-
PDF
- Split View
-
Views
-
Cite
Cite
Xiangxia Zeng, Yingying Ren, Kang Wu, Qifeng Yang, Sun Zhang, Donghao Wang, Yateng Luo, Nuofu Zhang, Association Between Smoking Behavior and Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis, Nicotine & Tobacco Research, Volume 25, Issue 3, March 2023, Pages 364–371, https://doi.org/10.1093/ntr/ntac126
Close - Share Icon Share
Abstract
To systematically review the association between smoking behavior and obstructive sleep apnea (OSA).
PubMed, Medline, the Cochrane Library, EMBASE, and Scopus databases were used to conduct this review. The two researchers independently screened the literatures, conducted the quality assessment, and data extraction according to the inclusion and exclusion criteria. The RevMan 5.3 was used to analysis the apnea hypopnea index (AHI) index, min saturation of oxyhemoglobin (SaO2), Epworth Sleepiness Scale (ESS) score, and oxygen desaturation index (DOI) and publication bias analysis to assess the effect of smoking on OSA patients. Furthermore, we performed subgroup of the severity of OSA, different countries of sample origin (western countries or eastern countries), and pack-years (PYs < 10 or PYs ≥ 20) to analyze the heterogeneity.
Thirteen studies were included in this analysis that conformed to inclusion criteria and exclusion criteria. Totally 3654 smokers and 9796 non-smokers have participated. The meta-analysis of 13 studies demonstrated that AHI levels were significantly higher in smoker group compared with non-smoker, ESS scores were also significantly higher in smoker group compared with non-smoker, min SaO2 levels were obviously lower in smoker group compared with non-smoker, however, DOI levels hadn’t significantly different between two groups. The subgroup analysis showed that there was an association between severe OSA, eastern countries, pack-years, and smoking.
Smoking behavior is a significant association with OSA. Heavy smokers with histories of more than 20 PYs were at a higher risk of OSA. Moreover, patient with severe OSA exhibited a significantly association with smoking compared with patients with mild or moderate OSA.
The relationship between smoking and OSA was controversial, especially, whether smoking increase or aggravate the risk of OSA. In our review and meta-analysis, we demonstrated that smoking behavior is a significant association with OSA. Heavy smokers with histories of more than 20 PYs were at a higher risk of OSA. Moreover, patient with severe OSA exhibited a significant association with smoking compared with patients with mild or moderate OSA. More prospective long-term follow-up studies about effect of quit smoking on OSA are recommended to establish the further relationship.
Introduction
Obstructive sleep apnea (OSA) is a syndrome caused by the collapse of the upper airway.1 Apnea attacks more than 30 times or apnea hypopnea index (AHI) ≥ 5 times/h during 7 h of sleep at night, moreover, the patients were accompanied by clinical symptoms such as snoring at night and sleepiness during the day.2,3 Epidemiological studies showed that the incidence rate of adult OSA was 14% male and 5% female among the 30–70 years old American population.4 Benjafiled et al. indicated that the prevalence of OSA maybe 936 million people, moreover, China is now with the highest OSA prevalence in the world and has nearly 176 million OSA population.5
OSA is related to a variety of diseases and complications, such as coronary disease, hypertension, diabetes, cerebral apoplexy, gastroesophageal reflux disease (GERD), orthodontic anomalies, and malignant tumor.6–10 They had common pathophysiological mechanisms, such as sympathetic activation, oxidative stress, and systemic inflammation.11,12 Moreover, the risk factors for OSA had obesity, sex, hyperglycemia, upper respiratory tract stenosis, hypertension, hyperlipidemia, etc.13 As we all know, smoking can lead to various respiratory diseases (chronic obstructive pulmonary disease, chronic bronchitis, chronic pulmonary heart disease, etc.), cardiovascular diseases (coronary heart disease, hypertension, etc.), and malignant tumor diseases (lung cancer, nasopharyngeal carcinoma, oral cancer, etc.). Notably, the relationship between smoking and OSA was controversial, especially, whether smoking increase or aggravate the risk of OSA. Wisconsin Sleep Cohort study showed that smokers were more difficult to start and maintain sleep compared with non-smokers, and smokers were more prone to poor sleep quality symptoms such as difficulty in waking up in the morning, sleep over time, and non-restorative sleep.14 On the other hand, Gothe et al.15 found that smoking could reduce the times of apnea, while Zevin Shoshana et al.16 indicated that nicotine had no obvious impact on the OSA population. It is an urge to provide more evidence about the association between smoking behavior and OSA.
This systematic review investigates the association between smoking behavior and OSA, on the other hand, we also analyze whether smoking behavior would aggravate the severity of OSA and whether the relationship was affected by region and smoking years.
Methods
Standard Protocol Approvals and Registrations
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.17 This review was registered in the Prospero database (PROSPERO, registration number CRD42021281692).
Search Strategy
We used PubMed, Medline, the Cochrane Library, EMBASE, and Scopus databases till September 2021. The search strategy contained keywords, medical subject headings, and free text, as follows:
(“Sleep Apnea, Obstructive” [MeSH] OR (Apneas, Obstructive Sleep[Title/Abstract]) OR (OSAs[Title/Abstract]) OR (Sleep Apneas, Obstructive[Title/Abstract]) OR (OSA Syndrome[Title/Abstract]) OR (OSA[Title/Abstract]) OR (OSAHS[Title/Abstract]) OR (Syndrome, Sleep Apnea, Obstructive[Title/Abstract]) OR (Sleep Apnea Syndrome, Obstructive[Title/Abstract]) OR (Apnea, Obstructive Sleep[Title/Abstract]) OR (Sleep Apnea Hypopnea Syndrome[Title/Abstract]) OR (Syndrome, OSA[Title/Abstract]) OR (Upper Airway Resistance Sleep Apnea Syndrome[Title/Abstract]) OR (Syndrome, Upper Airway Resistance, Sleep Apnea[Title/Abstract]))
(“smoking behavior”[MeSH] OR (smoking behavior[Title/Abstract]) OR (smoking[Title/Abstract]) OR (nicotine[Title/Abstract]) OR(cigarettes[Title/Abstract]) OR (tobacco[Title/Abstract]))
#1 and #2
(#3) NOT (review[Publication Type])
Study Selection
Two researchers(Xiangxia Zeng and Yingying Ren) independently screen literature and extract data. They will discuss questions and submit them to the third researcher (Nuofu Zhang) when they are confronted with divergence. We will contact the original author when encountered missing data.
Inclusion and Exclusion Criteria
Inclusion Criteria
(1) Participants in the study included were adults(18 years old), (2) the study was performed using normally adults, who hadn't mental or physical diseases, (3) the diagnosis of OSA was based on polysomnography (PSG) measurement and clinical symptoms. Namely, AHI ≥ 5 and OSA-related symptoms; or AHI ≥ 15 with/without OSA-related symptoms, which could diagnose OSA, (4) The literature showed the association between OSA and smoking, and (5) the researches were cross-sectional or longitudinal design.
Exclusion Criteria
(1) Central sleep apnea syndrome, (2) sleeping pill usage, (3) alcohol abuse, (4) narcolepsy, (5) chronic obstructive pulmonary disease, (6) basic oxygen saturation <90%, (7) use of oral appliances, and (8) had a history of surgery to treat apnea.
Data Extraction
We collected information of each article, such as first author, publication year, study design, size of participants, age of each group, number of female and male, body mass index (BMI), study duration, apnea hypopnea index (AHI), and main outcome.
Assessment of the Quality
The assessment of the quality of the literature complied with the Cochrane handbook. In our study, we assessed the quality of the articles based on the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) standard.18 The quality of articles was divided into high quality (low-risk bias), medium quality (unclear risk bias), and low quality (high-risk bias). The publication bias of included studies was assessed by funnel plot and Egger’s test. Sensitivity analyses were conducted to assess the stability of the results.
Statistical Methods
In our study, Review Manager Software (version 5.3) and Stata (version 15.0; StataCorp, College Station, TX, USA) were used for meta-analysis. Continuous variables were described by the mean difference (MD) between final values, binary variables were described by relative risk (RR) or odds ratio (OR), and 95% confidence interval (95% CI) was calculated. The random-effects model was conducted if significant heterogeneity was found (p < .05); otherwise, the fixed-effects model was applied (p ≥ .05). We used I2 index to assess heterogeneity. I2 ≥ 50 was considered as moderate-to-high heterogeneity. We conducted subgroup analyses based on OSA severity, PSG parameters, region, and pack-years when heterogeneity existed. Sensitivity analyses were directed to assess the influence of the individual study on the overall estimate. OSA severity, according to their AHI values, was classified as mild(5/h ≤ AHI < 15/h), moderate (15/h ≤ AHI < 30/h), or severe (AHI ≥ 30/h). We analyzed the symmetry of a funnel plot to evaluate possible small sample effects and used Begg’s and Egger’s tests to evaluate publication bias in the included studies.
Results
Study Selection
A total of 696 literature related to this study were preliminarily obtained. The literature were further screened according to the inclusion and exclusion criteria. After reading the full text, 52 literature that did not meet the inclusion conditions were removed. Among them, 28 reported incomplete data, six were review literature and five were non-case–control literature. Finally, 13 qualified literature were included.19–31 The flow diagram of study selection was shown in Figure 1. Furthermore, the characteristics and the quality assessment of included 13 studies were listed in Supplementary Table 1.
In the 13 qualified literature conformed to inclusion criteria and exclusion criteria, totally 3654 population who was smoking, and 9796 population who was non-smoking. This study included nine retrospective studies,20,21,24–31 one cross-sectional study,19 one case–control study,22 and one descriptive study.23 Moreover, the quality of eight articles was high to medium quality,21,23–25,27–30 quality of five literature was low quality.19,20,22,26,31
Meta-Analysis Results
Meta-analysis of AHI level in smoker compared with non-smoker and ever-smoker was shown in Figure 2. The result demonstrated that AHI levels were significantly association with smoking (95% CI = 0.43–9.25; p = .03) (Figure 2, A), furthermore, we investigated the meta-analysis of AHI level in smoker compared with ever-smoker, and the result showed that there was no significant difference between smoker and former smoker (MD = −0.29, 95% CI = −3.12–2.53; p = .84) (Figure 2, B). Seven studies19,21,25,26,28–30 presented Epworth Sleepiness Scale (ESS) score in smoker compared with non-smoker, the test for overall effect showed that ESS score was significance difference between two groups (95% CI = 0.54–1.05; p < .00001, Figure 3). Eight studies19–21,25–29 reported that min saturation of oxyhemoglobin (SaO2) in smoker compared with non-smoker, the test for overall effect showed that min SaO2 was significance difference between two groups (95% CI = −4.82–0.96; p = .003, Figure 3, A). Three studies reported21,24,29 the oxygen desaturation index (DOI) in smoker compared with non-smoker, the result demonstrated that there was no significant difference between two groups (95% CI = −4.26–0.78; p = .18, Figure 3, A).
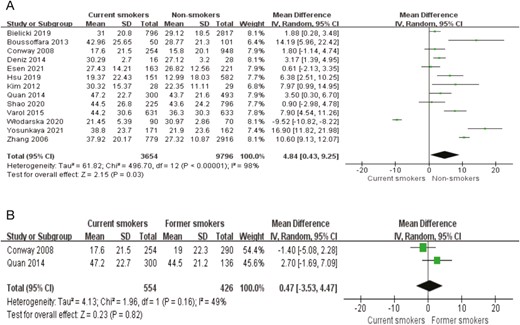
Meta-analysis of apnea hypopnea index (AHI) level. (A) Meta-analysis of AHI level in current smokers compared with nonsmokers; (B) Meta-analysis of AHI level in current smokers compared with former smokers.

Subgroup analysis. (A) Subgroup analysis of Epworth Sleepiness Scale (ESS), min SaO2 and ODI level in smoker compared with nonsmoker (random effect model); (B) Subgroup of obstructive sleep apnea (OSA) population and smoking incidence, according to OSA severity; (C) Subgroup of western and eastern of OSA population and smoking incidence; and (D) Subgroup of OSA population and package/years (PYs).
Subgroup Analysis
We tried to select different factors for subgroup analysis to find the source of heterogeneity between studies as much as possible. We performed subgroup analysis based on the severity of OSA. We divided into mild OSA, moderate OSA, and severe OSA. The result demonstrated that there was an association between severe OSA populations and smoking (p = .03), however, there was no association between mild OSA, moderate OSA populations, and smoking (p > .05)(Figure 3, B). On the other hand, subgroup analysis was conducted according to different countries of sample origin, the results demonstrated that the studies were divided into two subgroups: western countries and eastern countries. The subgroup analysis showed that eastern countries supported the association between smoking and OSA population (p = .003, p < .05), but western countries showed that there was no significant association between smoking and OSA population (p = .08, p > .05) (Figure 3, C). Furthermore, we presented the number of packages smoked per day (PYs, PYs = the number of packages per day × the year smoked) (PYs < 10 or PYs ≥ 20) to conduct a subgroup analysis. According to the results, those smokers who pack-years was no more less than 20 were significant association with OSA population (fixed-effects model, test for overall effect: p = .02). The overall effect indicated that there was heterogeneity among these subgroups (MD = −4.38, 95% CI = [−7.95,−0.81], I2 = 25%, and p = .02)(Figure 3, D).
Sensitivity Analysis
We excluded two study21,31 from the meta-analysis of AHI levels in smokers compared with non-smokers for sensitivity analysis, so as to further explore the reasons of the high heterogeneity between studies. We found that two studies was excluded among the thirteen studies,19–31 the heterogeneity of the meta-analysis had changed significantly (MD = 2.5, 95% CI = [1.59,3.41], I2 = 33%, and p < .00001) (Figure 4, A). The two articles may be the reason of the high heterogeneity. Moreover, we read two literature, the possible reasons may be the risk of bias, that is patients were governed by personal preferences to include grouping. On the other hand, the quality of those two literature is low, which may be cause high heterogeneity.

Sensitivity analysis and test for publication bias. (A) Sensitivity analysis of apnea hypopnea index (AHI) level in smoker compared with non-smoker; (B) The Egger’s test for publication bias; (C) The Begg’s test for publication bias; and (D) The trim and fill method.
Risk of Bias
The funnel plot showed that there was no publication bias. Moreover, the results of Egger’s test and Begg’s test showed that no publication bias with p = .436 and p = .428 (Figure 4, B and C). Trim and fill results showed that the pooled mean difference was 0.549 (95% CI = −0.409–0.218, p < .0001) in random-effects model. There is no significant difference before and after trim and fill (Figure 4, D). Therefore, no study needed to be statistically corrected for funnel plot asymmetry.
Discussion
The present meta-analysis which included 13 studies and 13 450 participants demonstrated that there is a statistically significant association between smoking behavior and the incidence of OSA, especially, the severity of OSA and pack-years. However, the association between quit smoking (former smoking) and the incidence of OSA had no statistically significant results. The subgroup analysis suggested that eastern countries supported the association between smoking and OSA population, and there was an association between severe OSA and smoking, however, there was no statistically significant association between mild OSA, moderate OSA, and smoking. Furthermore, those smokers who pack-years was no more less than 20 were significant association with OSA population.
As shown in our findings, smoking aggravated OSA. In the 3654 smokers, the average AHI level in the smoking group was higher than that in non-smoking group (95% CI = 0.43–9.25; p = .03). And the AHI level of those smokers who pack-years was no more less than 20 was higher than those smokers who pack-years was less than 10 (95% CI = [−7.95,−0.81], I2 = 25%, and p = .02), this indicated that longer smoking times and heavy smoking, the more severe OSA. Furthermore, the ESS score in the smoking group was higher compared with non-smoking group (95% CI = 0.54–1.05; p < .00001), and the min SaO2 in the smoking group was decreased compared with non-smoking group (95% CI = −4.82–0.96; p = .003), this indicated that smoking can increase daytime sleepiness and the severity of OSA, this is consistent with most researches.21,24,26,28 Moreover, Yosunkaya et al.19 confirmed that the smoking index increased by 1 unit, the AHI increased by 15.3% and the minimum oxygen saturation decreased by 2.6%. Pathological mechanism what smoking affected OSA population may be related to sleep structure disorder. As we all know, frequent arousal is one of the key factors leading to the aggravation of sleep-disordered breathing and the occurrence of OSA, according to the research of American health and nutrition, smokers have sleep structure disorder,32 Conway et al.30 also demonstrated that smoking group had a higher arousal index(22 ± 19, p < .05) and hypoxemia during sleep, which support our results. On the other hand, tobacco exposure can cause inflammation of nasal mucosa, impaired sensitivity, and increased nasal resistance. The thickening and edema of the lamina propria of uvula mucosa in smokers lead to upper airway stenosis and airway obstruction, moreover, smoke exposure stimulates bronchial mucosa, it also causes acute airway reflex contraction, increases airway resistance, and increases the collapsibility of upper airway during sleep, eventually, those inflammatory reaction may increase the risk of OSA.33
Noteworthy, we demonstrated that among severe OSA population, there is positive association between smoking and the severity of OSA (95% CI = 1.05–3.33, p = .03), but excluding mild and moderate OSA population. Moreover, the former smoking couldn’t decrease the AHI level compared with the smoking population (95% CI = −3.12–2.53, p = .84), which was consistent with the finding of Varol et al.26 In the study of Varol et al.,26 they showed that smoking index increased significantly as the increasing AHI, suggested that the more severe hypoxemia is, the more severe OSAHS is. The upper airway muscle activity in severe OSA population had decreased and neural regulation had serious changes,34 while the nicotine has little effect on upper airway muscle activity and neural regulation among the severe OSA population. On the other hand, according to our results, quit smoking couldn’t impact on OSA, which was inconsistent with some studies.30,32,35 The reasons we considered maybe: the definition of time of quit smoking was quite a difference which may cause the bias, furthermore, the sample of meta-analysis of AHI level in smoker compared with ever-smoker is relatively small, we couldn’t declare that quit smoking had no significant impact on OSA. More prospective long-term follow-up studies about the effect of quit smoking on OSA are recommended.
Undeniably, there had some limitations in our study. Firstly, most of the included studies are retrospective studies, there maybe exist bias such as selection bias, implementation bias, and result measurement bias, etc. We tried to reduce those differences by including case–control studies and descriptive studies. Secondly, the subgroup analysis of pack-years didn’t analyze the 10 ≤ PYs < 20, because there was only one study24 which investigated the PYs > 10 and PYs ≤ 10, this is not enough to perform the subgroup analysis. Thirdly, the studies about whether improving OSA affected the smoking status were less, we couldn’t perform the subgroup of this matter. Hence, more clinical evidence of larger prospective studies are needed to identify the association between smoking, quit smoking, and OSA.
Conclusions
In conclusion, there is a close relationship between smoking and OSA. Heavy smokers with histories of more than 20 PYs were at a higher risk of OSA. Moreover, patients with severe OSA exhibited a significant association with smoking compared with patients with mild or moderate OSA. Although quit smoking may be non-association with OSA, more clinical evidence of larger prospective studies are needed to identify the association between smoking, quit smoking, and OSA.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://dbpia.nl.go.kr/ntr.
Funding
The study was supported by grants from Guangdong Medical Science and Technology Research Fund Project (nos. B2019103). National Key Research and Development Program of China (Grant No. 2018YFC1313600 & 2016YFC0901102), Program Natural Science Foundation of Guangdong Province, China (Grant No.2019A1515010981).
Acknowledgments
X.Z. and Y.R. contributed equally. X.X.Z. designed the study. Y.Y.R and Q.F.Y. extracted the data and ran the analysis. X.X.Z. wrote the first draft of the article. D.H.W., S.Z., W.K., Y.T.L. contributed significantly. All authors read and approved the final manuscript.
Disclosure Statements
Financial disclosure: The authors declare no financial conflicts of interest. Non-financial disclosure: No potential conflicts of interest relevant to this article were reported.
Data Availability
The data in the study was obtained from PubMed, web of science, the Cochrane Library, VIP, CN KI, Wanfang Data, and CBM databases.
Ethics Approval
Not applicable.




Comments