-
PDF
- Split View
-
Views
-
Cite
Cite
Kenneth A Perkins, The 2022 Ferno Award Address: CrEATE, an Efficient Crossover Evaluation of Addiction Treatment Efficacy, Nicotine & Tobacco Research, Volume 25, Issue 1, January 2023, Pages 77–85, https://doi.org/10.1093/ntr/ntac139
Close - Share Icon Share
Abstract
Dozens of drugs have been evaluated in recent decades for initial evidence of efficacy to aid smoking cessation (i.e. “early Phase 2” testing, according to U.S. FDA terminology), with the vast majority failing to show efficacy. Even small randomized clinical trials (RCTs), the most common early Phase 2 tests, are costly undertakings, made more unappealing by their high likelihood of failure. At the same time, another early Phase 2 approach, acute tests of drug effects on surrogate endpoints such as withdrawal or craving severity, are more practical but have little predictive clinical validity. Described here is an innovative procedure that optimally combines the validity of clinical trials with the practical advantages of surrogate endpoint studies to more efficiently determine whether or not a novel drug warrants continued clinical development. This CrEATE procedure, or Crossover Evaluation of Addiction Treatment Efficacy, does so by assessing short-term quit success in smokers highly motivated to quit when briefly treated with active drug versus placebo in a crossover design, so that quit efficacy from both conditions is compared within participants. The program to develop and evaluate CrEATE demonstrates its sensitivity to efficacy from all three FDA-approved first-line cessation medications (NRT, varenicline, bupropion), tested here as model drugs, as well as specificity in identifying lack of efficacy with a drug known to be ineffective for cessation (modafinil). CrEATE has subsequently been used to evaluate a few novel interventions, concluding they lack efficacy in increasing quit success. Future directions for the potential utility of CrEATE are provided.
Implications: The ability of CrEATE to reach a Go/No Go decision more quickly and with far less cost lowers the risk of failure, meaning widespread use of the procedure should encourage the evaluation of more novel candidate drugs. With its greater efficiency, failed tests, unfortunately the most likely outcome in early Phase 2 studies, will cause less waste of resources. At the same time, CrEATE tests that indicate a novel treatment has efficacy will justify the substantial time and expense of moving forward to evaluate the drug in late Phase 2 RCTs.
Introduction
This paper is adapted from an address given at the 2022 SRNT meeting after receiving the Ove Ferno Award for “groundbreaking advances in clinical research.” It outlines the rationale and validation of a new procedure to more efficiently evaluate new treatments for initial evidence of efficacy in aiding smoking cessation, a focus of clinical research very relevant to the career of Ove Ferno,1 for whom the award is named. As explained in this paper, such a procedure should accelerate progress by more quickly and inexpensively identifying compounds that do, or do not, warrant the substantial expenditure of resources and time to formally test them in large randomized clinical trials (RCTs).
Despite a very steady decline in smoking prevalence in the UnitedStates. over the last 60 years, nearly 40 million Americans still smoke, and the number of tobacco users worldwide increased over that same period to nearly 1.2 billion adults, or a prevalence of about 20%. The global death toll now stands at almost 7.7 million per year, one every 4 seconds, and is likely to continue rising over the next few decades, to 10 million annually.2 The annual economic costs of treating smoking-attributable diseases globally now totals more than one-half trillion dollars (U.S.).3 Given the widely recognized risks of smoking, long making it the greatest preventable cause of mortality, it is not surprising that most smokers express a desire to quit. Yet, relatively few actually follow through and attempt to stop in any given year, and those who do try often experience rapid failure and relapse back to smoking. Thus, sustained quit success with individual attempts to quit is atypical, even with the most effective current treatments.4,5 The likely persistence of high health costs due to tobacco use, particularly via combustible cigarette smoking, makes it imperative that new treatments continue to be developed and evaluated, along with other tobacco control policies, if a substantial decline in the burden is to be a reality.6,7
Rationale for New Procedure
As with the entire drug development process faced by pharmaceutical companies, evaluating the efficacy of novel drugs for treating smoking cessation can be viewed as an exercise in failure, not unlike the outcomes of individual quit attempts. Across all treatment indications for all new experimental drugs, it is estimated that only one of roughly 30 novel compounds will succeed through all phases of development required by the Food and Drug Administration (FDA) to become a newly approved medication. Those phases start with preclinical testing in nonhuman animal models, followed by submission of the Investigational New Drug (IND) application and “first in human” Phase 1 testing of safety/toxicity, on to early and late Phase 2 tests of efficacy of the drug for the intended indication (i.e. health problem being treated), through to Phase 3 testing to broadly confirm that clinical efficacy with diverse patient subgroups, and finally approval for marketing.8 (Not relevant here is Phase 4 post-marketing to evaluate additional information on side effects, prescribing methods, etc.) Typically, one-third of experimental compounds will fail in preclinical testing and another third will fail in Phase 1 safety testing, all before any evaluation of the drug’s clinical efficacy in treating humans.9,10 And yet, despite that sharp decline in viable compounds early on, testing for initial efficacy in Phase 2 poses a far greater hurdle, with even higher risks of failure. Generally, of all drugs succeeding in Phase 1 and reaching Phase 2 testing, 70%–80% are then unsuccessful, mostly due to lack of evidence of efficacy,10–12 and so do not proceed to Phase 3, thus being dropped from further consideration.
This common pattern in the drug development process documents that failure of new drugs to show adequate clinical efficacy in treatment is the rule, rather than the exception. These dismal rates of success across drug development phases apply to all compounds, including drugs intended to treat smoking cessation. Of the several dozen compounds tested for initial evidence of efficacy to treat smoking over the past few decades, only three have been approved as first-line smoking cessation medications (the formulations of nicotine replacement therapy, or NRT, bupropion, varenicline),4,6,13 and no drug candidates evaluated for efficacy have proceeded to FDA approval in over 15 years.
On the other hand, a true “failure” in early Phase 2 testing is important to document. The accurate determination that a novel compound does not show efficacy in Phase 2 to justify continuing the drug to Phase 3 testing is critical to avoid wasting the enormous costs and time of the large, often multi-site trials needed in Phase 3.14 Such commitment of resources is prohibitive unless the drug is very likely to be efficacious with the large and broad patient population tested, as required for FDA approval. Nevertheless, the high rate of failure in Phase 2 tests of efficacy has its own damaging consequences, that of knowing beforehand the low chances of success and realizing the costs required to conduct the vast majority of such trials will be for nought and fail to advance development of a promising new treatment. Current typical Phase 2 evaluations are estimated to involve roughly $10 million and several years per study,15 with a few hundred treatment-seeking smokers (i.e. those intending to quit permanently) completing the trials, depending on the anticipated effect size of the test drug (beyond placebo or other comparison treatment16). With the track record of failure in Phase 2 being so high, the costs required may simply discourage attempts to evaluate some potentially promising drugs by pharma without a compelling justification, such as the proposed mechanism or an unmet market, or by noncommercial academic researchers lacking external funding, etc.
Partly for that reason, short-cuts are occasionally taken during or after success in Phase 1 to try and get an early signal on whether the drug will be efficacious. One approach is conducting pilot trials of smoking cessation with small samples randomized to conditions (i.e. a few dozen treatment-seekers, rather than a few hundred) to cut costs and save time. Yet, small quit trials, by definition, are often inadequately powered to detect a significant difference, or targeted effect size, for dichotomous quit or not quit outcomes due to active versus placebo treatments, leading to possibly unreliable findings about the active drug’s clinical efficacy.9,11
A second typical approach is assessing acute self-report or behavioral measures thought strongly related to the quitting process in response to short-term exposure to drug versus placebo, sometimes as secondary outcomes during short-term Phase 1 safety testing studies. Often called “surrogate” endpoints or measures,17,18 these are usually assessments of withdrawal and craving relief during brief periods of enforced smoking abstinence, or perhaps the magnitude of reduction in ad lib smoking behavior during acute exposure to the treatment conditions, to compare responses between the novel drug versus placebo. Less withdrawal or craving, or ad lib smoking, due to active versus placebo is believed to provide some evidence of a positive “signal” suggestive of clinical efficacy. Because the acute studies in this second approach are not randomized cessation trials (as in the first approach), they are nearly always done with non-quitting smokers (i.e. those not seeking help to quit but rather paid for volunteering), who are usually more practical to recruit for short-term studies. Unfortunately, such surrogate studies are often not clinically predictive, as acute withdrawal or craving responses,19,20 or reductions in ad lib smoking,21,22 due to brief drug versus placebo exposure in those not trying to quit are not robust. As a result, most of these acute assessments of drug effects in non-quitting smokers are not consistent with the cessation efficacy of those drugs administered to treatment-seeking smokers over weeks or months during formal randomized cessation trials,13 as with many surrogate endpoints of health harms from tobacco use.23
Thus, given the very high likelihood of failure in initial tests of clinical efficacy for drugs proceeding from Phase 1 to Phase 2, a more efficient early Phase 2 procedure, requiring lower costs, a smaller sample, and a shorter time to validly determine whether the drug has a positive signal, could produce substantial savings of resources, time, and other costs. Such a procedure was the objective of the primary project to be outlined here, which we label “CrEATE,” for “Crossover Evaluation of Addiction Treatment Efficacy.” We see the goal of a CrEATE study not as replacing all Phase 2 clinical testing and providing the sole determination of whether a novel drug should proceed to Phase 3 testing (and the required very large RCTs). Rather, the goal is to provide a strongly informed Go/No Go decision, quickly and with low cost, about moving forward at all to further evaluate the novel drug of interest, particularly to conducting the first formal RCT (i.e. now in late Phase 2) to compare the active drug’s efficacy in aiding cessation versus placebo. Positive findings (i.e. significant evidence of therapeutic efficacy for cessation) in CrEATE for an active novel drug versus placebo (or other comparison; see “Future Directions”) would predict that proceeding to a late Phase 2 RCT with that drug has a good chance of success, pending other practical issues. Negative findings in a CrEATE study, by contrast, would indicate little likelihood of therapeutic efficacy of that drug in a formal RCT, and argue against proceeding any further to preserve resources for more promising opportunities.
In other words, because the procedure is shorter and much less costly than even a small randomized trial, findings from CrEATE should be highly informative in determining that the novel medication being evaluated is, or is not, sufficiently efficacious for smoking cessation as to warrant the costs and time of late Phase 2 RCT testing. As such, the CrEATE procedure should accelerate medication development by increasing the efficiency by which promising drugs do, and unpromising drugs do not, proceed to the expensive and lengthy RCTs characterizing late Phase 2 (and Phase 3) testing.
CrEATE: Optimizing Advantages of Small RCTs and Surrogate Studies
Design of CrEATE Procedure
We designed this early Phase 2 procedure in a manner to increase its practicality in terms of lower cost and duration while maintaining a valid signal regarding the outcome result identifying the drug’s likely clinical efficacy for cessation. To accomplish this, we sought to combine the validity of adequately powered RCTs with the practical advantages of short-term surrogate endpoint studies. To ensure validity, participants are smokers already stating reliably (in at least two separate assessments) their intention to make a permanent attempt to quit smoking within the next 2–3 months (i.e. very similar to treatment-seekers in RCTs). The primary outcome measure in CrEATE studies is number of days they achieve 24-hour abstinence, confirmed by the stringent biochemical verification cutoff of expired-air carbon monoxide (CO) <5 ppm.24
To enhance practicality, a CrEATE study is much shorter and requires far smaller sample sizes than RCTs. First, the “practice quit” period in which participants attempt to stop smoking is only 4–5 days (following any dose run-up days needed). They are encouraged to learn how to cope with urges to smoke and avoid lapsing during the practice quit period, in preparation for when they make a permanent quit attempt. (As noted below in “Study 1-NRT,” a benefit for volunteers in all CrEATE studies is later receiving a full regimen of open-label medication plus counseling to make a permanent quit attempt after they complete participation in the formal CrEATE study.). Prior large clinical trials with FDA-approved medications have shown that, the greater the number of days “quit” in the first week of treatment after the quit date, the better the quit outcomes at the end of treatment (2–3 months later), as well as at 6-month follow-ups.25 This observation supports other literature demonstrating the longer-term predictive clinical validity of initial quit success.26,27 Using the continuous measure of number of quit days as the primary outcome also greatly enhances statistical power, rather than the dichotomous measure of quit or not quit at specific times during and after treatment (e.g. point-prevalence).28
Second, the sample sizes are smaller because both drug conditions being compared are presented (i.e. active vs. placebo, in counter-balanced order) to all participants in a crossover design, which nevertheless provides adequate power29 and is practical due to the brief “practice” quit periods with both drug conditions. The first drug condition ends after the last day of the week-long quit period. As informed during the consent process prior to their participation, all participants who are still quit by then must resume smoking ad lib during the 1–2 weeks between conditions for drug washout and a return to baseline smoking, prior to the second drug condition and quit period. They are informed of this requirement so that both treatment conditions can be given a fair test of their potential efficacy to aid in quitting. (All CrEATE studies described here were approved by the University of Pittsburgh Institutional Review Board.) This abbreviated evaluation of quit days in each drug condition allows for a short total study duration of only 6 weeks if a drug requires more than a few days for dose run-up, 4 weeks otherwise (one week baseline, one week trying to quit while taking active or placebo). See the timeline in Figure 1 for participants in a standard CrEATE study comparing one active drug (versus placebo) requiring a dose run-up week prior to the practice quit week. (Assessment days during the baseline and dose run-up weeks are flexible, given the need for typical pre-quit measures such as craving, withdrawal, or cigarettes per day, as well as the potential need to monitor initial adverse reactions and/or compliance with the novel drug.)
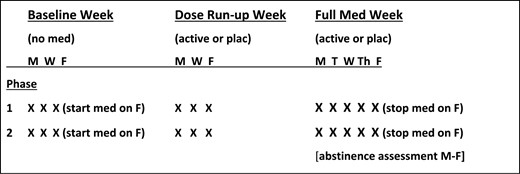
Timeline for participation in a standard CrEATE study testing efficacy in one novel active medication versus placebo. Completing the study consists of two phases, each 3 weeks long, differing only in active versus placebo. Abstinence is assessed daily during the practice quit “Full Med Week” (range of 0–5 quit days per week). Dose run-up week is only needed if the test drug’s pharmacokinetics require it. The frequency of visits before the practice quit week is flexible given other pre-quit assessments of interest. X = assessment/visit.
The crossover design allows for a far smaller sample size, not simply because one group gets both treatments, but due to the high within-subject correlation of ability to quit on active versus placebo. This correlation is high because individual difference characteristics influencing a smoker’s general ability to initiate quitting, such as the severity of nicotine dependence or strength of quit motivation, are identical for each drug condition since the same participants receive both conditions. With identical characteristics of those attempting to quit on active versus placebo, the sole cause for any differences in quit success during the two quit periods must be the drug conditions per se.29 The equation to determine how many participants in a crossover design would have power comparable to the number in an RCT is: NWithin = (1-rho)/2 x NRandomized (rho is the within-subject correlation of quit days during active versus placebo).30 For example, if there was absolutely no association of an individual’s ability to quit with active versus placebo (i.e. only the treatment mattered, not individual differences), rho would be 0, and the sample size for a crossover would simply be one-half that of an RCT, as the same n per condition in the RCT would receive both conditions in the crossover. Yet, our research has confirmed that treatment-seeking smokers consistently and strongly differ individually in their ability to quit regardless of treatment, generally rho =.50. Therefore, in the equation above, (1–0.5)/2, or 0.25, only one-fourth as many, in a crossover sample has power comparable to that of an RCT. Figure 2 illustrates how the CrEATE early Phase 2 procedure may effectively act as a bridge to combine: (1) the practicality of non-clinical Phase 1 testing of a drug’s acute safety in small groups of non-quitting smokers, with (2) the validity of formal randomized clinical trials with larger groups of treatment-seeking smokers in Phase 2 and Phase 3.
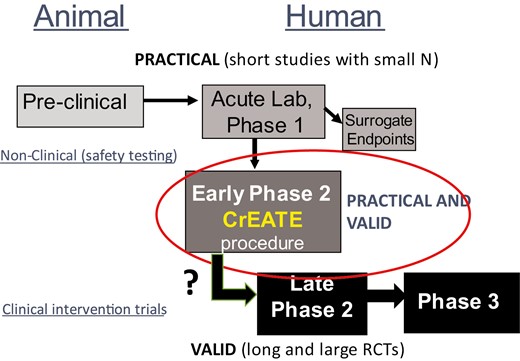
Schematic to illustrate how the CrEATE early Phase 2 procedure may more effectively bridge the practicality of Phase 1 testing in non-quitting smokers with the validity of formal randomized clinical trials (RCTs) of Phase 2 and Phase 3 in quitting smokers.
To summarize the greater efficiency of a CrEATE study versus a standard Phase 2 RCT: with rho = .50, as we have found, a sample size of 50 in CrEATE would have power comparable to an RCT involving about 200 treatment-seeking smokers (100 per condition); study duration for participants is 4–6 weeks in CrEATE versus more than 6 months in an RCT (2–3 months of treatment, follow-up at 6 months post quit date); and estimated cost per study of ≤ $1 million for CrEATE (based on direct costs for our five NIH-funded studies) versus perhaps $10 million (as reported for typical Phase 2 studies for all indications15).
Research to Validate and Finalize the CrEATE Procedure
Aside from confirming validity and feasibility, the primary goal of our project to develop and validate the CrEATE procedure was to identify the optimum participant characteristics that would maximize sensitivity to a medication’s efficacy for smoking cessation relative to placebo. Three studies employed the FDA-approved first-line cessation medications of NRT patch, varenicline, and bupropion, each compared to placebo, to serve as model drugs known to have efficacy over placebo in RCTs. The key question in identifying appropriate study participants was whether these drugs would show efficacy only in those with currently high “intrinsic” quit motivation, as in treatment-seeking smokers, or would even non-treatment seeking smokers low in “intrinsic” motivation, the majority of the smoking population, be sensitive to the drugs’ efficacy. A related question was whether the cause of high quit motivation mattered in an effective medication’s sensitivity for aiding abstinence. In other words, simply paying non-treatment seeking smokers to quit each day, as with contingency management treatment to reinforce abstinence from substance use,31 would increase their immediate quit motivation in “extrinsic” fashion, perhaps providing the same sensitivity to medication efficacy. If so, smokers not seeking treatment but willing to participate in a study offering monetary reinforcement of quitting could greatly simplify recruitment.
Study 1-NRT
Our first study recruited a total of 140 smokers, with high (n = 47) versus low (n = 93) intrinsic quit motivation, defined as planning to quit within the next 2 months versus no interest in quitting within the next 6 months. To attract smokers already intending to quit soon to participate in a crossover study involving only temporary “practice” quit periods, we offered all participants a full complement of open-label NRT patch and brief behavioral counseling to aid them in a permanent quit attempt after completing the study. (This follow-up contact was solely an optional benefit of participating, along with relatively modest payment per visit, as the formal research assessments ended before this post-study treatment contact.) The study design was a mixed between- and within-groups design. Along with the separate recruitment of smokers high versus low in intrinsic quit, each of these subgroups was randomized to high (n = 71) versus low (n = 69) extrinsic quit motivation manipulations, by providing $12 versus 0 per quit day, forming a 2 × 2 between-groups comparison of high/low intrinsic and high/low extrinsic quit motivation.
All received both treatment conditions of 21 mg NRT patch versus placebo patch, in counter-balanced order, for the within-groups crossover comparison of active versus placebo effects on aiding days quit. The two 2-week study phases each involved one week of ad lib smoking baseline followed by one week of patch application every weekday morning while trying to quit smoking, as CO was assessed at every Monday–Friday study visit. Regardless of quit status on Friday, all removed the patch after the visit and resumed ad lib smoking to repeat the two weeks on the other drug condition. The most important factor to assess sensitivity to active medication on increasing quit days was the interaction of each between-groups type of high versus low quit motivation (intrinsic and/or extrinsic) with the within-groups condition of active versus placebo treatment.
Results showed that active NRT versus placebo and high versus low extrinsic quit motivation each had significant main effects in increasing quit days, as expected based on the extensive prior clinical research documenting quit efficacy with NRT and with contingency management intervention. More important, the interaction of NRT versus placebo treatment by high versus low intrinsic quit motivation was highly significant, as hypothesized,32 with the efficacy of NRT patch in aiding quit days found only with smokers high, and not low, in intrinsic quit motivation (treatment-seekers). These differences in sensitivity to NRT versus placebo as a function of intrinsic quit were observed regardless of the high versus low extrinsic quit manipulation (i.e. interaction of NRT versus placebo by high versus low extrinsic quit was not significant). Also not significant was the three-way interaction of NRT versus placebo × high versus low intrinsic × high versus low extrinsic quit motivation conditions.
Study 2-Varenicline
Because any new early Phase 2 procedure would need to be applicable to evaluating efficacy in a variety of new compounds, not just NRT, Study 2 sought to cross-validate these results with a different model drug, varenicline. The goal was to confirm that those with high intrinsic quit motivation are the optimum sample to recruit for CrEATE studies. Thus, conditions virtually identical to those of Study 1 were followed, as smokers high (n = 57) or low (n = 67) in intrinsic quit motivation were randomized to the same high (n = 61) versus low (n = 63) extrinsic quit motivation manipulation. Two 3-week phases were conducted, with an ad lib smoking baseline week preceding a dose run-up week while continuing to smoke ad lib, followed by a week in which all tried to quit each weekday, with CO<5 ppm again assessed daily Mon-Fri to confirm quit status. (Open-label varenicline and counseling were offered to make a permanent quit attempt after their CrEATE study participation was completed.)
Similar to Study 1, the main effects of varenicline versus placebo and high versus low extrinsic quit motivation were significant, as was high versus low intrinsic quit motivation. Again, the interaction of high versus low intrinsic quit by varenicline versus placebo treatment was significant (p = .05), confirming that sensitivity to the efficacy of active medication for quitting was greater in those with high intrinsic quit motivation, regardless of the extrinsic quit manipulation.33 Although the interaction was significant, varenicline treatment did increase quit days over placebo in smokers low in intrinsic quit motivation, perhaps consistent with the widespread clinical observation of varenicline’s robust efficacy as a partial agonist of nicotine receptors in blunting acute reinforcement from nicotine per se.34 Yet, we would argue that such robust efficacy could not be assumed in most novel compounds being evaluated in early Phase 2 testing, again suggesting that use of smokers high in intrinsic quit motivation is essential to ensure a sample sensitive to a drug’s efficacy in aiding cessation.
Study 3-Bupropion and Modafinil
Studies 1 and 2 confirmed that those high in intrinsic quit motivation (i.e. treatment-seeking smokers), were more sensitive to the efficacy of the cessation medications of NRT patch and varenicline within the CrEATE procedure. Yet, we also needed to ensure that CrEATE provided specificity in detecting lack of efficacy in drugs known to be ineffective in aiding cessation. After all, the history of Phase 2 testing shows that nearly 4 out of 5 novel drugs will fail to show efficacy, and one could argue that documenting lack of efficacy will be the more likely outcome of such testing. Accurate detection of failures early in the drug development process is critical to prevent the waste of resources on drugs not likely to warrant proceeding to costly late Phase 2 or Phase 3 evaluation.
Thus, Study 3 examined cessation efficacy from bupropion, the only remaining FDA-approved first-line medication, as well as from modafinil, a drug FDA-approved for wakefulness but shown ineffective for cessation in an RCT.35 These positive (bupropion) and negative control (modafinil) treatment conditions were compared to placebo, forming a double-crossover variation in the within-groups component of the CrEATE procedure. Because of the dose run-up week needed for bupropion and modafinil, this study involved three 3-week phases, or 9 weeks in all, again with drug conditions administered in random order between subjects. Also different from the prior studies, only smokers with high intrinsic quit motivation (n = 45) were recruited in a solely within-groups design, as we had already shown this to be the subgroup most sensitive to cessation medication efficacy. (A third test of the between-groups manipulations of high versus low intrinsic and high versus low extrinsic quit motivations seemed unnecessary. Similar to the prior studies, open-label bupropion and counseling were offered to make a permanent quit attempt after their CrEATE study participation was completed.) As hypothesized, bupropion did, and modafinil did not increase quit days versus placebo, confirming that the CrEATE procedure was again sensitive to another known efficacious quit medication, and it was also specific in identifying lack of efficacy in a known ineffective quit medication.36
Conclusions from CrEATE Development and Validation Project
As shown in Figure 3, this project to evaluate and finalize a more efficient early Phase 2 procedure to detect initial efficacy for aiding smoking cessation in novel compounds confirmed that high intrinsic quit motivation was essential to reliably identify the sensitivity and specificity of active drug versus placebo. We also showed in Studies 1 and 2 that manipulating extrinsic quit motivation did not matter in terms of treatment efficacy (consistent with earlier research37). Thus, efforts to detect efficacy in novel treatments clearly must be conducted in smokers already exhibiting high intrinsic quit motivation; other subgroups of smokers will certainly be less sensitive to any treatments, likely minimizing detection of efficacy, if it exists.38 To facilitate testing in smokers with sufficiently high intrinsic motivation to quit, it may be worthwhile to include motivational interviewing39 for all participants, especially those with only moderate motivation, just before the start of their first week of formal study participation.
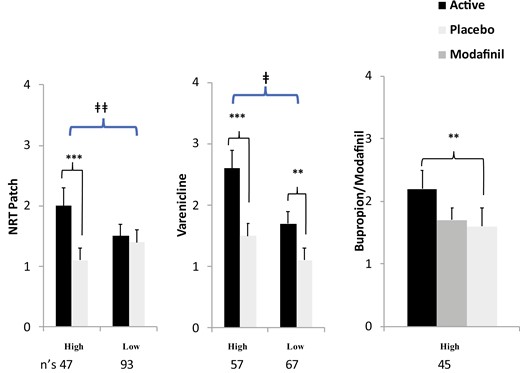
Mean (SE) quit days by drug conditions in smokers with high versus low intrinsic quit motivation. (** p ≤ .01, *** p < .001 for difference from placebo within-groups. ǂ p = .05, ǂǂ p < .01 for drug x quit motivation interaction).
Another conclusion from the project was to confirm the substantial statistical power of the crossover design. For example, just 45 treatment-seeking participants in Study 3 were needed to show a significant efficacy for quit days with bupropion over placebo. The within-subject correlation of days quit with each condition was r =.59, and so the N = 45 in this CrEATE study had power comparable to 220 treatment-seeking smokers, 110 in each condition, in an RCT (i.e. 45 = [(1−.59)/2] × 220). Finally, the feasibility of other methodological details from the CrEATE procedure were documented, such as few missed sessions during the quit weeks (1%) and relatively modest drop-out rates (mean of 22%13), as well as <1% dropping out due to refusal to continue after quitting in the first study phase (just 1, out of 192 high intrinsic quit and 160 low intrinsic quit motivation participants across the three studies). Feasibility is also suggested for the availability of recruiting smokers planning to quit within 2–3 months, as national survey data indicate that 16% of all adult smokers state they intend to quit within the next 1 month and another 35% plan on quitting in more than 1 but less than 12 months.40
Subsequent CrEATE Tests of Initial Efficacy in Novel Drugs
With confirmation that the optimum sample for recruitment into a CrEATE study is smokers with high intrinsic motivation to quit within the next few months, we proceeded to begin using the procedure for its ultimate purpose, to examine initial evidence of efficacy in novel drugs potentially useful to help smokers quit. Our first such test was for repurposing fenofibrate, FDA-approved for lipid control and related to other fibrates, one of which was shown to significantly decrease nicotine self-administration in primate and rodent models.41 We conducted a 4-week CrEATE procedure similar to our NRT study (Study 1, above), with two 2-week phases each involving several days of ad lib smoking baseline and several days of dose run-up, then the quit week. (Bupropion and counseling were offered for making a permanent quit attempt after completing the study.) Results clearly showed no differences between fenofibrate versus placebo on any measure, with nearly 2.0 quit days per week for each treatment condition.42
A second CrEATE study to evaluate a novel compound for evidence of quit efficacy involved an α7 nicotine receptor positive allosteric modulator (PAM), JNJ-39393406, an experimental drug initially developed by Janssen to improve cognitive function in schizophrenia. Galantamine, another α7 PAM, decreased nicotine self-administration in rodents,43 as did this α7 PAM compound (“JNJ”) in preliminary research (D. Brunzell, unpublished observations), consistent with other findings on α7 receptor-based compounds and functions relevant to nicotine reinforcement.44,45 Because this JNJ compound also might moderate cognitive deficits due to nicotine withdrawal, especially in smokers with schizophrenia, we proposed to test its potential efficacy for cessation in smokers high in quit motivation who did or did not (i.e. “healthy”) have schizophrenia. Parallel CrEATE studies were conducted at the Univ of Pittsburgh with separate groups of such smokers participating. Procedures mirrored our earlier Study 2, testing varenicline (above), with two 3-week phases, involving ad lib smoking baseline, dose run-up while ad lib smoking, then the quit week on active JNJ or placebo. (Open-label bupropion and counseling were offered for a permanent quit after completing study participation.) Results again showed no differences in cessation (or in withdrawal) between JNJ and placebo, in both groups of smokers.46
While disappointing, these two CrEATE studies successfully completed the intended objective of early Phase 2 testing, determining whether or not the novel drug had evidence of efficacy for aiding smoking cessation above placebo, and both did so very quickly and efficiently. As previously noted, the past history of Phase 2 testing has shown nearly 80%, or 4 out of 5 novel drugs, will fail to show efficacy and not warrant proceeding to Phase 3 clinical testing, making failure in these two early Phase 2 tests unsurprising. Their lack of efficacy is also likely very reliable, given the sensitivity of this CrEATE procedure to efficacy in known effective cessation medications, although further research on other, related compounds may produce different efficacy outcomes.44,45
Future Directions
Likely Characteristics of Drugs Well Suited to CrEATE Procedure
Among possible future directions for employing CrEATE to more efficiently advance pharmaceutical development, ideal drug candidates to evaluate for efficacy with cessation include repurposing selective medications already approved for other treatment indications.4 Immediate prior examples are bupropion in the 1990s, originally approved as an antidepressant,47 fenofibrate approved for lipid control and then evaluated for cessation with CrEATE above,42 or novel drugs first examined in Phase 2 for other indications but abandoned, as with the JNJ compound we tested for cessation with CrEATE.46 Many others have been evaluated for repurposing with smoking cessation in RCTs but showed little to no efficacy.4 Because drugs for repurposing have completed Phase 1 testing, little additional preparation should be needed to proceed to Phase 2 testing of their efficacy for smoking cessation using our CrEATE procedure.
Other classes of products that might be evaluated for efficacy in aiding cessation of smoking combustible cigarettes include non-smoked (and non-medicinal) nicotine products such as new oral pouches or gums,48 and heat-not-burn tobacco.49 These are in addition to electronic cigarettes, which have already demonstrated some efficacy for smoking cessation in RCTs,50 and thus may be past the point of testing for evidence of initial efficacy in early Phase 2. Also possible may be evaluations of these products for efficacy with harm reduction, or a reduced amount of smoking if not complete abstinence,51 assuming a reliable acute measure of reduced exposure is feasible and predictive of long-term behavior change. (For example, we found similar mean declines of 75% in cigarettes per day and 50% in CO, as well as similar quit days, in high intrinsic quit smokers during the practice quit periods with either fenofibrate or placebo in CrEATE, showing acute reductions in harm with either condition in the study documenting no efficacy for fenofibrate per se.42)
Other Conditions Potentially Suited to Use of CrEATE
Generally, the CrEATE procedure should also be most useful when evaluating the potential efficacy of a drug intended to treat any population of smokers that might be difficult to recruit in large numbers, as necessary for any RCTs. These might include smokers with certain comorbid conditions such as schizophrenia,46 other suspected individual difference characteristics in sensitivity to a drug’s efficacy,52 or smokers differing in specific genotypes of interest (e.g. precision medicine).53,54 These and other hypotheses about subgroup differences in sensitivity to efficacy would warrant adding between-groups factors to the study designs, along with within-groups evaluation of the active versus comparison conditions, as in Studies 1 and 2 here manipulating high versus low intrinsic or extrinsic quit motivations. Yet, each subgroup could be tested using the CrEATE procedure to efficiently compare differences in efficacy between the subgroups (rather than randomizing much larger samples of each subgroup to active or placebo).
Although taking longer to complete than our standard two-comparison CrEATE procedure, the three-comparison double-crossover procedure (as in Study 3, above) may have substantial practical value, by efficiently evaluating efficacy in novel interventions relative to that of comparison treatments (as well as versus placebo). Examples include concurrent comparisons of efficacy between a novel drug versus another active medication of known efficacy that might be a competing alternative treatment in the marketplace; a novel drug more effective than placebo but less effective than an existing medication may not be an attractive option to continue pursuing and so may not warrant further clinical evaluation. Another example could be to evaluate two different doses of a novel compound, to consider which one provides a better balance between efficacy versus risks of adverse events, in preparation for procedures in a planned large RCT. A third example is in evaluating superior efficacy from combination treatments versus monotherapy, such as a novel drug plus a known effective medication, or two effective treatments versus either one alone.55
Potential Refinement and Extension of CrEATE
Especially relevant for studies involving extended durations of participation in the double-crossover variation,36 further streamlining procedures to reduce participant burden may encourage widespread use of CrEATE. For example, daily confirmation of abstinence via CO was obtained in all our studies noted above solely by research assistants during regularly scheduled in-person study visits every weekday during the periods in which participants attempted to quit. Recent technological advances provide simpler validation of daily CO remotely, such as via emailing of videotaped self-assessment of CO56,57 or with new mobile phone-based CO monitors (e.g. iCO58,59), precluding the need for in-person visits simply to check CO values. Remote CO validation of recent smoking exposure should enhance recruitment and minimize drop-outs, given the lower burden of participation, reducing the risk of an underpowered study.11 Such methodology may also allow easier remote assessments of secondary measures during treatment conditions, such as craving and withdrawal.
Beyond these potential new directions in using CrEATE, broader applications of this crossover procedure may be possible. Although thus far used only to evaluate efficacy in drugs for smoking cessation, it may be applicable to evaluating efficacy in drugs for treating other substance dependence problems, such as alcohol, opiates, etc., or even to stop using electronic cigarettes and end all nicotine product use.60 Perhaps speculative is the possibility the CrEATE procedure might be used to evaluate the efficacy of novel non-drug interventions for smoking cessation (or other drug dependence problems), or of behavioral manipulations to improve compliance with a medication regimen.61
However, CrEATE procedures modified for purposes other than evaluating the efficacy of novel drugs for smoking cessation would need to be validated. Some factors may preclude such use of the procedure, if outcome measures such as biochemical confirmation of abstinence (e.g. as with daily CO in CrEATE) are unavailable or impractical. A second limiting factor may be insufficient evidence that short-term abstinence during brief attempts to abstain under treatment conditions (e.g. practice quit days) shows clinical validity in predicting long-term outcome results. Third, credible “placebo” or nonspecific conditions would be needed to compare with active treatments in order to maintain effective blinding, which may be difficult with non-drug interventions. Finally, a novel drug’s characteristics may also limit the use of CrEATE, such as those with pharmacokinetics requiring very long dose run-up or washout periods, extending the duration of each treatment phase beyond what is practical, or drugs with prominent and persistent carryover effects may confound the validity of results from placebo conditions that follow active drug conditions. Others expected to have very long-term actions, such as vaccines, may preclude any crossover design.
Conclusion
The enormous global health costs due to the consequences of combustible tobacco smoking argues for the importance of increasing efforts to develop more robust treatments to quit smoking. One way to help accelerate that development is to more rapidly assess the efficacy of those novel treatments. This early Phase 2 CrEATE procedure offers a very efficient method to identify those novel drugs that do or do not warrant the substantial investment of time and resources to evaluate their efficacy in formal RCTs. Even rapid determination of those lacking efficacy, which drug development history shows is the vast majority of candidate drugs, can speed up the pace of progress and preserve resources so they are more narrowly focused on drugs very likely to succeed in late Phase 2 RCTs. Furthermore, research may demonstrate broader application of the CrEATE procedure to evaluating the efficacy of novel interventions for other treatment indications.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://dbpia.nl.go.kr/ntr.
Funding
Research reported in this publication was supported by the National Institutes of Health under grant awards P50 CA084718 and P50 CA143187 from National Cancer Institute, and UH3 TR000958 from National Center for Advancing Translational Sciences. Preparation of this paper was also supported by U01 DA054882 from National Institute on Drug Abuse. Views expressed are those of the author and do not necessarily represent the position of NIH or FDA.
Author Contributions
K. P. oversaw all the reported research as PI of the studies described.
Declaration of Interests
The author has no potential conflicts of interest to report.
Acknowledgments
The author thanks colleagues Caryn Lerman, Roy Chengappa, and Darlene Brunzell in collaborations to plan the key studies described here. He also thanks the research staff who recruited all participants, conducted the day-to-day assessments and treatment visits, and aided with data analysis for these studies, particularly Carolyn Fonte, Joshua Karelitz, Melissa Mercincavage, Nancy Jao, Valerie Michael, Jessica Briski, and Margaret Boldry.




Comments