-
PDF
- Split View
-
Views
-
Cite
Cite
Tzu Tsun Luk, Yee Tak Derek Cheung, Helen Ching-han Chan, Patrick Wai-yin Fok, Kin Sang Ho, Chu Dik Sze, Tai Hing Lam, Man Ping Wang, Mobile Chat Messaging for Preventing Smoking Relapse Amid the COVID-19 Pandemic: A Pilot Randomized Controlled Trial, Nicotine & Tobacco Research, Volume 25, Issue 2, February 2023, Pages 291–297, https://doi.org/10.1093/ntr/ntac045
Close - Share Icon Share
Abstract
The ongoing COVID-19 pandemic had reduced access to traditional, in-person smoking cessation treatment. We examined the feasibility, acceptability, and potential effectiveness of mobile chat messaging in preventing smoking relapse in smokers who have recently quit smoking.
In this assessor-blinded, pilot randomized controlled trial in five cessation clinics, we recruited adult daily smokers who had been receiving cessation treatments and abstained for 3 to 30 days. The intervention group received real-time, personalized chat messaging on relapse prevention via WhatsApp for 3 months. The control group received generic text messaging on the harms of smoking and benefits of quitting for 3 months. The primary outcome was carbon monoxide–validated abstinence at 6 months post-treatment initiation. The trial was registered with ClinicalTrials.gov (NCT04409496).
From June to July 2020, 108 of 130 (83%) eligible subjects were randomized to the intervention (N = 54) or control (N = 54) groups. The retention rate was 93% at 3 months (end of treatment) and 85% at 6 months. In the intervention group, 80% of participants responded to the chat messages at least once; 43% continuously engaged with the intervention over the 3-month intervention period. By intention-to-treat, validated abstinence at 6 months was higher in the intervention than control group (31% vs. 22%), with a relative risk of 1.72 (95% CI = 0.91% to 3.23%; p = .09) after adjusting for pre-quit nicotine dependence, duration of abstinence, and cessation treatment at baseline.
This pilot trial showed the feasibility and acceptability of mobile chat messaging for relapse prevention with preliminary evidence on its effectiveness in increasing validated abstinence.
Smoking relapse is the most likely outcome of smoking cessation attempts and an undertreated problem. This pilot trial showed the feasibility and acceptability of personalized chat messaging via WhatsApp for relapse prevention in recent abstainers amid the COVID-19 pandemic. The higher carbon monoxide–validated abstinence rate in participants who received chat messaging than controls showed preliminary evidence on the effectiveness of the intervention. Fully powered trials are warranted to test the intervention.
Introduction
Most smokers who made quit attempts and achieved short-term abstinence return to smoking (relapse) over time, even when aided by effective cessation treatment. A pooled analysis of 43 randomized controlled trials (RCTs) showed that only 32%, 20%, and 16% of smokers who received 12 weeks of varenicline, bupropion, and nicotine replacement therapy (NRT), respectively, remained abstinent at 6 months after the quit date.1 A Cochrane review of relapse prevention interventions did not find traditional behavioral strategies, including self-help materials, telephone counseling, and group therapy, effective in increasing abstinence at 6 months or longer (relative risk [RR] = 0.98).2
Recently, mobile health (mHealth) has become a new avenue for behavioral cessation support, with a Cochrane review showing that text messaging via short message service (SMS) is effective in increasing abstinence at 6 months or longer by 54% in smokers.3 However, evidence on mHealth interventions for relapse prevention has remained scarce. Our PubMed search using the keywords of smoking, relapse, digital health, and their synonyms identified only one pilot trial on smokers who recently quit smoking (recent abstainers), which found a non-significantly higher 6-month biochemically validated abstinence in those who received online social group support versus usual care only (26% vs. 15%; p = .14).4
Chat messaging apps (eg, WhatsApp, WeChat) are increasingly popular alternative to SMS for mobile messaging and employed for delivery of health care interventions.5 For instance, the penetration rate of WhatsApp was 83.6% in Hong Kong.6 The interface of chat messaging apps could facilitate more engaging mobile conversations compared with SMS. Our formative qualitative study on current smokers showed the acceptability and feasibility of delivering interactive, real-time support via chat messaging apps for smoking cessation.7 Our subsequent trial confirmed the beneficial effect of chat-based cessation support combined with brief intervention in increasing abstinence in smokers.8 The feasibility and effectiveness of chat messaging for relapse prevention in recent abstainers has remained untested.
The ongoing COVID-19 pandemic has provided both opportunities and challenges to promote smoking cessation. Emerging studies showed that smoking is associated with poor COVID-19 prognosis,9 and smokers who believed so were more likely to make quit attempts.10 Yet, smokers exposed to unverified claims in social media that smoking can protect against COVID-19 were more likely to smoke more.11 The practice of social distancing had constrained access to clinic-based smoking cessation services after the onset of the COVID-19 outbreak. The increased psychological distress associated with the pandemic might also increase the risk of relapse.12 To maintain remote cessation support and prevent smoking relapse amid the pandemic, we developed a mobile chat messaging intervention for recent abstainers and conducted a pilot RCT to examine the feasibility, acceptability, and potential effectiveness in recent abstainers.
Methods
Study Design and Setting
We conducted a parallel-group, pilot RCT in Tung Wah Group of Hospitals Integrated Centre on Smoking Cessation (ICSC), a government-funded smoking cessation service in Hong Kong.13 The clinic-based service provides free cessation treatment, including behavioral support, NRT, and varenicline. Following the COVID-19 outbreak (Jan 2020), selected clients could also enroll in the “Mail to Quit” program of ICSC to receive mailed NRT with telephone support to avoid in-person contacts. The trial was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 20-356) and registered with ClinicalTrials.gov (NCT04409496). We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline and its extension to randomized pilot and feasibility trials.14
Participant Recruitment
From June 2 to July 30, 2020, a research nurse approached and screened potential participants who were attending smoking cessation service in all five clinics under the ICSC. Counselors at the ICSC also referred the clients of the Mail to Quit program to the research team for screening and recruitment. To be eligible, the participants needed to be Hong Kong residents aged 18 years or older, smoked daily, and had abstained for 3 to 30 days, enrolled in a smoking cessation program under the ICSC, owned a mobile phone with a mobile instant messaging app (eg, WhatsApp) installed, and able to communicate in Chinese. Subjects with communication problems because of physical or cognitive conditions were excluded. Some subjects met all the eligibility criteria except having stopped smoking for at least 3 days during screening. These subjects were re-contacted 3 days after their target quit date and then randomized if they reported having quit smoking for at least 3 days.
Randomization and Blinding
Participants were individually randomized (1:1) to the intervention or control group according to a randomization list generated by an independent biostatistician, with random permuted blocks of 2, 4, or 6 to achieve similar numbers of participants in both groups. For allocation concealment, the sequence was not disclosed to the research nurse involved in participant recruitment. Blinding of participants and the counselor who delivered the interventions were not possible because of the nature of intervention. Outcome assessors and statistical analysts were blinded to the treatment allocation.
Intervention Group
Participants in both groups received standard smoking cessation treatment, including behavioral support, NRT, bupropion, and varenicline as appropriate. The intervention group additionally received chat messaging on smoking relapse prevention through WhatsApp for 3 months after randomization, in which a counselor interacted with a participant in real-time and provided cessation information and advice. The design of the intervention was similar to our previous trial of chat messaging for promoting quitting,8 but the content focused on relapse prevention. To initiate conversations between the counselors and participants, 18 regular messages were sent to the participants with a tapering schedule over the 3-months intervention period: from 2 messages per week in the first month to alternating frequency of 1 and 2 messages per week in the second month and 1 message per week in the last month. The reduction in message frequency avoided abrupt withdrawal of the intervention, which might not be pleasant to the participants. The messages covered advice on how to manage the 5 main reasons for relapse per the US Tobacco Use and Dependence Guideline,9 including (1) lack of cessation support, (2) negative mood or depression, (3) strong or prolonged withdrawal symptoms, (4) weight gain, and (5) smoking lapses (slips). The messages also included information about the increased risk of COVID-19 severity (admission to intensive care unit, use of ventilators) and deaths in smokers according to the available evidence at the time of the study.9 We also warned that smoking is a known cause of respiratory infections and that the hand-to-mouth motion of smoking and mask removal could expose smokers to corona virus.
Based on the responses from the participants via chat messaging, the counselor provided personalized relapse prevention support by using behavioral change techniques (BCTs) for smoking cessation.15 These included BCTs that promote motivation (eg, strengthen ex-smoker identity [BCT code: BM8]), maximize self-regulatory capacity (eg, advise on avoiding social cues for smoking [BS11]), promote adjuvant activities (eg, advise on stop-smoking medication [A1]), and other supportive BCTs that facilitate information gathering (eg, assess withdrawal symptoms [RI4]) and communication (eg, elicit and answer questions [RC3]). Due to resource constraints, the participants were informed that the counselor could only interact with them during office hours. The Supplementary Material shows some selected WhatsApp conversations from the trial on how BCTs were applied.
Control Group
In addition to standard smoking cessation treatment, the control group received 18 text messages with the same duration (12 weeks) and tapering schedule as the regular instant messages in the intervention group. The messages covered generic information about the hazards of smoking and benefits of quitting.
Outcome Measures
Participant data were collected by questionnaire at baseline and telephone interview at 3 and 6 months after intervention initiation. Nicotine dependence was assessed before quitting by Fagerström Test for Nicotine Dependence.
To assess feasibility, we calculated the recruitment rate (number of subjects randomized/ number of eligible subjects) and retention rate (number of subjects responded to follow-up/ number of subjects randomized) at 3 months and 6 months. The use and acceptability of the intervention were assessed using the conversation log retrieved from the instant messaging app; we counted the number of participants who responded to the chat message at least once during the 3-month intervention period and those who blocked the contact with the counselor in the instant messaging app. Continuous engagement was defined as having responded to the chat message at least once in the first, second, and third month of the intervention. In both groups, we also assessed the perceived usefulness of the chat/ text message in promoting (1) motivation to quit, (2) knowledge in preventing relapse, and (3) perception of being supported by others, each on a scale of 1 (not useful at all) to 5 (very useful).
False reporting of abstinence has been reported in RCTs that involved intensive treatment16 and digital health intervention.17 Therefore, the primary outcome for examining intervention effectiveness was biochemically validated abstinence at 6 months after intervention initiation,18 confirmed by an exhaled carbon monoxide level of 3 parts per million or below. This cutoff gives a lower rate of misclassifying smokers as abstainers (3%) than the usual cut-offs of 8 ppm (14%) and 10 ppm (21%).19 Since blinding of the participants was not possible in this trial, biochemical validation could also reduce potential performance bias and strengthen the validity of the findings. Participants who reported 7-day point-prevalence abstinence (PPA) at 6 months were invited to participate in the test and reimbursed HK$300 (≈US$38.5) for their traveling and time expense.
Secondary outcomes were self-reported, including 6-month prolonged abstinence with no more than 5 lapses permitted at 6 months; 7-day PPA at 3 and 6 months; and relapse, defined as the use of any tobacco product for 7 consecutive days, at 3 and 6 months.18 Other outcomes included change in self-efficacy to quit smoking, assessed by the Chinese version of the Smoking Self-Efficacy Questionnaire 12,20 and tobacco withdrawal, assessed by the Chinese version of the Minnesota Tobacco Withdrawal Scale.21
Data Analyses
Since this was a pilot trial, a formal sample size calculation was not conducted. A sample size of 100 participants was deemed sufficient to assess the feasibility and inform the sample size of a definitive trial.
Formal hypothesis testing for intervention effectiveness is not recommended in pilot trials, which are typically underpowered to detect statistical significance.14, 22 Nonetheless, we compared the primary outcome of 6-month biochemically validated abstinence between the two groups to examine potential effectiveness and to estimate the effect size required for sample size calculation in a full-scale trial. The analysis was preliminary and should be interpreted with caution. We used Poisson regression with robust variance to calculate the RR of the intervention effect,23 adjusting for baseline characteristics that predict smoking cessation.24,25 These included pre-quit nicotine dependence,26 duration of abstinence,27 and concurrent cessation treatment.28 The denominators included all randomized participants (ie, intention-to-treat), assuming participants with missing data were non-abstinent. Complete case analyses were also conducted by excluding participants with missing outcomes. All feasibility outcomes and secondary outcomes were reported descriptively using mean ± SD or number of participants (%) as appropriate. All analyses were conducted in Stata/MP version 15.1.
Results
From June 2 to July 31, 2020, 108 of 130 (83.1%) eligible subjects provided consent and were randomized into either the intervention (n = 54) or control group (n = 54) (Figure 1). The participants were mostly male (75.0%; n = 81), with a mean ± SD age of 45.1 ± 10.9 years. Table 1 shows that there were significant between-group differences in duration of abstinence (p = .026) and heated tobacco product use (p = .027). The mean score on Fagerström Test for Nicotine Dependence and the proportion of participants who received varenicline/bupropion were greater in the intervention group than in the control group, but the differences were not significant (p > .17).
| . | Total (N = 108) . | Intervention group (N = 54) . | Control group (N = 54) . | p value . |
|---|---|---|---|---|
| Mean ± SD age, years | 45.1 ± 10.9 | 46.2 ± 11.0 | 44.0 ± 10.9 | .32 |
| Sex | ||||
| Male | 81 (75.0%) | 41 (75.9%) | 40 (74.1%) | |
| Female | 27 (25.0%) | 13 (24.1%) | 14 (25.9%) | |
| Education level | .64 | |||
| Junior secondary | 35 (33.0%) | 15 (28.8%) | 20 (37.0%) | |
| Senior secondary | 41 (38.7%) | 22 (42.3%) | 19 (35.2%) | |
| Tertiary | 30 (28.3%) | 15 (28.8%) | 15 (27.8%) | |
| Mean ± SD duration of smoking, years | 27.4 ± 11.9 | 27.7 ± 12.3 | 27.1 ± 11.6 | .80 |
| Mean ± SD cigarette per day | 20.8 ± 9.8 | 21.2 ± 9.9 | 20.4 ± 9.8 | |
| Mean ± SD score on Fagerström Test for Nicotine Dependencea | 5.6 ± 2.4 | 5.9 ± 2.4 | 5.3 ± 2.4 | .17 |
| Previous 24-hour quit attempt | .17 | |||
| Within 12 months | 17 (15.7%) | 10 (18.5%) | 7 (13.0%) | |
| More than 12 months ago | 80 (74.1%) | 36 (66.7%) | 44 (81.5%) | |
| Never | 11 (10.2%) | 8 (14.8%) | 3 (5.6%) | |
| Mean ± SD duration of abstinence, days | 11.8 ± 8.0 | 10.1 ± 6.3 | 13.5 ± 9.1 | .026 |
| Current cessation treatment | .28 | |||
| Behavioral support only | 3 (2.8%) | 2 (3.7%) | 1 (1.9%) | |
| NRT monotherapy | 66 (61.1%) | 29 (53.7%) | 37 (68.5%) | |
| Combined NRT | 13 (12.0%) | 6 (11.1%) | 7 (13.0%) | |
| Varenicline/Bupropion | 26 (24.1%) | 17 (31.5%) | 9 (16.7%) | |
| Use of heated tobacco productb | .027 | |||
| Never | 76 (70.4%) | 32 (59.3%) | 44 (81.4%) | |
| Not in the past 30 days | 20 (18.5%) | 15 (27.8%) | 5 (9.3%) | |
| Past 30 day | 12 (11.1%) | 7 (13.0%) | 5 (9.3%) |
| . | Total (N = 108) . | Intervention group (N = 54) . | Control group (N = 54) . | p value . |
|---|---|---|---|---|
| Mean ± SD age, years | 45.1 ± 10.9 | 46.2 ± 11.0 | 44.0 ± 10.9 | .32 |
| Sex | ||||
| Male | 81 (75.0%) | 41 (75.9%) | 40 (74.1%) | |
| Female | 27 (25.0%) | 13 (24.1%) | 14 (25.9%) | |
| Education level | .64 | |||
| Junior secondary | 35 (33.0%) | 15 (28.8%) | 20 (37.0%) | |
| Senior secondary | 41 (38.7%) | 22 (42.3%) | 19 (35.2%) | |
| Tertiary | 30 (28.3%) | 15 (28.8%) | 15 (27.8%) | |
| Mean ± SD duration of smoking, years | 27.4 ± 11.9 | 27.7 ± 12.3 | 27.1 ± 11.6 | .80 |
| Mean ± SD cigarette per day | 20.8 ± 9.8 | 21.2 ± 9.9 | 20.4 ± 9.8 | |
| Mean ± SD score on Fagerström Test for Nicotine Dependencea | 5.6 ± 2.4 | 5.9 ± 2.4 | 5.3 ± 2.4 | .17 |
| Previous 24-hour quit attempt | .17 | |||
| Within 12 months | 17 (15.7%) | 10 (18.5%) | 7 (13.0%) | |
| More than 12 months ago | 80 (74.1%) | 36 (66.7%) | 44 (81.5%) | |
| Never | 11 (10.2%) | 8 (14.8%) | 3 (5.6%) | |
| Mean ± SD duration of abstinence, days | 11.8 ± 8.0 | 10.1 ± 6.3 | 13.5 ± 9.1 | .026 |
| Current cessation treatment | .28 | |||
| Behavioral support only | 3 (2.8%) | 2 (3.7%) | 1 (1.9%) | |
| NRT monotherapy | 66 (61.1%) | 29 (53.7%) | 37 (68.5%) | |
| Combined NRT | 13 (12.0%) | 6 (11.1%) | 7 (13.0%) | |
| Varenicline/Bupropion | 26 (24.1%) | 17 (31.5%) | 9 (16.7%) | |
| Use of heated tobacco productb | .027 | |||
| Never | 76 (70.4%) | 32 (59.3%) | 44 (81.4%) | |
| Not in the past 30 days | 20 (18.5%) | 15 (27.8%) | 5 (9.3%) | |
| Past 30 day | 12 (11.1%) | 7 (13.0%) | 5 (9.3%) |
NRT = nicotine replacement therapy; SD = standard deviation.
aPossible scores ranged from 0 to 10, with higher scores indicating greater nicotine dependence.
bTobacco products that generate aerosol for inhalation by heating processed tobacco leaf.
| . | Total (N = 108) . | Intervention group (N = 54) . | Control group (N = 54) . | p value . |
|---|---|---|---|---|
| Mean ± SD age, years | 45.1 ± 10.9 | 46.2 ± 11.0 | 44.0 ± 10.9 | .32 |
| Sex | ||||
| Male | 81 (75.0%) | 41 (75.9%) | 40 (74.1%) | |
| Female | 27 (25.0%) | 13 (24.1%) | 14 (25.9%) | |
| Education level | .64 | |||
| Junior secondary | 35 (33.0%) | 15 (28.8%) | 20 (37.0%) | |
| Senior secondary | 41 (38.7%) | 22 (42.3%) | 19 (35.2%) | |
| Tertiary | 30 (28.3%) | 15 (28.8%) | 15 (27.8%) | |
| Mean ± SD duration of smoking, years | 27.4 ± 11.9 | 27.7 ± 12.3 | 27.1 ± 11.6 | .80 |
| Mean ± SD cigarette per day | 20.8 ± 9.8 | 21.2 ± 9.9 | 20.4 ± 9.8 | |
| Mean ± SD score on Fagerström Test for Nicotine Dependencea | 5.6 ± 2.4 | 5.9 ± 2.4 | 5.3 ± 2.4 | .17 |
| Previous 24-hour quit attempt | .17 | |||
| Within 12 months | 17 (15.7%) | 10 (18.5%) | 7 (13.0%) | |
| More than 12 months ago | 80 (74.1%) | 36 (66.7%) | 44 (81.5%) | |
| Never | 11 (10.2%) | 8 (14.8%) | 3 (5.6%) | |
| Mean ± SD duration of abstinence, days | 11.8 ± 8.0 | 10.1 ± 6.3 | 13.5 ± 9.1 | .026 |
| Current cessation treatment | .28 | |||
| Behavioral support only | 3 (2.8%) | 2 (3.7%) | 1 (1.9%) | |
| NRT monotherapy | 66 (61.1%) | 29 (53.7%) | 37 (68.5%) | |
| Combined NRT | 13 (12.0%) | 6 (11.1%) | 7 (13.0%) | |
| Varenicline/Bupropion | 26 (24.1%) | 17 (31.5%) | 9 (16.7%) | |
| Use of heated tobacco productb | .027 | |||
| Never | 76 (70.4%) | 32 (59.3%) | 44 (81.4%) | |
| Not in the past 30 days | 20 (18.5%) | 15 (27.8%) | 5 (9.3%) | |
| Past 30 day | 12 (11.1%) | 7 (13.0%) | 5 (9.3%) |
| . | Total (N = 108) . | Intervention group (N = 54) . | Control group (N = 54) . | p value . |
|---|---|---|---|---|
| Mean ± SD age, years | 45.1 ± 10.9 | 46.2 ± 11.0 | 44.0 ± 10.9 | .32 |
| Sex | ||||
| Male | 81 (75.0%) | 41 (75.9%) | 40 (74.1%) | |
| Female | 27 (25.0%) | 13 (24.1%) | 14 (25.9%) | |
| Education level | .64 | |||
| Junior secondary | 35 (33.0%) | 15 (28.8%) | 20 (37.0%) | |
| Senior secondary | 41 (38.7%) | 22 (42.3%) | 19 (35.2%) | |
| Tertiary | 30 (28.3%) | 15 (28.8%) | 15 (27.8%) | |
| Mean ± SD duration of smoking, years | 27.4 ± 11.9 | 27.7 ± 12.3 | 27.1 ± 11.6 | .80 |
| Mean ± SD cigarette per day | 20.8 ± 9.8 | 21.2 ± 9.9 | 20.4 ± 9.8 | |
| Mean ± SD score on Fagerström Test for Nicotine Dependencea | 5.6 ± 2.4 | 5.9 ± 2.4 | 5.3 ± 2.4 | .17 |
| Previous 24-hour quit attempt | .17 | |||
| Within 12 months | 17 (15.7%) | 10 (18.5%) | 7 (13.0%) | |
| More than 12 months ago | 80 (74.1%) | 36 (66.7%) | 44 (81.5%) | |
| Never | 11 (10.2%) | 8 (14.8%) | 3 (5.6%) | |
| Mean ± SD duration of abstinence, days | 11.8 ± 8.0 | 10.1 ± 6.3 | 13.5 ± 9.1 | .026 |
| Current cessation treatment | .28 | |||
| Behavioral support only | 3 (2.8%) | 2 (3.7%) | 1 (1.9%) | |
| NRT monotherapy | 66 (61.1%) | 29 (53.7%) | 37 (68.5%) | |
| Combined NRT | 13 (12.0%) | 6 (11.1%) | 7 (13.0%) | |
| Varenicline/Bupropion | 26 (24.1%) | 17 (31.5%) | 9 (16.7%) | |
| Use of heated tobacco productb | .027 | |||
| Never | 76 (70.4%) | 32 (59.3%) | 44 (81.4%) | |
| Not in the past 30 days | 20 (18.5%) | 15 (27.8%) | 5 (9.3%) | |
| Past 30 day | 12 (11.1%) | 7 (13.0%) | 5 (9.3%) |
NRT = nicotine replacement therapy; SD = standard deviation.
aPossible scores ranged from 0 to 10, with higher scores indicating greater nicotine dependence.
bTobacco products that generate aerosol for inhalation by heating processed tobacco leaf.
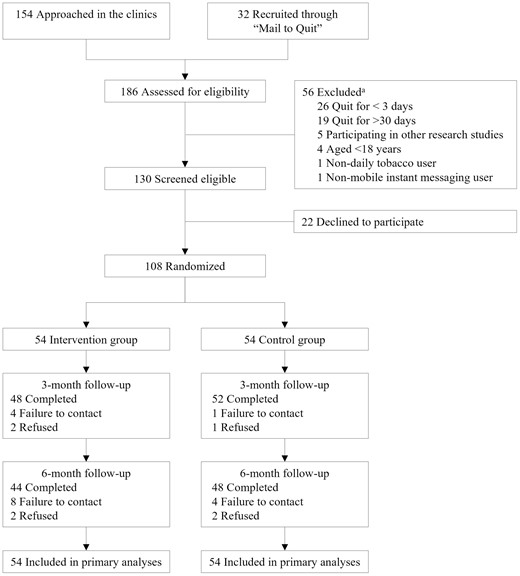
CONSORT diagram. aSome subjects had more than two reasons for exclusion.
The retention rates were 92.6% (100 of 108) at 3 months and 85.2% (92 of 108) at 6 months, and the differences between the two groups were not significant (p > .14). Table 2 shows that, by intention-to-treat, the primary outcome of biochemically validated abstinence rate at 6 months was 31.4% (17 of 54) in the intervention group versus 22.2% (12 of 54) in the control group, with RR of 1.72 (95% CI .91–3.23; p = .094) adjusting for pre-quit nicotine dependence, duration of abstinence and concurrent treatment at baseline. The results from complete case analyses were similar (RR=1.86; 95% CI 0.99–3.49; p = .054). The participation rate in the in-person biochemical validation test was 46.3% (31 of 67), and 1 participant in each group did not pass the validation.
The Primary Outcome of Biochemically Validated Abstinence at 6 Months in Both Groups
| . | Biochemically validated abstinence at 6 months, n/N (%) . | . | RR (95% CI) . | . |
|---|---|---|---|---|
| Intervention group | Control group | Crude model | Adjusted modela | |
| Intention-to-treatb | 17/54 (31.4%) | 12/54 (22.2%) | 1.42 (0.75–2.68) | 1.72 (0.91–3.23) |
| Complete case analysesc | 17/44 (38.6%) | 12/48 (25.0%) | 1.55 (0.83–2.87) | 1.86 (0.99–3.49) |
| . | Biochemically validated abstinence at 6 months, n/N (%) . | . | RR (95% CI) . | . |
|---|---|---|---|---|
| Intervention group | Control group | Crude model | Adjusted modela | |
| Intention-to-treatb | 17/54 (31.4%) | 12/54 (22.2%) | 1.42 (0.75–2.68) | 1.72 (0.91–3.23) |
| Complete case analysesc | 17/44 (38.6%) | 12/48 (25.0%) | 1.55 (0.83–2.87) | 1.86 (0.99–3.49) |
CI = confidence interval; RR = relative risk.
aAdjusted for pre-quit nicotine dependence, duration of abstinence, and cessation treatment at baseline.
b Included all randomized participants; those with missing outcome were assumed to be non-abstinent.
cRandomized participants with missing outcomes were excluded.
The Primary Outcome of Biochemically Validated Abstinence at 6 Months in Both Groups
| . | Biochemically validated abstinence at 6 months, n/N (%) . | . | RR (95% CI) . | . |
|---|---|---|---|---|
| Intervention group | Control group | Crude model | Adjusted modela | |
| Intention-to-treatb | 17/54 (31.4%) | 12/54 (22.2%) | 1.42 (0.75–2.68) | 1.72 (0.91–3.23) |
| Complete case analysesc | 17/44 (38.6%) | 12/48 (25.0%) | 1.55 (0.83–2.87) | 1.86 (0.99–3.49) |
| . | Biochemically validated abstinence at 6 months, n/N (%) . | . | RR (95% CI) . | . |
|---|---|---|---|---|
| Intervention group | Control group | Crude model | Adjusted modela | |
| Intention-to-treatb | 17/54 (31.4%) | 12/54 (22.2%) | 1.42 (0.75–2.68) | 1.72 (0.91–3.23) |
| Complete case analysesc | 17/44 (38.6%) | 12/48 (25.0%) | 1.55 (0.83–2.87) | 1.86 (0.99–3.49) |
CI = confidence interval; RR = relative risk.
aAdjusted for pre-quit nicotine dependence, duration of abstinence, and cessation treatment at baseline.
b Included all randomized participants; those with missing outcome were assumed to be non-abstinent.
cRandomized participants with missing outcomes were excluded.
Table 3 shows that about half of the participants in both groups reported being abstinent throughout the 6-month follow-up period. Scores on Minnesota Tobacco Withdrawal Scale decreased in both groups from baseline to 3 and 6 months, indicating a continued reduction in nicotine withdrawal symptoms. Scores on Smoking Self-Efficacy Questionnaire 12 increased in both groups from baseline to 3 months and sustained at 6 months, indicating increased self-efficacy to resist smoking.
| . | Intervention group (n = 54) . | Control group (n = 54) . | p value . |
|---|---|---|---|
| Self-reported 6-month prolonged abstinence | 26 (48.1%) | 27 (50.0%) | .85 |
| Self-reported 7-day point-prevalent abstinence | |||
| 3 months | 39 (72.2%) | 39 (72.2%) | 1.00 |
| 6 months | 31 (57.4%) | 36 (66.7%) | .32 |
| Relapse ratea | |||
| 3 months | 10 (18.5%) | 11 (20.4%) | .81 |
| 6 months | 22 (40.7%) | 21 (38.9%) | .84 |
| Mean ± SD score on Minnesota Tobacco Withdrawal Scaleb | |||
| Baseline | 11.1 ± 7.0 | 10.1 ± 7.3 | .43 |
| 3 months | 6.4 ± 7.0 | 5.6 ± 6.3 | .96 |
| 6 months | 5.4 ± 7.5 | 3.7 ± 5.8 | .64 |
| Mean ± SD score on Smoking Self-Efficacy Questionnaire 12c | |||
| Baseline | 30.6 ± 11.6 | 30.6 ± 10.8 | .99 |
| 3 months | 48.2 ± 9.7 | 48.9 ± 9.8 | .78 |
| 6 months | 49.0 ± 13.2 | 51.7 ± 11.2 | .33 |
| . | Intervention group (n = 54) . | Control group (n = 54) . | p value . |
|---|---|---|---|
| Self-reported 6-month prolonged abstinence | 26 (48.1%) | 27 (50.0%) | .85 |
| Self-reported 7-day point-prevalent abstinence | |||
| 3 months | 39 (72.2%) | 39 (72.2%) | 1.00 |
| 6 months | 31 (57.4%) | 36 (66.7%) | .32 |
| Relapse ratea | |||
| 3 months | 10 (18.5%) | 11 (20.4%) | .81 |
| 6 months | 22 (40.7%) | 21 (38.9%) | .84 |
| Mean ± SD score on Minnesota Tobacco Withdrawal Scaleb | |||
| Baseline | 11.1 ± 7.0 | 10.1 ± 7.3 | .43 |
| 3 months | 6.4 ± 7.0 | 5.6 ± 6.3 | .96 |
| 6 months | 5.4 ± 7.5 | 3.7 ± 5.8 | .64 |
| Mean ± SD score on Smoking Self-Efficacy Questionnaire 12c | |||
| Baseline | 30.6 ± 11.6 | 30.6 ± 10.8 | .99 |
| 3 months | 48.2 ± 9.7 | 48.9 ± 9.8 | .78 |
| 6 months | 49.0 ± 13.2 | 51.7 ± 11.2 | .33 |
SD = standard deviation.
aDefined as having smoked for seven consecutive days after baseline.
bPossible scores ranged from 0 to 35, with higher scores indicating greater tobacco withdrawal symptoms.
cPossible scores ranged from 12 to 60, with higher scores indicating greater self-efficacy to resist smoking.
| . | Intervention group (n = 54) . | Control group (n = 54) . | p value . |
|---|---|---|---|
| Self-reported 6-month prolonged abstinence | 26 (48.1%) | 27 (50.0%) | .85 |
| Self-reported 7-day point-prevalent abstinence | |||
| 3 months | 39 (72.2%) | 39 (72.2%) | 1.00 |
| 6 months | 31 (57.4%) | 36 (66.7%) | .32 |
| Relapse ratea | |||
| 3 months | 10 (18.5%) | 11 (20.4%) | .81 |
| 6 months | 22 (40.7%) | 21 (38.9%) | .84 |
| Mean ± SD score on Minnesota Tobacco Withdrawal Scaleb | |||
| Baseline | 11.1 ± 7.0 | 10.1 ± 7.3 | .43 |
| 3 months | 6.4 ± 7.0 | 5.6 ± 6.3 | .96 |
| 6 months | 5.4 ± 7.5 | 3.7 ± 5.8 | .64 |
| Mean ± SD score on Smoking Self-Efficacy Questionnaire 12c | |||
| Baseline | 30.6 ± 11.6 | 30.6 ± 10.8 | .99 |
| 3 months | 48.2 ± 9.7 | 48.9 ± 9.8 | .78 |
| 6 months | 49.0 ± 13.2 | 51.7 ± 11.2 | .33 |
| . | Intervention group (n = 54) . | Control group (n = 54) . | p value . |
|---|---|---|---|
| Self-reported 6-month prolonged abstinence | 26 (48.1%) | 27 (50.0%) | .85 |
| Self-reported 7-day point-prevalent abstinence | |||
| 3 months | 39 (72.2%) | 39 (72.2%) | 1.00 |
| 6 months | 31 (57.4%) | 36 (66.7%) | .32 |
| Relapse ratea | |||
| 3 months | 10 (18.5%) | 11 (20.4%) | .81 |
| 6 months | 22 (40.7%) | 21 (38.9%) | .84 |
| Mean ± SD score on Minnesota Tobacco Withdrawal Scaleb | |||
| Baseline | 11.1 ± 7.0 | 10.1 ± 7.3 | .43 |
| 3 months | 6.4 ± 7.0 | 5.6 ± 6.3 | .96 |
| 6 months | 5.4 ± 7.5 | 3.7 ± 5.8 | .64 |
| Mean ± SD score on Smoking Self-Efficacy Questionnaire 12c | |||
| Baseline | 30.6 ± 11.6 | 30.6 ± 10.8 | .99 |
| 3 months | 48.2 ± 9.7 | 48.9 ± 9.8 | .78 |
| 6 months | 49.0 ± 13.2 | 51.7 ± 11.2 | .33 |
SD = standard deviation.
aDefined as having smoked for seven consecutive days after baseline.
bPossible scores ranged from 0 to 35, with higher scores indicating greater tobacco withdrawal symptoms.
cPossible scores ranged from 12 to 60, with higher scores indicating greater self-efficacy to resist smoking.
In the intervention group, 43 of 54 (79.6%) participants responded to the chat messages at least once during the 3 months intervention period; 23 of 54 (42.6%) responded to the chat messages at least once in the first, second, and third month of the chat messages, suggesting continued engagement with the intervention. Validated abstinence at 6 months was higher in those with continued engagement with chat messaging versus those without (39.1% vs 25.8%; p = .30). No participant blocked the contact with the counselor in WhatsApp. Compared with the control group, the intervention group had slightly higher ratings on the perceived usefulness of the messages in promoting motivation to quit (3.5 vs 3.2; p = .17), knowledge in preventing relapse (3.4 vs 3.2; p = .39), and perception of being supported by others (4.0 vs 3.8; p = .42) on a scale of 1 to 5.
Discussion
We have first reported a pilot RCT of personalized chat messaging intervention for relapse prevention, which was an adaptation to the ongoing COVID-19 pandemic for maintaining remote cessation support despite disruptions to clinic-based services. The high recruitment rate (83%) and retention rates at 3 months (end of intervention; 93%) and 6 months (85%) indicated the feasibility of recruiting and retaining recent abstainers under the pandemic. We also found a higher 6-month biochemically validated abstinence in recent abstainers who received chat messaging on relapse prevention than the controls. The effect size of about 50% to 70% increase in validated abstinence based on the crude and adjusted models was consistent with prior trials of text messaging for smoking cessation in smokers.3 Nonetheless, the evidence was preliminary given the small sample and the pilot trial design. Fully-powered trials are warranted to establish the intervention’s effectiveness.
Most (79.6%) participants in the intervention group responded to the chat messaging at least once, and 42.6% responded over the 3-month intervention period. These figures were high compared with the intervention engagement rate (17%) observed in our previous trial of chat messaging for promoting quitting in smokers in the community.8 The discrepancy likely reflected the greater commitment to quit among participants in the pilot trial, as they had already achieved short-term abstinence, than smokers proactively recruited in the community. The higher 6-month validated abstinence rate in those who continued to interact with the counselor via WhatsApp versus those who did not (39.1% vs 25.8%) also corroborate prior findings that mHealth intervention engagement was associated with increased abstinence.8,29 Perceived usefulness of the messages and perceived psychosocial support, which has been found mediating the effect of text messaging on quitting,30 were also higher in the intervention group than the control group. These results supported the acceptability of chat messaging for relapse prevention.
An experimental study showed that communicating the increased risk of COVID-19 vulnerability in smokers could discourage smoking.31 Although the regular messages in the intervention group included information about the link between smoking and COVID-19 risk, it is not possible to determine their contribution to the cessation outcomes relative to other messages on relapse prevention.
We had considered and decided against offering no additional intervention beyond usual care (ie, behavioral support and pharmacotherapy) in the control group. Using a usual care control might lead to greater performance bias and differential attrition because of the imbalanced contact time and attention given to the participants. Therefore, the control group also received duration-matched text messaging as attention control. Since text messaging support is not a regular cessation practice and may also increase abstinence in the control group,3 this approach might have led to underestimation of the real-word effectiveness of chat messaging for relapse prevention.
This trial had some limitations. First, the pilot trial was purposefully small for examining the feasibility and acceptability of delivering chat messaging to recent abstainers and thus underpowered to detect effectiveness. We analyzed the intervention effect on validated abstinence for planning a full-scale trial; the results should not be used as definite evidence to inform clinical practice. Second, there were some imbalances in baseline characteristics, which are common in small trials.14 However, adjusting for baseline characteristics did not attenuate the effect size. Third, despite the higher validated quit rate in the intervention than the control group, the self-reported secondary outcomes showed no or small differences. The telephone follow-up rates were lower in the intervention than the control group (81.5% vs 88.9% at 6 months). This, coupled with the use of intention-to-treat analysis with missing outcome imputed as smoking, gave a more conservative estimate of self-reported outcomes in the intervention group and thus between-group differences. Finally, our participants were mostly males, which reflected the male predominance of smoking in Hong Kong and Asia.32 The generalizability of the findings to other populations is uncertain.
While ample evidence has shown the effectiveness of text messaging support for smoking cessation, our trial provided initial evidence to support chat messaging in preventing smoking relapse, an understudied problem. If confirmed by fully-powered trials, chat messaging could be implemented to maintain continuous relapse prevention support under the COVID-19 pandemic, which is expected to last for a prolonged period. With its high scalability and accessibility, chat messaging could also address health disparity related to the low coverage of traditional in-person cessation treatment in remote or resource-poor settings, where the development of information and communicate technologies often outpace that of health infrastructure. The findings could also inform the development of chatbots (computer programs that can simulate inter-personal conversations) to provide low-cost, automated chat messaging relapse prevention support.
Smoking relapse is the most likely outcome of smoking cessation attempts and an undertreated problem. This pilot trial showed the feasibility and acceptability of personalized chat messaging via WhatsApp for relapse prevention in recent abstainers amid the COVID-19 pandemic. The higher carbon monoxide-validated abstinence rate in participants who received chat messaging than controls showed preliminary evidence on the effectiveness of the intervention.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://dbpia.nl.go.kr/ntr.
Acknowledgments
We would like to thank the staff at Tung Wah Group of Hospitals Integrated Centre on Smoking Cessation for their assistance and the participants for their participation in the trial.
Funding
This trial did not receive any funding. Tung Wah Group of Hospitals Integrated Centre on Smoking Cessation was funded by the Tobacco and Alcohol Control Office, Department of Health, Government of Hong Kong Special Administrative Region.
Declaration of Interests
The authors have no conflicts of interest to report.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.




Comments
Do you mean 31% abstinence rate is good enough for people participated in a messaging type of intervention? 69% had not stopped regular smoking! In addition, the control group also had 22% abstinence in usual care.
In addition, 6 months abstinence rate is not a long enough time to evaluate abstinence for smoking, is it?
Is this statistical significance to be a misleading example?