), >17.56 (females), obesity >19.57 (males), >19.36 (females)* | |
| Boys—T0 | 60 | Before pregnancy | |||||
| Boys—T1 | 144 | First trimester | |||||
| Girls—T0 | 71 | Before pregnancy | |||||
| Girls—T1 | 168 | First trimester | |||||
| Toschke et al.27 | – | 8 765 | Before pregnancy | Breastfeeding, parental education, low birth weight, and prematurity. | 1997 | 5–7 | BMI: overweight ≥90th and obesity ≥97th percentile for age and sex |
| Toschke et al.28 | – | 4 974 | First trimester | Breastfeeding, educational level, parental obesity, watching televisions, playing electronic games occasionally, physical activity, and high infant weight gain. | 2001–2002 | 5–7 | BMI: overweight >17.55 (males), >17.34 (females), obesity >19.78 (males), >19.65 (females)* |
*International Obesity Task Force by Cole et al. Establish a standard definition for child overweight and obesity worldwide: international survey. BMJ (2000).
**Overweight and obesity percentiles established by the Centers for Disease Control and Prevention.
***Overweight and obesity percentiles established by the World Health Organization.
BMI, body mass index; SD, standard deviation; cigs, cigarettes; T0, before pregnancy; T1, first trimester.
Association data were analyzed for both children of continued smokers and quitters with the reference group being children of nonsmokers. Presentation of the extracted data in the analyzed articles determined how quitter subgroups were defined. Associations were directly extracted when available and otherwise calculated from relevant data reported in the article. As a measure of quality control, we manually calculated ORs when the data on the risk of outcome was available to compare with unadjusted ORs reported in the article. For articles that only reported absolute BMI without BMI z-score, we calculated the BMI z-score by sex and age based on WHO Growth Standards22,23 and then mean difference between groups.
We considered three associations, including ORs of overweight, ORs of obesity, and mean difference in BMI z-score, obtained from unadjusted and adjusted models. The unadjusted model included models labeled in articles as crude/unadjusted or if unavailable then models adjusted for only age and/or sex. We used the most adjusted model in the article if multiple models were present in the study.
Statistical Analysis
We calculated pooled ORs and pooled BMI z-score mean differences with their 95% CIs using random effect models in forest plots (“metan” package in Stata). The weight of studies was determined by the inverse variance of outcome associations. I2 values were used to determine the percent of total variation in outcome associations because of the heterogeneity of the studies instead of chance.24 Our primary analysis was conducted among all studies that included children of quitters with the reference group being children of mothers who did not smoke during pregnancy. Our secondary analysis was conducted among the subset of studies that included both children of continued smokers and children of quitters (“matched quitters”) with the reference group being children of nonsmokers. Funnel plots were created to determine if there was publication bias. Analyses were performed using Stata 12.0 software (StataCorp, College Station, Texas).
Subgroup Analysis
We conducted two subgroup analyses based on timing of quitting and the definition of overweight. The subgroups by timing of quitting included quitting before pregnancy, in the first trimester, and quitting during pregnancy (mixed timing, mostly first trimester). The quitting during pregnancy subgroup was a mixture of first, second, and third trimester quitters with most quitters being from the first trimester. The quitting during pregnancy subgroup was necessary since some studies did not separate their results based on each trimester. We calculated pooled outcome associations for each subgroup in a forest plot and compared the associations using Z-tests. In the main analysis, we defined overweight and obesity to be ≥85th percentile and ≥95th percentile, respectively. In addition, for studies in which obesity was excluded from the overweight definition (eg, 85th–<95th percentile), we labeled them as the subgroup “obesity excluded” and compared them to the subgroup of “obesity included” (≥85th percentile). Similarly, ORs of overweight in these subgroups were pooled in a forest plot and Z-tests were used to test the differences.
Assessment of Bias
After the data were extracted, two reviewers individually performed a bias assessment using the Newcastle Ottawa Scale for cohort studies.25 Specifically, each article was rated based on 3 major categories with various number of assessment criteria: selection (representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at the start of study), comparability (comparability of cohorts on the basis of design or analysis), and outcome (assessment of outcome, was follow-up long enough for outcomes to occur, adequacy of follow-up cohorts). One point was given if an assessment criterion was met. The maximum score for each study was 9. A score from 7 to 9 was considered to have a low risk of bias, 4 to 6 to have a moderate risk of bias, and 0 to 3 to have a high risk of bias.
Once the scores were calculated by the two reviewers, each criteria underwent a comparison to assess any differences in scoring. The paired t-tests showed (p = .141) no significant differences between the two reviewers’ total scores. Any discrepancy in scoring was resolved by a third researcher. Out of the 10 final articles, 9 had a low risk of bias, 1 had a moderate risk of bias, and none had a high risk of bias (Supplementary Table 6).
Results
Study Characteristics
A total of 10 articles were included in the meta-analysis and all of them were cohort studies (Table 1). Two articles were from North America,14,26 six from Europe,4,18,27–30 one from Asia,31 and one from Australia.32 There was a total of 71 393 (18 550 boys and 52 843 girls) children born between 1991 and 2006 in all included studies. The sex imbalance was mostly caused by a large study with girls only.14 Child age ranged from 2 to 18 years old.
Primary Analysis
In the combined samples across all studies, the overall prevalence of overweight was 11.7% (5076/43 206) and the prevalence of obesity was 2.3% (770/33 950). The results for quitters and continued smokers with ORs or mean differences in BMI z-score can be found in Table 2 (pooled outcome associations) and Supplemental Table 1 (individual studies). The pooled unadjusted OR for overweight in children of quitters vs nonsmokers was 1.36 (95% CI: 1.14, 1.62) (N = 48 819) (Figure 1). There was a moderate heterogeneity (I2) of 56.0% across studies. Pooled adjusted OR for overweight was 1.29 (1.11, 1.50) (N = 51 412) with a moderate heterogeneity of 50.6% (Supplementary Figure 8). The corresponding unadjusted OR for obesity was 1.65 (1.17, 2.32) (N = 38 915) with heterogeneity of 62.1% (Figure 2), while the adjusted OR was 1.51 (1.09, 2.10) (N = 43 424) with a moderate heterogeneity of 53.3% (Supplementary Figure 17). The unadjusted mean difference in BMI z-score was 0.51 (0.41, 0.61) (N = 7807) with a low I2 of 31.2% (Supplementary Figure 19), and the adjusted mean difference in BMI z-score was 0.03 (−0.04, 0.10) (N = 10 098) with a low I2 of 10.9% (Supplementary Figure 24).
Pooled Associations Between Maternal Smoking Status During Pregnancy and Child Weight Status Outcomes
| Outcome . | Adjustment . | Pooled association measurement . | Nonsmokers . | Continued smokers . | Quitters . | Quitters (match studies with continued smoke data) . |
|---|---|---|---|---|---|---|
| Overweight | Unadjusted | OR (95% CI) | Reference | 1.44 (1.27, 1.64) | 1.36 (1.14, 1.62) | 1.39 (1.15, 1.68) |
| Overweight | Most adjusted | OR (95% CI) | Reference | 1.28 (1.16,1.42) | 1.29 (1.11, 1.50) | 1.32 (1.13, 1.55) |
| Obesity | Unadjusted | OR (95% CI) | Reference | 1.94 (1.38, 2.73) | 1.65 (1.17, 2.32) | 1.65 (1.17, 2.32) |
| Obesity | Most adjusted | OR (95% CI) | Reference | 1.59 (1.38, 1.84) | 1.51 (1.09, 2.10) | 1.51 (1.09, 2.10) |
| BMI z-score | Unadjusted | Mean difference (95% CI) | Reference | 0.64 (0.58, 0.70) | 0.51 (0.41, 0.61) | 0.51 (0.36, 0.66) |
| BMI z-score | Most adjusted | Mean difference (95% CI) | Reference | 0.11 (0.05, 0.17) | 0.03 (−0.04, 0.10) | 0.01 (−0.06, 0.08) |
| Outcome . | Adjustment . | Pooled association measurement . | Nonsmokers . | Continued smokers . | Quitters . | Quitters (match studies with continued smoke data) . |
|---|---|---|---|---|---|---|
| Overweight | Unadjusted | OR (95% CI) | Reference | 1.44 (1.27, 1.64) | 1.36 (1.14, 1.62) | 1.39 (1.15, 1.68) |
| Overweight | Most adjusted | OR (95% CI) | Reference | 1.28 (1.16,1.42) | 1.29 (1.11, 1.50) | 1.32 (1.13, 1.55) |
| Obesity | Unadjusted | OR (95% CI) | Reference | 1.94 (1.38, 2.73) | 1.65 (1.17, 2.32) | 1.65 (1.17, 2.32) |
| Obesity | Most adjusted | OR (95% CI) | Reference | 1.59 (1.38, 1.84) | 1.51 (1.09, 2.10) | 1.51 (1.09, 2.10) |
| BMI z-score | Unadjusted | Mean difference (95% CI) | Reference | 0.64 (0.58, 0.70) | 0.51 (0.41, 0.61) | 0.51 (0.36, 0.66) |
| BMI z-score | Most adjusted | Mean difference (95% CI) | Reference | 0.11 (0.05, 0.17) | 0.03 (−0.04, 0.10) | 0.01 (−0.06, 0.08) |
OR, odds ratio; CI, confidence interval; BMI, body mass index.
Pooled Associations Between Maternal Smoking Status During Pregnancy and Child Weight Status Outcomes
| Outcome . | Adjustment . | Pooled association measurement . | Nonsmokers . | Continued smokers . | Quitters . | Quitters (match studies with continued smoke data) . |
|---|---|---|---|---|---|---|
| Overweight | Unadjusted | OR (95% CI) | Reference | 1.44 (1.27, 1.64) | 1.36 (1.14, 1.62) | 1.39 (1.15, 1.68) |
| Overweight | Most adjusted | OR (95% CI) | Reference | 1.28 (1.16,1.42) | 1.29 (1.11, 1.50) | 1.32 (1.13, 1.55) |
| Obesity | Unadjusted | OR (95% CI) | Reference | 1.94 (1.38, 2.73) | 1.65 (1.17, 2.32) | 1.65 (1.17, 2.32) |
| Obesity | Most adjusted | OR (95% CI) | Reference | 1.59 (1.38, 1.84) | 1.51 (1.09, 2.10) | 1.51 (1.09, 2.10) |
| BMI z-score | Unadjusted | Mean difference (95% CI) | Reference | 0.64 (0.58, 0.70) | 0.51 (0.41, 0.61) | 0.51 (0.36, 0.66) |
| BMI z-score | Most adjusted | Mean difference (95% CI) | Reference | 0.11 (0.05, 0.17) | 0.03 (−0.04, 0.10) | 0.01 (−0.06, 0.08) |
| Outcome . | Adjustment . | Pooled association measurement . | Nonsmokers . | Continued smokers . | Quitters . | Quitters (match studies with continued smoke data) . |
|---|---|---|---|---|---|---|
| Overweight | Unadjusted | OR (95% CI) | Reference | 1.44 (1.27, 1.64) | 1.36 (1.14, 1.62) | 1.39 (1.15, 1.68) |
| Overweight | Most adjusted | OR (95% CI) | Reference | 1.28 (1.16,1.42) | 1.29 (1.11, 1.50) | 1.32 (1.13, 1.55) |
| Obesity | Unadjusted | OR (95% CI) | Reference | 1.94 (1.38, 2.73) | 1.65 (1.17, 2.32) | 1.65 (1.17, 2.32) |
| Obesity | Most adjusted | OR (95% CI) | Reference | 1.59 (1.38, 1.84) | 1.51 (1.09, 2.10) | 1.51 (1.09, 2.10) |
| BMI z-score | Unadjusted | Mean difference (95% CI) | Reference | 0.64 (0.58, 0.70) | 0.51 (0.41, 0.61) | 0.51 (0.36, 0.66) |
| BMI z-score | Most adjusted | Mean difference (95% CI) | Reference | 0.11 (0.05, 0.17) | 0.03 (−0.04, 0.10) | 0.01 (−0.06, 0.08) |
OR, odds ratio; CI, confidence interval; BMI, body mass index.
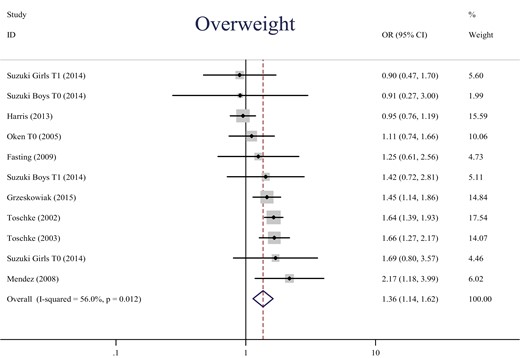
Forest plot of pooled unadjusted OR for overweight in children of quitters (vs nonsmokers during pregnancy). OR, odds ratio; CI, confidence interval; T0, before pregnancy; T1, first trimester. Weights are from random effects analysis.

Forest plot of pooled unadjusted OR for obesity in children of quitters (vs nonsmokers during pregnancy) OR, odds ratio; CI, confidence interval. Weights are from random effects analysis.
Secondary Analysis of Continued Smoking vs Quitting
A secondary analysis was conducted among a subset of studies with data on both children of continued smokers and quitters. The pooled unadjusted OR for overweight was 1.39 (95% CI: 1.15, 1.68) (N = 48 144) in the matched quitter group and 1.44 (95% CI: 1.27, 1.64) (N = 54 323) for the continued smoking group, compared to the nonsmoking group (Supplementary Figures 2 and 3). The pooled adjusted OR for overweight was 1.32 (95% CI: 1.13, 1.55) (N = 50 737) in the matched quitter group and 1.28 (1.16, 1.42) (N = 56 598) in the continued smoking group (Supplementary Figures 9 and 10). The corresponding pooled unadjusted OR for obesity was 1.65 (95% CI: 1.17, 2.32) (N = 38 915) in the matched quitter group and 1.94 (95% CI: 1.38, 2.73) (N = 44 337) for the continued smoking group, compared to the nonsmoking group (Figure 2, Supplementary Figure 15). The pooled adjusted OR for obesity was 1.51 (95% CI: 1.09, 2.10) (N = 43 424) in the matched quitter group and 1.59 (1.38, 1.84) (N = 49 198) in the continued smoking group (Supplementary Figures 17 and 18).
Subgroup Analysis
For the pooled outcome associations by timing of quitting subgroups, the pooled unadjusted OR for overweight in children of mothers quitting before pregnancy was 1.46 (1.15, 1.85) (N = 9921) I2 10.8%, during first trimester was 1.52 (1.27, 1.82) (N = 12 595) I2 0.0%, and for quitting during pregnancy 0.97 (0.79, 1.20) (N = 27 957) I2 23.8% (Supplementary Figure 4). The corresponding pooled unadjusted OR for obesity in children of mothers quitting before pregnancy was 1.67 (1.26, 2.21) (N = 7461), during first trimester was 2.41 (1.49, 3.90) (N = 4077), and for quitting during pregnancy 1.21 (0.85, 1.72) (N = 27 377) (Supplementary Figure 16, Supplementary Table 2). The corresponding pooled unadjusted mean difference in BMI z-score in children of mothers quitting before pregnancy was 0.49 (0.33, 0.65) (N = 675), during first trimester was 0.59 (0.48, 0.70) (N = 8653), and for quitting during pregnancy 0.38 (0.21, 1.00) (N = 580) (Supplementary Figure 23, Supplementary Table 4). As discussed above Z-tests were completed to analyze the pooled outcome associations for subgroups of timing of quitting. Significant differences were found between the “quitting during pregnancy” and the “first trimester” subgroups in unadjusted and adjusted OR for overweight, unadjusted OR for Obesity, and unadjusted mean difference in BMI z-score, with the same direction as overweight (“first trimester”>“quitting during pregnancy”). Other pairwise comparisons were not significant including the comparison between the “first trimester” and the “before pregnancy” subgroups. The p-values between these two subgroups ranged from 0.197 to 0.943 across all unadjusted and adjusted outcomes (Supplementary Tables 3 and 5). Additionally, there was a significant difference between “quitting during pregnancy” and “before pregnancy” in unadjusted OR for overweight. Additional Z-tests were completed to analyze the subgroups of children of quitters based on two different definitions of overweight, that is, “obesity included” and “obesity excluded.” Similar results were found between them.
Publication Bias Analysis
Funnel plots for outcome associations are included in Supplementary Figures 28-45. We did not see evidence of publication bias in these plots except in those for unadjusted (Supplementary Figure 36) and adjusted (Supplementary Figure 39) ORs for obesity in continued smokers (vs nonsmokers). Both were asymmetrical with more studies on the right side of the plot and therefore we performed a publication bias test (the Begg’s test) on these groups which showed a p-value of .050 for unadjusted ORs for obesity in continued smokers and a p-value of .188 for adjusted ORs for obesity in continued smokers. Therefore, the results from the former group should be viewed with reservations as the test indicates it has publication bias.
Discussion
Summary of Key Results
In this meta-analysis, we systematically reviewed 10 studies on the associations between smoking cessation during pregnancy and childhood overweight/obesity. We found fairly consistent evidence across most studies. Compared to children of nonsmokers, the pooled OR for obesity was 1.65 among children of quitters, while 1.94 among children of continued smokers. A similar trend, but to less extent, was also observed for the pooled ORs for overweight (1.36 vs 1.44). Although there is still an increased risk in of childhood overweight (1.46) and childhood obesity (1.67) in children of mothers who quit before pregnancy, timing of cessation may moderate harm reduction from quitting smoking. Unexpectedly however, we found the most reduction of risk in the quitting during pregnancy subgroup (OR 0.97 [0.79, 1.20], that is, not significantly different from the nonsmoker reference group) but not in the before or in the first trimester subgroup. Our meta-analysis suggested that children of quitters still have elevated risks of childhood overweight/obesity compared to children of nonsmokers, but lower than children of continued smokers.
Interpretation
Previous meta-analyses have shown a correlation between smoking during pregnancy and the development of overweight and obesity during childhood.9,10 This puts a further emphasis on the direct effect that tobacco smoke has on not only the mother, but on the fetus as well. Quitting smoking can mitigate some future smoking-related adverse effects during fetal development. In our supplemental analysis of continued smokers, children of mothers who continued smoking showed seemingly higher ORs for overweight and obesity than the ORs for children of mothers who quit.
Biological Mechanisms
One hypothesized biological explanation for overweight and obesity can be potentially explained via the chemical exposure to nicotine itself influencing mechanistic changes, for example DNA methylation of cytosine-guanine dinucleotide (CpG) sites on genes such as: RUNX3, CYP1A1, and NR3C1 to only name a few.33 More specifically, dysregulation of the CYP1A1 gene has been linked to obesity development.34 Other effects include rapid catch-up growth,32 intrauterine exposure resulting in alterations in the hypothalamus leading to poorer satiation and higher appetite35,36 potentially associated with the thrifty phenotype theory, and nicotine influencing body size through the hypothalamic pituitary-axis.14 Some of these adverse effects of maternal smoking on weight-related biological mechanisms of the fetus may be mitigated by smoking cessation,17 depending on the baseline smoking level and timing of smoking cessation (see below).
Timing of Smoking Cessation
Throughout the years, smoking cessation has shown some potential weight normalization among children of smokers. In addition, the timing of smoking cessation can further limit the detrimental effects tobacco exposure may have. Our study has shown children of mothers who quit mostly in the first trimester (the quitting during pregnancy subgroup) (OR 0.97) and children of mothers who quit in the first trimester (OR 1.52) to have different risks of childhood overweight suggesting that the timing of quitting may influence its effect on childhood obesity. However, caution should be taken while drawing conclusions since the quitting during pregnancy subgroup only contained two studies, even though it had a large sample size (27 957).
The ideal timing for maternal smoking cessation during pregnancy to maximize its harm reduction on offspring growth has not been clearly established in the literature. The existing evidence points to sometime up to the second trimester. For example, a previous study showed that newborns of quitters in the first trimester had a birth weight that was most similar to newborns of nonsmokers, whereas newborns of quitters in the second (−161 g) or third trimester had decreased birth weight (−248 g).37 Another study found mothers who quit during the first (1.04 [0.76, 1.43]) or the second trimester (0.83 [0.43, 1.61]) to have a normalized risk of small-for-gestational-age birth compared to children of nonsmoking mothers.38 Similar results have been found for infant growth. For example, our team has shown, 27 weeks of pregnancy, by the end of the second trimester to be the latest timing that smoking cessation can occur to reduce the risk of rapid infant BMI z-score gain.17
Biological Mechanisms
The biological mechanisms through which maternal smoking during pregnancy is associated with childhood obesity remain unclear. One plausible mediator is rapid infant weight or BMI gain, which can be supported by the Thrifty Phenotype hypothesis.39 According to this hypothesis, early metabolic alterations because of environmental insults such as prenatal exposure to maternal smoking can occur to adapt to the disadvantaged intrauterine environments. If the disadvantaged intrauterine environments last for a long time, some of these prenatal metabolic alterations may continue after birth and have long-lasting health effects including obesity development. These alterations include energy metabolism becoming more conservative because of the limited resources.
Prenatal exposure to maternal smoking can lead to restricted blood flow to the placenta, and result in decreased supply of oxygen and nutrients to the fetus.40 Carbon monoxide (CO) from maternal smoking also competes with binding to hemoglobin, further reducing the oxygen supply.41 Compromised supply of oxygen and nutrients may activate the development of the thrifty phenotype. To some extent, maternal smoking cessation can prevent or correct the thrifty phenotype caused by decreased supply of oxygen and nutrients. For example, alterations to insulin metabolism in the liver are some of the effects nicotine exposure can have on the fetus.42 These would lead to a more active glucose-insulin pathway, resulting in fat storage even with low or normal levels of insulin. Since the liver begins to develop at 4 weeks of gestation and the basic structure is formed by the end of the first trimester,43 smoking cessation before the complete development can substantially assist in limiting the potential alterations that can occur. This possibility is supported by our meta-analysis showing children of quitters during the first trimester of pregnancy have a lower risk of childhood obesity than children of continued smokers.
The fetal weight growth velocity starts to increase rapidly at 16 weeks and peaks at 35 weeks of gestation which is within the time frame for the second and third trimester.44 Before this timeframe, the fetus’s requirement for energy is not as high, so the thrifty phenotype may not be triggered at this time. Interestingly, children of quitters in the during pregnancy subgroup (mixed timing, mostly first trimester), have a closer to normal risk of obesity. This suggests that the extent to which maternal smoking cessation can prevent or correct the smoking-induced thrifty phenotype depends on dosage of previous exposure to maternal smoking and timing of smoking cessation. Specifically, fetuses of quitters in the quitting during pregnancy subgroup would receive a substantial amount of exposure to nicotine that still allows the fetal body to function normally, but not enough to trigger the thrifty phenotype, resulting in a “lifelong lean phenotype” after birth. Fetuses of late pregnancy quitters or continued smokers would receive an excess amount of nicotine exposure that can trigger the development of the thrifty phenotype. After birth, these children would no longer have in utero exposure to high levels of nicotine in the postnatal environment and have access to more plentiful oxygen and nutrients. As a result of the mismatch between prenatal and postnatal environment, they would experience rapid infant weight or BMI gain, and gradually develop a high risk of obesity. The thrifty phenotype vs “lifelong lean phenotype” can help explain the high risk of overweight and obesity among continued smokers vs the normalized risk among quitting during pregnancy subgroup. However this does not explain why the quitting before pregnancy and first trimester subgroups do not have this normalized risk compared to nonsmokers whereas the quitting during pregnancy (mixed timing, mostly first trimester) does. Other biological mechanisms may be involved, we are not sure at this time.
Methodological Considerations
Adjustment of Confounders
Although adjusting for confounders (for example socioeconomic status) is necessary, the inconsistency and variation in adjusted confounders across previous studies made the adjusted ORs difficult to compare and interpret. Therefore, we calculated both unadjusted and adjusted pooled outcome associations, however we focused on unadjusted. Some studies only adjusted for up to four confounders, whereas others adjusted for up to eight confounders. Thus, utilizing the unadjusted would be more beneficial since it is more comparable. Additionally in some studies, researchers adjusted for potential mediators which could substantially underestimate the total effect of smoking cessation. For instance, one study28 adjusted for breastfeeding and rapid infant weight gain. These variables have been shown to have a protective effect on overweight/obesity.45 Breastfeeding and rapid infant weight gain mediate the pathway through which maternal smoking cessation may increase the risk of childhood obesity.46 A study has also shown that a shorter duration of breastfeeding and increased tobacco use is associated with catch-up growth.47
Inconsistent Definition of Exposure and Outcome
Some studies grouped their continued smokers with later quitters. For example,18,26,27,31 persistent smokers were defined as mothers who smoked any time past the first trimester. This mixture could have led to a dilution of effects seen between persistent smokers and quitters.18 National survey data48–50 does show an increasing prevalence in maternal smokers who quit during the last trimester. In addition, some studies did not have a clear, concise definition of overweight and obesity. Multiple guidelines on obesity and overweight were utilized (CDC, WHO51), which led to different thresholds that had to be met for the proper classification.
Measurement Bias
All the studies used questionnaires to assess smoking status/cessation, this can lead to self-reporting bias in all the study populations since they were not confirmed with a biological marker. Biases can include actual smoking status (quitter vs continued smoker) or timing of quitting (earlier vs later). For example, some mothers may over report their quitting status while others may report quitting earlier than they actually did. These biases can contribute to an explanation behind why children of quitters can still have an elevated risk, diluting the true effects of smoking cessation. The self-questionnaires have affected the ascertainment of exposure in selection by implementing bias, however this has not affected the overall quality of the study.
Strengths and Limitations
One major strength of this study was being the first meta-analysis that has looked at the relationship of smoking cessation, along with timing of cessation, with the risk of childhood overweight and obesity. This research was able to fill in research gaps and allow for insight into other risks that may have been overlooked regarding maternal smoking. Second, having the amount of children that were analyzed throughout these studies, over 70 000 participants, provides high validity into the results seen. Third, we were able to provide subgroup analysis on the stratified categories of timing of quitting and continued smoking paradigms, further strengthening the conclusions reached by our team. Fourth, we also looked at measures such as BMI z-score, which is not talked about in related meta-analyses involving this topic. These strengths have provided a more in-depth analytical representation of measures of the studies.
The first limitation was the potential overlapping timing of quitting among the trimesters of pregnancy. Two of the studies14,18 in our meta-analysis contained the “quitting during pregnancy” subgroup which consisted of the first, second, and third trimester quitters. This was separated from the “first trimester” subgroup which consisted exclusively of the first trimester quitters. With the additional variability in the “quitting during pregnancy” subgroup, we were unable to completely separate the influence of smoking cessation during specific trimesters of pregnancy. Second, there was insufficient information on average cigarettes per day or secondhand smoke exposure. Only three articles14,30,32 reported the number of continued smokers at different number ranges of cigarettes per day as we reported in Table 1. Offspring secondhand smoke exposure in childhood was only considered in two articles, one adjusting for current maternal smoking at outcome assessment4 and the other adjusting for childhood exposure to paternal smoking.14 Third, the three online search engines utilized may have missed some literature that was not included in their databases. For instance, while we included all articles published in the selected databases with no limit on language in our eligibility criteria, we missed articles without an English abstract as required to be included in PubMed, EMBASE, and CENTRAL databases. This might have led us to not have all articles in this field for analysis. We did however use a translator for the two non-English full text articles found in these databases. Fourth, a limitation to this study was that we did not include any gray literature including thesis papers, dissertations, or textbook chapters. Fifth, our review did not address postpartum smoking relapse, which is common.52,53 Last, there was no uniform use of control groups across the studies with some being labeled as “nonsmokers” and others “never smoker.” Therefore, we chose to use the term of “nonsmoker” to include all of these groups to be inclusive of all controls used. The control groups defined by the original articles were: four articles with the control group being never smoking in the lifetime,4,18,26,31 two articles with the control group being not smoking a year before pregnancy and through to delivery, and the other four articles with the control group not smoking 3 months prior to or at conception. For the three articles with a quitting subgroup of women who quit smoking before pregnancy26,27,31 they did have a separate control group who never smoked in lifetime26,31 or did not smoke in the year before pregnancy.27 Therefore, within each individual article, there was no overlap between the quitting before pregnancy subgroup and the nonsmoker control group. Specifically, Oken’s quitting before pregnancy subgroup quit smoking at least 3 months before pregnancy.26 Suzuki’s quitting before pregnancy subgroup simply reported quitting before pregnancy.31 Toschke’s quitting before pregnancy subgroup smoked sometime in the year before pregnancy but not during pregnancy, which meant they quit within the year before pregnancy.27
Conclusion
In conclusion, our analysis using nonsmokers as a reference group, shows quitters had a higher risk of overweight/obesity in their children, but the risk might not be as high as continued smokers. Timing of maternal smoking cessation would also have an impact on the risk of childhood overweight/obesity: quitting before or within (mostly) the first trimester would be most beneficial in decreasing the risk.
Of the total number of global smokers, 8–10% are pregnant mothers, with a majority of them considered moderate to heavy smokers in daily frequency,54 so this study shows the importance of reducing the risk of early childhood overweight/obesity through maternal smoking cessation. Overall, our study has shown that maternal smoking cessation and its timing plays an important role in overweight/obesity development in children.
Future research can be focused on tracking smoking status through the entirety of the pregnancy to gather more data on quitters in later trimesters. Having a clear definition of overweight and obesity would also be beneficial in evaluating the true effects of smoking. Biological mechanisms can be more focused on in future research, as well.
Funding
This work was supported through the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under R40MC31880 entitled “Socioeconomic disparities in early origins of childhood obesity and body mass index trajectories” (PI, Xiaozhong Wen). Dr. Wen’s time effort was also supported by Clinical and Translational Science Award (CTSA) Pilot Study support from National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) grant UL1TR001412; and R21 exploratory research support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH grant R21HD091515 (both awarded to Xiaozhong Wen).
Declaration of Interests
No financial disclosures were reported by the authors of this paper.
Acknowledgments
We appreciate the help from Nell Aronoff, librarian and liaison to the Jacobs School of Medicine and Biomedical Sciences, State University of New York at Buffalo, on search strategies for literature.




Comments