-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica M Powers, Stephen A Maisto, Michael J Zvolensky, Bryan W Heckman, Joseph W Ditre, Longitudinal Associations Between Pain and Use of Cigarettes and E-cigarettes in the Population Assessment of Tobacco and Health (PATH) Study, Nicotine & Tobacco Research, Volume 25, Issue 3, March 2023, Pages 404–411, https://doi.org/10.1093/ntr/ntac197
Close - Share Icon Share
Abstract
Pain has been implicated in the onset and maintenance of nicotine addiction, and there is initial cross-sectional evidence of covariation between pain and the use of cigarettes and e-cigarettes. The goals of the current study were to: (1)test pain severity as a predictor of initiating co-use of cigarettes and e-cigarettes, (2)examine longitudinal associations between pain and use/co-use of cigarettes and e-cigarettes, (3)generate the first prevalence rate data regarding cigarette and e-cigarette use as a function of pain, and (4)examine gender as a moderator of these associations.
Data were drawn from Waves 1–4 of the Population Assessment of Tobacco and Health Study (2013–2018).
Among exclusive cigarette smokers at Wave 1 (n = 7719), pain severity was associated with a greater likelihood of and faster trajectory to initiating co-use of cigarettes and e-cigarettes (ps < .05). A significant pain × gender interaction (p < .05) revealed this prospective relationship was stronger among women. Among adult respondents who provided at least three waves of data (n = 24 255), greater Wave 1 pain severity was positively associated with e-cigarette use, cigarette smoking, and co-use of cigarettes and e-cigarettes at Waves 2, 3, and 4 (ps < .001). At Wave 4 (n = 33 822), adults with moderate or severe pain endorsed rates of e-cigarette and cigarette use almost two times greater versus no or low pain (ps < .001).
Collectively, these findings provide evidence that pain likely serves as an important candidate risk factor for the initiation and maintenance of cigarette and e-cigarette use.
This is the first prospective study to show that pain serves as an important risk factor for initiation and maintenance of cigarette and e-cigarette use over time. Weighted prevalence estimates further demonstrated that individuals with moderate or severe pain endorsed rates of cigarette and e-cigarette use and co-use approximately two times greater compared to those with no or low pain. These findings highlight a subpopulation of nicotine users more susceptible to greater healthcare burden, nicotine dependence, and physical impairment. Nicotine users with comorbid pain may benefit from integrated interventions that address pain in the context of cessation.
Introduction
Nicotine use and pain are highly prevalent, frequently co-occur, and engender a combined economic impact of more than $635 billion in the United States each year.1–3 Prevalence estimates indicate that rates of cigarette smoking are up to three times greater among persons with pain (vs. the general population),1,2 and nearly 60% of individuals with tobacco use disorder also meet the criteria for chronic pain.4 Pain and nicotine use are posited to interact in the manner of a positive feedback loop, leading to the worsening of both conditions over time.5–7 There is evidence regular consumption of tobacco can increase the risk for worsening and maintenance of pain.8 Conversely, pain can also serve as a potent motivator of nicotine use consumption. Meta-analytic evidence indicates that nicotine (delivered via tobacco and other methods) produces small-to-moderate acute analgesic effects,9 and pain patients readily identify pain-coping as a primary reason for tobacco use.10
Despite mounting evidence of covariation between pain and nicotine use, a majority of research to date has focused exclusively on combustible cigarette smoking. However, up to 11 million adults in the US endorse the current use of electronic cigarettes (e-cigarettes),11 reflecting the unprecedented uptake and popularity of e-cigarette use nationwide. Cross-sectional data have shown that, among e-cigarette users, pain, and pain-related factors are associated with greater e-cigarette dependence and less adaptive cessation-related cognitions (e.g. perceived barriers to quitting, negative abstinence outcome expectancies).12–14 Furthermore, almost half of all nicotine users report concurrent use of multiple nicotine products, with tobacco cigarettes + e-cigarettes currently recognized as the most common combination.11 Multiple nicotine product uses (vs. single product use) have been associated with greater nicotine exposure,15 more severe nicotine dependence,16 and a greater number of cigarettes smoked per day.17 In two cross-sectional studies conducted by our research team, cigarette smokers with pain (vs. no pain) were found to be three times more likely to endorse co-use of e-cigarettes,18 and more severe pain was positively associated with co-use of cigarettes and e-cigarettes among individuals with chronic pain.19 We hypothesized that the presence of pain may contribute to efforts aimed at supplementing overall nicotine intake; however, we are not aware of prior prospective research examining whether pain predicts the uptake of e-cigarettes among current cigarette smokers or whether trajectories of cigarette and e-cigarette co-use differ as a function of pain over time.
The goal of the current study was to examine pain in relation to the use and co-use of cigarettes and e-cigarettes across four annual waves of nationally representative cohort data collected via the Population Assessment of Tobacco and Health (PATH) Study. Although prior research has examined longitudinal associations between pain and other substance use (e.g. alcohol, cannabis, and painkillers, sedatives, tranquilizers),20 we are not aware of any prior work that focused on the pain in relation to the use of cigarettes or e-cigarettes using longitudinal PATH study data. First, we examined pain severity as a predictor of e-cigarette co-use initiation among individuals who identified as exclusive tobacco cigarette smokers at Wave 1. We hypothesized that greater pain severity at wave 1 would be associated with faster latency to initiation of co-use across Waves 2–4. Second, we examined longitudinal associations between pain severity at Wave 1 and use of cigarettes and e-cigarettes across Waves 2–4. We hypothesized that greater pain severity at Wave 1 would subsequently be associated with greater likelihood of endorsing: (1) exclusive e-cigarette use, (2) exclusive combustible cigarette smoking, and (3) co-use of cigarettes and e-cigarettes at Waves 2, 3, or 4. We also examined if pain severity at Wave 1 predicted escalations in the heaviness of cigarette and e-cigarette use across Waves 2–4. Third, we applied recommended population weights to generate the first prevalence rates of cigarette and e-cigarette use and co-use as a function of moderate or severe pain status (vs. no or low pain) using data collected at Wave 4. Finally, analyses were conducted to examine gender as a moderator of these hypothesized outcomes. Reciprocal conceptualizations of pain and nicotine use have identified the importance of considering gender as a third variable.5,6 For example, the pain has been more strongly associated with perceived barriers to cessation and negative outcome expectancies among male (vs. female) e-cigarette users21; however, there is also evidence that women with pain endorse greater tobacco use and nicotine dependence.4,22
Method
Data Source
This study employed data from four waves of the PATH Study, a nationally representative longitudinal cohort study of 45 971 civilians, noninstitutionalized adults, and youth in the United States.23 Funded via a partnership between the FDA’s Center for Tobacco Products, NIDA, and NIH, the purpose of the PATH Study is to contribute evidence to inform the regulation of tobacco products and support efforts to reduce the burden of tobacco-related death and disease. A four-stage stratified area probability sample design was utilized for data collection. Wave 1 of data was collected in 2013–2014 and Wave 4 was collected in 2018–2019. Initial reliability and validity estimates indicate high levels of agreement across interviews,24 and response rates were above 73% for all four waves. The current analyses included only adults aged 18 years or older at Wave 1, given the prevalence of chronic pain among adults,25 and the minimum age to buy nicotine products in the United States.26 Further details of the PATH study are described elsewhere.23
Measures
Pain Severity
Pain severity was assessed at each wave via a single item (“In the past 7 days, how would you rate your pain on average on a scale from 0 to 10, where 0 is no pain and 10 is the worst pain imaginable”). Past one- and two-week pain ratings have been associated with greater persistence of pain,27 and have been strongly positively correlated with more comprehensive measures of pain status and severity.28 Previous research has also demonstrated clinically relevant associations between past-week pain and cigarettes per day and dependence scores.29,30 To aid interpretability of several analyses (e.g. survival curves), pain was dichotomized to moderate/severe pain over the past week (severity > 4/10) vs. no/low pain (severity ≤ 4/10). A cutoff of > 4/10 is commonly utilized in both the clinical and empirical literature to define co-occurring moderate pain, and we used this index in the current study.31
E-cigarette Use
Consistent with prior research using PATH data,32 current e-cigarette use at each wave was defined as every day and some days e-cigarette or ENDS users who reported using e-cigarettes fairly regularly. Participants were defined as exclusive e-cigarette users if they denied established cigarette smoking (described below) at the concurrent wave. Heaviness of e-cigarette use at each wave was assessed by asking participants to report the number of puffs they had taken from an e-cigarette or ENDS product today, yesterday or the day before yesterday. This item has demonstrated evidence of reliability and validity via associations with objective measures of nicotine use (e.g. saliva cotinine).33
Cigarette Smoking
Current established cigarette smokers at each wave included participants who endorsed smoking some days or every day and reported more than 100-lifetime cigarettes. Participants were defined as exclusive cigarette users if they met these criteria and denied established use of e-cigarettes at the concurrent wave. To assess the heaviness of cigarette use, smokers reported the number of cigarettes per day on days smoked.
Cigarette and E-cigarette Co-use
Participants who endorsed at the same wave: (1) current established cigarette smoking, and (2) current established use of e-cigarettes were defined as co-users for that period of data collection. Initiation of e-cigarette co-use was defined as current established cigarette smokers who did not endorse current established use of e-cigarettes at Wave 1, who subsequently endorsed current established e-cigarette use at a following wave (W2, W3, W4). Latency to initiation of e-cigarette co-use was measured in years (waves).
Demographic Variables
Participants reported a variety of sociodemographic characteristics at each wave, including age, gender, ethnicity, race, education, employment status, and annual income. Gender was assessed using a binary item (male, female). Even though response options were male and female, we elected to use the term “gender” to describe this item, since it is how the item was posed to participants.
Data Analysis
All analyses were conducted using Stata 17. Age, gender, race, annual income, ethnicity, and education were included as covariates in all models given significant correlations with cigarette and e-cigarette use among Wave 1 respondents (ps < .001). First, cox proportional hazard models were conducted to examine pain severity at Wave 1 as a predictor of latency to initiating e-cigarette co-use (yes, no) over Waves 2–4. Participants included respondents who endorsed exclusive cigarette smoking at Wave 1 and completed at least 3 waves of data collection (n = 7719). Differences in survival curves were determined using the log-rank test. Pain severity was dichotomized using a standard cutoff described above (>4/10) to aid in the interpretability of survival curves.
Second, generalized estimating equations (GEEs) were utilized to determine if Wave 1 pain severity was subsequently associated with greater likelihood of endorsing use and co-use of cigarettes and e-cigarettes at Wave 2, 3, or 4. Participants included adult respondents who provided data at Wave 1 and completed at least 3 Waves of data collection (n = 24 255). GEE models were also utilized to examine if pain severity at wave 1 predicted escalations in heaviness of cigarette and e-cigarette use from Wave 2–4.
Third, cross-sectional analyses were conducted utilizing data from Wave 4 to examine weighted prevalence rates of: (1) exclusive e-cigarette use, (2) exclusive cigarette smoking, and (3) co-use of cigarettes and e-cigarettes as a function of pain status. Multinomial logistic regression was first conducted to determine the relative risk of cigarette and e-cigarette use as a function of pain status, with non-users of cigarettes and e-cigarettes utilized as the comparison group.
Fourth, to test gender as a moderator, a pain × gender interaction term was included in step 2 of all models specified above. Significant interaction terms were then probed by examining the predicted values of the outcome at each level of gender (i.e. male, female). Per recommendations, the PATH Study population and replicate weights were used in all models.23 All estimates in this study were calculated with balanced repeated replication methods using a Fay’s adjustment value of 0.3.34,35 Analyses conducted across four waves of data utilized the Wave 1 cohort all wave weights, whereas cross-sectional analyses conducted at Wave 4 utilized the Wave 4 single wave weights.36
Results
Sample Characteristics
There were 32 320 adult (aged 18 years and above) respondents who provided data at Wave 1. Unweighted sample characteristics were examined. Approximately 34% (n = 10 385) of the sample were current exclusive cigarette smokers, approximately 3% (n = 579) were current exclusive e-cigarette users, and approximately 5% (n = 1017) were co-users of cigarettes and e-cigarettes. Mean pain severity was 2.06 (SE = 0.01, 95% CI [2.03 to 2.09]), and approximately 19% of the sample met the criteria for moderate/severe pain (pain severity > 4/10; n = 6093). All unweighted sociodemographic, nicotine use, and pain characteristics at Wave 1 are presented in Table 1.
Unweighted Sample Characteristics of Adults at Wave 1 of the PATH Study (N = 32 320)
| . | Total sample . | No or Low pain . | Moderate or severe pain . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Gender | |||
| Male | 16 322 (50.50) | 13 468 (51.74) | 2751 (45.15) |
| Female | 15 993 (49.48) | 12 562 (48.26) | 3342 (54.85) |
| Race | |||
| White | 23 879 (73.88) | 19 451 (74.73) | 4304 (70.64) |
| Black | 5052 (15.63) | 3861 (14.83) | 1147 (18.82) |
| Other | 3384 (10.47) | 2718 (10.44) | 642 (10.54) |
| Ethnicity | |||
| Hispanic | 5560 (17.21) | 4612 (17.72) | 889 (14.59) |
| Age | |||
| 18–24 y | 9110 (28.19) | 8111 (31.16) | 943 (15.48) |
| 25–34 y | 6337 (19.61) | 5367 (20.62) | 931 (15.28) |
| 35–44 y | 4930 (15.26) | 3942 (15.14) | 967 (15.87) |
| 45–54 y | 4846 (15.00) | 3509 (13.48) | 1306 (21.43) |
| 55–64 y | 3971 (12.29) | 2783 (10.69) | 1172 (19.24) |
| 65–74 y | 2117 (6.55) | 1591 (6.11) | 511 (8.39) |
| 75 y and older | 993 (3.07) | 722 (2.77) | 263 (4.32) |
| Education level | |||
| Less than high school | 4233 (13.10) | 2870 (11.03) | 1323 (21.71) |
| High school graduate, GED | 9765 (30.21) | 7596 (29.18) | 2118 (34.76) |
| Some college or associate’s | 11 300 (34.96) | 9199 (35.34) | 2061 (33.83) |
| Bachelor’s degree | 4498 (13.92) | 4116 (15.81) | 369 (6.06) |
| Advanced degree | 2311 (7.15) | 2117 (8.13) | 189 (3.10) |
| Past-year annual income | |||
| Less than $10 000 | 5668 (17.54) | 4004 (15.38) | 1631 (26.77) |
| $10 000–$24 000 | 6768 (20.94) | 5027 (19.31) | 1719 (28.21) |
| $25 000–$49 000 | 6670 (20.64) | 5505 (21.15) | 1,145 (18.79) |
| $50 000–$99 999 | 6140 (19.00) | 5391 (20.71) | 742 (12.18) |
| $100 000 or more | 3914 (12.11) | 3618 (13.90) | 288 (4.73) |
| Exclusive cigarette smoker | 10,385 (33.89) | 7397 (29.76) | 2929 (52.16) |
| Exclusive e-cigarette user | 579 (2.77) | 453 (2.53) | 121 (4.30) |
| Co-user of cigarettes + E-cigarettes | 1017 (4.76) | 674 (3.71) | 336 (11.04) |
| . | Total sample . | No or Low pain . | Moderate or severe pain . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Gender | |||
| Male | 16 322 (50.50) | 13 468 (51.74) | 2751 (45.15) |
| Female | 15 993 (49.48) | 12 562 (48.26) | 3342 (54.85) |
| Race | |||
| White | 23 879 (73.88) | 19 451 (74.73) | 4304 (70.64) |
| Black | 5052 (15.63) | 3861 (14.83) | 1147 (18.82) |
| Other | 3384 (10.47) | 2718 (10.44) | 642 (10.54) |
| Ethnicity | |||
| Hispanic | 5560 (17.21) | 4612 (17.72) | 889 (14.59) |
| Age | |||
| 18–24 y | 9110 (28.19) | 8111 (31.16) | 943 (15.48) |
| 25–34 y | 6337 (19.61) | 5367 (20.62) | 931 (15.28) |
| 35–44 y | 4930 (15.26) | 3942 (15.14) | 967 (15.87) |
| 45–54 y | 4846 (15.00) | 3509 (13.48) | 1306 (21.43) |
| 55–64 y | 3971 (12.29) | 2783 (10.69) | 1172 (19.24) |
| 65–74 y | 2117 (6.55) | 1591 (6.11) | 511 (8.39) |
| 75 y and older | 993 (3.07) | 722 (2.77) | 263 (4.32) |
| Education level | |||
| Less than high school | 4233 (13.10) | 2870 (11.03) | 1323 (21.71) |
| High school graduate, GED | 9765 (30.21) | 7596 (29.18) | 2118 (34.76) |
| Some college or associate’s | 11 300 (34.96) | 9199 (35.34) | 2061 (33.83) |
| Bachelor’s degree | 4498 (13.92) | 4116 (15.81) | 369 (6.06) |
| Advanced degree | 2311 (7.15) | 2117 (8.13) | 189 (3.10) |
| Past-year annual income | |||
| Less than $10 000 | 5668 (17.54) | 4004 (15.38) | 1631 (26.77) |
| $10 000–$24 000 | 6768 (20.94) | 5027 (19.31) | 1719 (28.21) |
| $25 000–$49 000 | 6670 (20.64) | 5505 (21.15) | 1,145 (18.79) |
| $50 000–$99 999 | 6140 (19.00) | 5391 (20.71) | 742 (12.18) |
| $100 000 or more | 3914 (12.11) | 3618 (13.90) | 288 (4.73) |
| Exclusive cigarette smoker | 10,385 (33.89) | 7397 (29.76) | 2929 (52.16) |
| Exclusive e-cigarette user | 579 (2.77) | 453 (2.53) | 121 (4.30) |
| Co-user of cigarettes + E-cigarettes | 1017 (4.76) | 674 (3.71) | 336 (11.04) |
Participants included adults (aged 18 years or older) who provided data at Wave 1 of the Population Assessment of Tobacco and Health (PATH) Study. Numbers may be less than N = 32 320 because of missing data and responses.
Unweighted Sample Characteristics of Adults at Wave 1 of the PATH Study (N = 32 320)
| . | Total sample . | No or Low pain . | Moderate or severe pain . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Gender | |||
| Male | 16 322 (50.50) | 13 468 (51.74) | 2751 (45.15) |
| Female | 15 993 (49.48) | 12 562 (48.26) | 3342 (54.85) |
| Race | |||
| White | 23 879 (73.88) | 19 451 (74.73) | 4304 (70.64) |
| Black | 5052 (15.63) | 3861 (14.83) | 1147 (18.82) |
| Other | 3384 (10.47) | 2718 (10.44) | 642 (10.54) |
| Ethnicity | |||
| Hispanic | 5560 (17.21) | 4612 (17.72) | 889 (14.59) |
| Age | |||
| 18–24 y | 9110 (28.19) | 8111 (31.16) | 943 (15.48) |
| 25–34 y | 6337 (19.61) | 5367 (20.62) | 931 (15.28) |
| 35–44 y | 4930 (15.26) | 3942 (15.14) | 967 (15.87) |
| 45–54 y | 4846 (15.00) | 3509 (13.48) | 1306 (21.43) |
| 55–64 y | 3971 (12.29) | 2783 (10.69) | 1172 (19.24) |
| 65–74 y | 2117 (6.55) | 1591 (6.11) | 511 (8.39) |
| 75 y and older | 993 (3.07) | 722 (2.77) | 263 (4.32) |
| Education level | |||
| Less than high school | 4233 (13.10) | 2870 (11.03) | 1323 (21.71) |
| High school graduate, GED | 9765 (30.21) | 7596 (29.18) | 2118 (34.76) |
| Some college or associate’s | 11 300 (34.96) | 9199 (35.34) | 2061 (33.83) |
| Bachelor’s degree | 4498 (13.92) | 4116 (15.81) | 369 (6.06) |
| Advanced degree | 2311 (7.15) | 2117 (8.13) | 189 (3.10) |
| Past-year annual income | |||
| Less than $10 000 | 5668 (17.54) | 4004 (15.38) | 1631 (26.77) |
| $10 000–$24 000 | 6768 (20.94) | 5027 (19.31) | 1719 (28.21) |
| $25 000–$49 000 | 6670 (20.64) | 5505 (21.15) | 1,145 (18.79) |
| $50 000–$99 999 | 6140 (19.00) | 5391 (20.71) | 742 (12.18) |
| $100 000 or more | 3914 (12.11) | 3618 (13.90) | 288 (4.73) |
| Exclusive cigarette smoker | 10,385 (33.89) | 7397 (29.76) | 2929 (52.16) |
| Exclusive e-cigarette user | 579 (2.77) | 453 (2.53) | 121 (4.30) |
| Co-user of cigarettes + E-cigarettes | 1017 (4.76) | 674 (3.71) | 336 (11.04) |
| . | Total sample . | No or Low pain . | Moderate or severe pain . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Gender | |||
| Male | 16 322 (50.50) | 13 468 (51.74) | 2751 (45.15) |
| Female | 15 993 (49.48) | 12 562 (48.26) | 3342 (54.85) |
| Race | |||
| White | 23 879 (73.88) | 19 451 (74.73) | 4304 (70.64) |
| Black | 5052 (15.63) | 3861 (14.83) | 1147 (18.82) |
| Other | 3384 (10.47) | 2718 (10.44) | 642 (10.54) |
| Ethnicity | |||
| Hispanic | 5560 (17.21) | 4612 (17.72) | 889 (14.59) |
| Age | |||
| 18–24 y | 9110 (28.19) | 8111 (31.16) | 943 (15.48) |
| 25–34 y | 6337 (19.61) | 5367 (20.62) | 931 (15.28) |
| 35–44 y | 4930 (15.26) | 3942 (15.14) | 967 (15.87) |
| 45–54 y | 4846 (15.00) | 3509 (13.48) | 1306 (21.43) |
| 55–64 y | 3971 (12.29) | 2783 (10.69) | 1172 (19.24) |
| 65–74 y | 2117 (6.55) | 1591 (6.11) | 511 (8.39) |
| 75 y and older | 993 (3.07) | 722 (2.77) | 263 (4.32) |
| Education level | |||
| Less than high school | 4233 (13.10) | 2870 (11.03) | 1323 (21.71) |
| High school graduate, GED | 9765 (30.21) | 7596 (29.18) | 2118 (34.76) |
| Some college or associate’s | 11 300 (34.96) | 9199 (35.34) | 2061 (33.83) |
| Bachelor’s degree | 4498 (13.92) | 4116 (15.81) | 369 (6.06) |
| Advanced degree | 2311 (7.15) | 2117 (8.13) | 189 (3.10) |
| Past-year annual income | |||
| Less than $10 000 | 5668 (17.54) | 4004 (15.38) | 1631 (26.77) |
| $10 000–$24 000 | 6768 (20.94) | 5027 (19.31) | 1719 (28.21) |
| $25 000–$49 000 | 6670 (20.64) | 5505 (21.15) | 1,145 (18.79) |
| $50 000–$99 999 | 6140 (19.00) | 5391 (20.71) | 742 (12.18) |
| $100 000 or more | 3914 (12.11) | 3618 (13.90) | 288 (4.73) |
| Exclusive cigarette smoker | 10,385 (33.89) | 7397 (29.76) | 2929 (52.16) |
| Exclusive e-cigarette user | 579 (2.77) | 453 (2.53) | 121 (4.30) |
| Co-user of cigarettes + E-cigarettes | 1017 (4.76) | 674 (3.71) | 336 (11.04) |
Participants included adults (aged 18 years or older) who provided data at Wave 1 of the Population Assessment of Tobacco and Health (PATH) Study. Numbers may be less than N = 32 320 because of missing data and responses.
Pain as a Predictor of Latency to Cigarette and E-cigarette Co-use Among Exclusive Cigarette Smokers at Wave 1
Among exclusive cigarette smokers at Wave 1 (n = 7719), cox regression analysis showed that greater pain severity was associated with a faster trajectory to initiation of cigarette and e-cigarette co-use at a subsequent wave (HOR = 1.05, SE = 0.01, 95% CI [1.03 to 1.08], p < .001). A significant pain × gender interaction in Step 2 (HOR = 0.95, SE = 0.02, 95% CI [0.90 to 0.99], p = .034) revealed that pain severity was more strongly associated with the latency of initiation of co-use among women (HOR = 1.06, SE = 0.02, 95% CI [1.03 to 1.09], p < .001) vs. men (HOR = 1.04, SE = 0.02, 95% CI [1.01 to 1.08], p = .011). Survival curves as a function of pain status (moderate/severe pain vs. no/low pain) are presented in Figure 1.
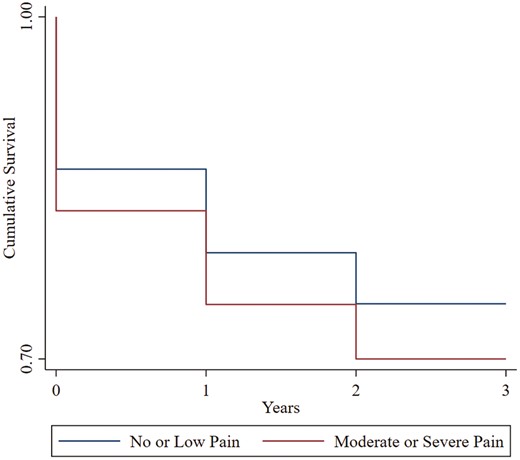
Kaplan–Meier survival curves of years (waves) to initiation of cigarette and e-cigarette co-use as a function of pain status at Wave 1. Participants included adult exclusive cigarette smokers at Wave 1 (n = 7719); models covaried for gender, age, race, past-year annual income, Hispanic ethnicity, and education level: Moderate/Severe Pain = > 4/10 pain severity at Wave 1; No or Low Pain = ≤ 4/10 pain severity at Wave 1.
Longitudinal Associations Between Pain Severity at Wave 1 and Use of Cigarettes and E-cigarettes at Waves 2, 3, and 4.
Cigarette and E-cigarette Use/Co-use at Waves 2, 3, and 4
Among all participants who completed at least 3 waves of data collection (n = 24 255), adjusted GEE models demonstrated that pain severity at Wave 1 was positively associated with greater odds of exclusive cigarette smoking, exclusive e-cigarette use, and co-use of cigarettes, and e-cigarettes at all subsequent waves (Wave 2, 3, and 4; ps < .001; see Table 2). A significant pain × gender interaction revealed that the association between pain severity and exclusive e-cigarette use was stronger among women at Wave 3 (AOR = 1.63, SE = 0.03, 95% CI [1.10 to 1.23], p < .001) and Wave 4 (AOR = 1.17, SE = 0.04, 95% CI [1.09 to 1.25], p < .001) in comparison to men (w3; AOR = 1.12, SE = 0.03, 95% CI [1.06 to 1.18], p < .001; w4: AOR = 1.14, SE = 0.03, 95% CI [1.07 to 1.20], p < .001). There also was a significant pain severity × gender interaction on co-use of cigarettes and e-cigarettes at Wave 3 (p = .031), such that the association between pain severity and co-use of cigarettes and e-cigarettes was stronger among women (AOR = 1.27, SE = 0.03, 95% CI [1.21 to 1.34], p < .001), relative to men (AOR = 1.23, SE = 0.03, 95% CI [1.17 to 1.30], p < .001).
Pain Severity and Gender at Wave 1 as Predictors of Use of Cigarettes and E-Cigarettes at Waves 2, 3, and 4.
| . | Exclusive cigarette smoking . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.14 | 0.011 | [1.12 to 1.16] | <.001** | 1.13 | 0.011 | [1.11 to 1.15] | <.001** | 1.14 | 0.011 | [1.12 to 1.16] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.97 | 0.014 | [0.95 to 1.00] | .063 | 0.97 | 0.014 | [.95 to 1.00] | .058 | 0.98 | 0.016 | [0.95 to 1.00] | .175 |
| . | Exclusive e-cigarette use . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.16 | 0.019 | [1.12 to 1.19] | <.001** | 1.14 | 0.020 | [1.10to 1.18] | <.001** | 1.154 | 0.024 | [1.11 to 1.20] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.035 | [0.87 to1.01] | .080 | 0.092 | 0.033 | [0.86 to 0.99] | .026* | 0.916 | 0.037 | [0.84 to 0.99] | .037* |
| . | Co-use of cigarettes + e-cigarettes . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.25 | 0.017 | [1.22 to 1.29] | <.001** | 1.26 | 0.023 | [1.21 to 1.30] | <.001** | 1.25 | 0.024 | [1.20 to 1.29] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.028 | [0.89 to 1.00] | .052 | 0.94 | 0.030 | [0.88 to 0.94] | .031* | 0.94 | 0.034 | [0.87 to 1.00] | .064 |
| . | Exclusive cigarette smoking . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.14 | 0.011 | [1.12 to 1.16] | <.001** | 1.13 | 0.011 | [1.11 to 1.15] | <.001** | 1.14 | 0.011 | [1.12 to 1.16] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.97 | 0.014 | [0.95 to 1.00] | .063 | 0.97 | 0.014 | [.95 to 1.00] | .058 | 0.98 | 0.016 | [0.95 to 1.00] | .175 |
| . | Exclusive e-cigarette use . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.16 | 0.019 | [1.12 to 1.19] | <.001** | 1.14 | 0.020 | [1.10to 1.18] | <.001** | 1.154 | 0.024 | [1.11 to 1.20] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.035 | [0.87 to1.01] | .080 | 0.092 | 0.033 | [0.86 to 0.99] | .026* | 0.916 | 0.037 | [0.84 to 0.99] | .037* |
| . | Co-use of cigarettes + e-cigarettes . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.25 | 0.017 | [1.22 to 1.29] | <.001** | 1.26 | 0.023 | [1.21 to 1.30] | <.001** | 1.25 | 0.024 | [1.20 to 1.29] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.028 | [0.89 to 1.00] | .052 | 0.94 | 0.030 | [0.88 to 0.94] | .031* | 0.94 | 0.034 | [0.87 to 1.00] | .064 |
Participants included adults who provided at least 3 waves of data (n = 24 255); all models covaried for gender, age, race, past-year annual income, Hispanic ethnicity, and education level; AOR = Adjusted Odds Ratio, SE = standard errors calculated using balanced repeated replication technique with a Fay’s adjustment of 0.3; * p < .05; ** p < .001.
Pain Severity and Gender at Wave 1 as Predictors of Use of Cigarettes and E-Cigarettes at Waves 2, 3, and 4.
| . | Exclusive cigarette smoking . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.14 | 0.011 | [1.12 to 1.16] | <.001** | 1.13 | 0.011 | [1.11 to 1.15] | <.001** | 1.14 | 0.011 | [1.12 to 1.16] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.97 | 0.014 | [0.95 to 1.00] | .063 | 0.97 | 0.014 | [.95 to 1.00] | .058 | 0.98 | 0.016 | [0.95 to 1.00] | .175 |
| . | Exclusive e-cigarette use . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.16 | 0.019 | [1.12 to 1.19] | <.001** | 1.14 | 0.020 | [1.10to 1.18] | <.001** | 1.154 | 0.024 | [1.11 to 1.20] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.035 | [0.87 to1.01] | .080 | 0.092 | 0.033 | [0.86 to 0.99] | .026* | 0.916 | 0.037 | [0.84 to 0.99] | .037* |
| . | Co-use of cigarettes + e-cigarettes . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.25 | 0.017 | [1.22 to 1.29] | <.001** | 1.26 | 0.023 | [1.21 to 1.30] | <.001** | 1.25 | 0.024 | [1.20 to 1.29] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.028 | [0.89 to 1.00] | .052 | 0.94 | 0.030 | [0.88 to 0.94] | .031* | 0.94 | 0.034 | [0.87 to 1.00] | .064 |
| . | Exclusive cigarette smoking . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.14 | 0.011 | [1.12 to 1.16] | <.001** | 1.13 | 0.011 | [1.11 to 1.15] | <.001** | 1.14 | 0.011 | [1.12 to 1.16] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.97 | 0.014 | [0.95 to 1.00] | .063 | 0.97 | 0.014 | [.95 to 1.00] | .058 | 0.98 | 0.016 | [0.95 to 1.00] | .175 |
| . | Exclusive e-cigarette use . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.16 | 0.019 | [1.12 to 1.19] | <.001** | 1.14 | 0.020 | [1.10to 1.18] | <.001** | 1.154 | 0.024 | [1.11 to 1.20] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.035 | [0.87 to1.01] | .080 | 0.092 | 0.033 | [0.86 to 0.99] | .026* | 0.916 | 0.037 | [0.84 to 0.99] | .037* |
| . | Co-use of cigarettes + e-cigarettes . | |||||||||||
| Wave 2 . | Wave 3 . | Wave 4 . | ||||||||||
| AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | AOR . | SE . | 95% CI . | p . | |
| Step 1 | ||||||||||||
| Pain severity | 1.25 | 0.017 | [1.22 to 1.29] | <.001** | 1.26 | 0.023 | [1.21 to 1.30] | <.001** | 1.25 | 0.024 | [1.20 to 1.29] | <.001** |
| Step 2 | ||||||||||||
| Pain severity × gender | 0.94 | 0.028 | [0.89 to 1.00] | .052 | 0.94 | 0.030 | [0.88 to 0.94] | .031* | 0.94 | 0.034 | [0.87 to 1.00] | .064 |
Participants included adults who provided at least 3 waves of data (n = 24 255); all models covaried for gender, age, race, past-year annual income, Hispanic ethnicity, and education level; AOR = Adjusted Odds Ratio, SE = standard errors calculated using balanced repeated replication technique with a Fay’s adjustment of 0.3; * p < .05; ** p < .001.
Heaviness of Cigarette and E-cigarette Use at Waves 2, 3, and 4
Pain severity at Wave 1 was positively associated with CPD at each following wave (Wave 2, 3, 4; ps < .001). However, pain severity at Wave 1 was not associated with changes in the heaviness of e-cigarette use (assessed via puffs per today, yesterday, or day before yesterday; β = 0.06, SE = 0.11, 95% CI [−0.15 to 0.27], p = .569) or cigarette use (assessed via CPD on days smoked; β = -.03, SE = 0.02, 95% CI [−0.06 to 0.01], p = .144) from Wave 2 to 4. There was no pain × gender interaction for change in the heaviness of e-cigarette use (β = −0.11, SE = 0.31, 95% CI [−0.89 to 0.33], p = .369) or cigarette smoking (β = 0.05, SE = 0.08, 95% CI [−0.10 to 0.20], p = .544) from Wave 2 to 4.
Prevalence of Cigarette and E-cigarette Use as a Function of Pain Status at Wave 4
Among all adult respondents at Wave 4 (n = 33 822), the multinomial logistic regression model examining pain status in relation to cigarette and e-cigarette use and co-use was statistically significant (p < .001). Specifically, moderate or severe pain (vs. no or low pain) was associated with the greater relative risk of endorsing exclusive e-cigarette use (RR = 1.47, SE = 0.18, 95% CI [1.16 to 1.88], p = .002), exclusive cigarette smoking (RR = 1.96, SE = 0.10, 95% CI [1.77 to 2.17], p < .001), and co-use of cigarettes and e-cigarettes (RR = 2.44, SE = 0.28, 95% CI [1.94 to 3.06], p < .001). Weighted prevalence statistics further revealed that individuals with moderate or severe pain endorsed rates of cigarette and e-cigarette use and co-use at rates approximately two times greater than those with no/low pain (see Figure 2). For example, approximately 45% of participants reporting moderate or severe pain were classified as currently established someday or everyday cigarette smokers, versus 22.42% of participants with no or low pain. There was a significant pain × gender interaction between co-use of cigarettes and e-cigarettes (p = .040) such that the association between pain and co-use of cigarettes and e-cigarettes was stronger among women (RR = 2.76, SE = 0.45, 95% CI [1.99 to 3.83], p < .001), relative to men (RR = 2.16, SE = 0.35, 95% CI [1.56 to 2.98], p < .001). There was no significant pain × gender interaction on the likelihood of exclusive cigarette or e-cigarette use (ps > .065).
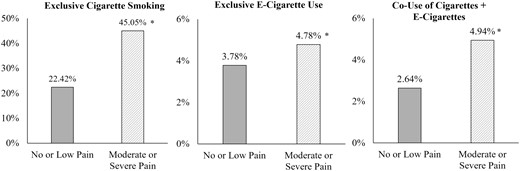
Prevalence of exclusive cigarette smoking, exclusive e-cigarette use, and co-use of cigarettes + e-cigarettes as a function of pain at Wave 4. All prevalence rates utilized population weights using balanced repeated replication technique with a Fay’s adjustment of 0.3; Moderate/Severe Pain = > 4/10 pain severity; No/Low Pain = ≤ 4/10 pain severity; * p < .05.
Discussion
This study examined prospective associations between pain and use of cigarettes and e-cigarettes utilizing four annual waves of national cohort data collected via the PATH Study. Results indicated that, among exclusive cigarette smokers at Wave 1, greater pain severity was associated with faster latency to initiation of e-cigarette and cigarette co-use. Pain severity at Wave 1 was also prospectively associated with exclusive cigarette smoking, exclusive e-cigarette use, and co-use of cigarettes and e-cigarettes at Waves 2, 3, and 4. Weighted prevalence statistics indicated that individuals with moderate or severe pain rates of cigarette and e-cigarette use approximately two times that observed among those with no or low pain.
Informed by initial cross-sectional findings that pain is associated with greater co-use of cigarettes and e-cigarettes,18,19 the current study is the first to document a prospective relationship between pain severity and initiation of cigarette and e-cigarette co-use. Nicotine produces acute analgesic effects,9 use of multiple nicotine products has been associated with greater overall nicotine exposure,15 and it is possible that smokers with more severe pain may have initiated co-use in an effort to increase overall nicotine intake (e.g. between combustible cigarettes or in instances when smoking is not permitted). That these individuals had a shorter latency to co-use initiation suggests that pain may be an important risk factor for nicotine use (via cigarettes and e-cigarettes) and that smokers with pain may be especially susceptible to polynicotine use.
Longitudinal analyses among the full sample revealed that pain severity at Wave 1 was associated with exclusive e-cigarette use, exclusive cigarette smoking, and co-use of cigarettes and e-cigarettes at all subsequent waves. Pain severity was also associated with a greater number of cigarettes smoked per day at all following waves, which is consistent with previous work demonstrating evidence of covariation between pain and heaviness of cigarette smoking.37 Although laboratory and ecological momentary assessment studies have established that the experience of pain can act as a situational motivator of nicotine use,38,39 we are not aware of any prior epidemiological research that tested whether pain predicts the use of nicotine over multiple years. These findings build upon accumulating research literature to suggest that the experience of pain can have enduring effects on the use of nicotine.
Prevalence estimates at Wave 4 indicated that rates of cigarette smoking, e-cigarette use, and co-use of cigarettes and e-cigarettes were approximately two times greater as a function of moderate or severe pain (vs. no or low pain). Approximately 45% of individuals with moderate or severe pain were combustible cigarette smokers (vs. 22% among those with no or low pain), which is consistent with prior estimates that have shown rates of cigarette smoking to be 2–3 times greater among pain populations (vs. the general population).1,2 Although the popularity of e-cigarette use and co-use of cigarettes and e-cigarettes was substantially smaller in comparison to combustible cigarette smoking, this discrepancy has been shown in other nationally representative samples.40 Regardless, approximately 5% of individuals with moderate/severe pain endorsed co-use of cigarettes and e-cigarettes in comparison to 2.5% of those with no/low pain, with similar rates observed for e-cigarette use. These findings suggest that co-occurring pain may function as a risk factor for patterns of nicotine use in addition to combustible cigarette smoking.
We also observed a significant interaction between gender and pain for initiation of co-use. Probing of the interaction revealed that: (1) greater pain was associated with a greater likelihood of—and faster initiation to—co-use regardless of gender, and (2) associations between pain and co-use initiation were strongest among women. We also observed a similar pattern of results for exclusive e-cigarette use and co-use of cigarettes and e-cigarettes at several waves, further underscoring the importance of considering how pain-nicotine use relations may differ between men and women. Indeed, these findings are consistent with a recently published study among 18 019 Danish adults, which found that associations between pain and cigarette smoking were stronger among females, relative to males.41 Research has also shown that among female smokers, but not male smokers, the greater pain is associated with greater cigarette dependence, greater perceived barriers to cessation, and greater cessation-related problems.42 Potential explanations include gender differences in pharmacologic effects of nicotine,43 pain processing,44 and psychological or sociocultural factors (e.g. gender role expectations).45,46 For example, women, relative to men, demonstrate lower experimental pain threshold and tolerance,43 and may be more sensitive to the antinociceptive effects of nicotine.42
Clinical implications include that the presence of pain may increase the risk for initiation of e-cigarette use among individuals who already smoke combustible tobacco cigarettes and thus increase nicotine consumption. Greater nicotine consumption has been linked to the onset and worsening of pain,6,8 and these findings highlight a subpopulation of nicotine users susceptible to greater healthcare burden, nicotine dependence, and physical impairment. An emerging literature suggests that pain severity should be assessed and addressed in the context of nicotine dependence and over the course of cessation.13,47 Nicotine users with comorbid pain, especially women, may also benefit from integrated interventions that address pain in the context of cessation.48–50 Integrated interventions for pain and nicotine use have incorporated pain-nicotine psychoeducation to develop discrepancy between continued nicotine use and desired pain outcomes.49 Other suggested components include teaching more adaptive skills for coping with pain in the absence of nicotine use, and increasing self-efficacy for quitting and remaining abstinent in the context of pain.
Several important limitations and related directions for future research should be noted. First, because PATH data collection is focused primarily on the measurement of nicotine and tobacco use, pain assessment is limited to a single item. Although past-week pain severity has demonstrated the utility and positive associations with cigarette dependence and pain persistence,28–30 additional research is needed using a more granular and clinically relevant pain assessment methodology (e.g. chronic pain, disability status, persistence, location, and etiology) and validated measures of pain severity (e.g. Graded Chronic Pain Scale).28 Second, these data were obtained via self-report, and future studies should include biochemical verification of nicotine use and validation of pain. Third, our aim of examining the initiation of cigarette and e-cigarette co-use focused on the first instance of reported co-use across Waves 2–4, and additional work is needed to examine pain as a predictor of more dynamic nicotine use patterns (e.g. switching from cigarettes to e-cigarettes and vice versa). Fourth, PATH Study data included four annual waves, and longitudinal research is needed to examine pain in relation to nicotine use over longer timeframes. Fifth, these analyses were focused on the pain in relation to cigarette and e-cigarette use, given the emerging literature in this domain. However, future research should examine associations between pain, gender, and other types of nicotine and tobacco use (e.g. cigars, smokeless tobacco) and substance use (e.g. alcohol, opioids, and cannabis). Sixth, gender was limited to a binary self-report item (male, female) and further research is needed to extend these findings to samples that include a broader range of gender identities (e.g. nonbinary, agender). Seventh, comorbid psychopathology (e.g. anxiety, depression), internalizing and externalizing tendencies, and several candidate transdiagnostic factors (e.g. anxiety sensitivity, pain-related anxiety) have been proposed as potential mechanisms in bidirectional pain-smoking associations.6 Prospective research is needed to examine these variables as moderators of associations between pain and use of cigarettes and e-cigarettes. Finally, prevalence estimates of combustible cigarette smoking in the sample were rather high (23% vs. 14% in general population estimates),11 which may be because of the inclusion of both someday and everyday smoking, and because PATH data collection purposefully oversamples nicotine users.23
In summary, the current results demonstrate that pain is likely an important risk factor for initiation and maintenance of cigarette and e-cigarette use utilizing four annual waves of annual cohort data collected via the PATH Study. Future research is needed to examine patterns and trajectories of nicotine product use among individuals with varying levels of pain.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://dbpia.nl.go.kr/ntr.
Funding
This research was supported by a National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH) Grant No. F31DA054717 awarded to Jessica M. Powers. Research reported in this publication was also supported by the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health (NIH) to the University of Houston under Award Number U54MD015946. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interests
The authors report no conflict of interest.
Data Availability
The data underlying this article is from the Population Assessment of Tobacco and Health (PATH) Study, public use files available at the National Addiction and HIV Data Archive Program: https://www.icpsr.umich.edu/web/NAHDAP/studies/36498




Comments