-
PDF
- Split View
-
Views
-
Cite
Cite
Omar Bushara, Alex Guzner, Elizabeth Bachman, Roger Stupp, Rimas V Lukas, Jessica W Templer, Tumor type, epilepsy burden, and seizure documentation: experiences at a single center neuro-oncology clinic, Neuro-Oncology Practice, Volume 8, Issue 5, October 2021, Pages 581–588, https://doi.org/10.1093/nop/npab032
Close - Share Icon Share
Abstract
Patients with both primary and metastatic brain tumors have significant seizure burden due to their tumor. The management of tumor-related epilepsy (TRE) and optimizing antiepileptic drug (AED) regimens requires collaboration between neurologists and seizure specialists, which is facilitated by seizure documentation in clinic notes. We aim to describe seizure incidence in patients seen in neuro-oncology clinical practice. Further, in the subset of those patients with TRE, we aim to analyze seizure documentation.
This is a retrospective review of patients with a primary or metastatic brain tumor seen in a neuro-oncology clinic in October 2019. Patients with TRE were included in the analysis of seizure documentation. These notes were analyzed for inclusion of seizure descriptors, terminology, AED regimens, and changes in management.
Of the full cohort of 356 patients, 199 (55.9%) had TRE. Anaplastic astrocytomas had the highest percentage of patients with TRE. The analysis of seizure documentation in patients with TRE revealed that the majority of notes (90.9%) mentioned seizures. Fewer notes (39.6%) provided additional descriptions of the seizures or commented on AED regimens (58.3%). In notes for patients who had seizures within the previous 6 months, seizure descriptors were more likely.
This study defines the TRE burden in a cohort of patients seen in neuro-oncology clinic. Among patients with TRE, our study shows that documentation of many aspects of the characteristics and management of patient seizures can be improved, which would facilitate further analysis of impact on patient care as well as future research.
Patients with both primary brain tumors and brain metastases have significant seizure burden due to their lesion(s), with up to 90% of patients having epilepsy as a consequence of their cancer.1–3 Tumor-related epilepsy (TRE) is also often resistant to medical management and presents a significant source of mortality and morbidity in patients with brain tumors.1–6 Currently, the underlying mechanisms of TRE are not fully understood, although higher rates of epilepsy have been reported in patients with low-grade gliomas, larger tumor volumes, frontal tumor location, and molecular markers such as the IDH1 mutation.1–5 It is likely that tumor biology plays a role in TRE. Oncometabolites have been demonstrated to facilitate seizures in preclinical models.7 It can also be hypothesized that direct tumor cell-neuronal interaction could comprise another mechanism.8,9
The management of these patients is often complex, requiring simultaneous oncological treatment (medical, radiotherapeutic, and surgical) as well as epilepsy management. As such, clear communication among expert providers is essential. Optimal treatment and management of epilepsy and the careful choice of a single agent or combination antiepileptic drug (AED) regimen requires collaboration between neurologists and seizure specialists. The understanding of potential drug interactions of AEDs, chemotherapy, and certain medications commonly utilized in patients undergoing cancer treatment. Furthermore, recognizing specific side effects related to the use of anti-seizure medications is important and requires mutual understanding of treatments prescribed by the other specialists.10–15
While brain tumor patients may frequently see multiple experts from various specialties, and these specialists may informally communicate and collaborate, there are no established best practices nor is there a consistent manner on how seizures and AED adverse effects are recorded and addressed. An important step towards improving cross-specialty collaboration is accurately characterizing and documenting the seizure burden of patients with primary or secondary tumors in the brain and central nervous system.
Improved documentation has implications both for clinical practice as well as future research. Standardized and detailed documentation of seizures at neuro-oncology clinic visits will allow for early identification of toxicities and drug interactions as well as identification and documentation of breakthrough seizures or seizure-associated symptoms. This information is needed to evaluate the efficacy of anti-seizure prophylaxis and to adapt and optimize treatment regimens. As both overly cautious prophylaxis and polypharmacy can result in a higher likelihood of medication adverse effects and under-treatment can result in breakthrough seizures, thorough documentation of patient progress is paramount.
More thorough seizure evaluation and documentation during neuro-oncology clinic visits would increase opportunities for clinical assessment of seizures and may improve documentation accuracy.16–19 Thus, adequate and accurate documentation of seizure characteristics, frequency, adherence to AED therapy, and monitoring of side effects are key to management of brain tumor patients, clinical epilepsy research, and longitudinal patient outcomes. Treatment side effects and effectiveness of seizure control should be distinguished from symptoms and manifestations of tumor progression, further underscoring the need for precise evaluation and documentation.19–23
The purpose of this study is to provide a descriptive analysis of seizure incidence in patients seen in neuro-oncology clinical practice. Further, in the subset of those patients with TRE, we aim to determine documentation of seizure frequency, seizure semiology, and AED regimen within neuro-oncology clinic notes.
Methods
Patient Selection
In this retrospective review, we reviewed all patients seen at Northwestern Medicine Lou and Jean Malnati Brain Tumor Institute neuro-oncology clinic in October 2019. Patients had to have a diagnosis of a primary brain tumor or brain metastases with one or more physical appointments with 1 of the 6 neuro-oncology physicians and 3 neuro-oncology nurse practitioners. Institutional IRB approval was waived as this study was a retrospective quality improvement project.
TRE was defined as patients having had a seizure occurring any time from 1 month prior to tumor diagnosis up to the time of the visit. This included patients who presented to medical attention due to a seizure, as well as patients who experienced seizures after their diagnosis. Duplicate patients who had more than 1 visit during October 2019 had their second visit removed. This yielded 199 patients to be included in the analyses (Figure 1).
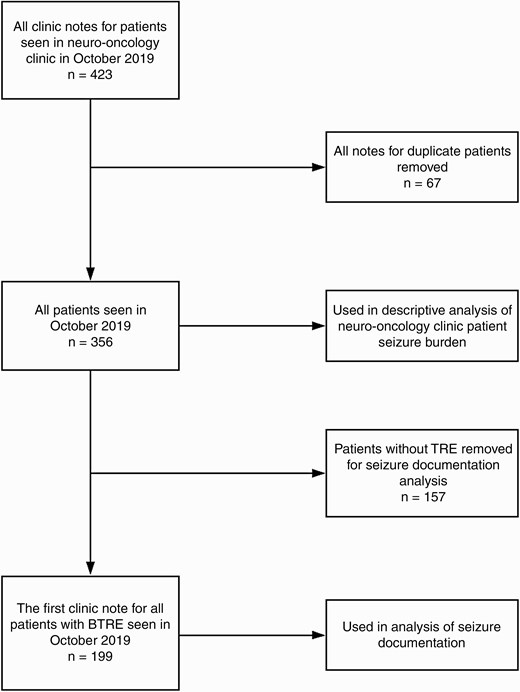
A total of 423 clinic notes were completed in the neuro-oncology clinic in October 2019. Sixty-seven of these notes were based on visits of duplicate patients. For duplicate patients, the first note was kept, and subsequent notes were not used in the analysis. Notes for which the primary purpose of the visit was to administer treatment or to provide treatment education rather than a full clinical evaluation were not utilized in our analysis. A total of 356 unique patients were used in the analysis of tumor types and seizure burden. Of these patients, 157 patients did not have TRE and were thus removed for the final analysis of seizure documentation. The remaining 199 patients were included in the analysis of seizure documentation. Abbreviation: TRE, tumor-related epilepsy.
Variables and Analysis
The tumor type, grade, tumor status, and the presence or absence of seizures were collected for all patients. Seizure burden based on tumor type and grade was compared using chi-squared tests. The progress notes for each patient visit in this timeframe were reviewed. Documentation in each note was assessed for the parameters listed in Table 1. The reviewed parameters included description and dates of seizures, location of seizure description within the note, and medication documentation. Of these variables, AED lists are the only component of the note that is able to be automatically generated—other variables must be either written by the provider at the time of note writing or copy forwarded from previous notes. Each variable was then analyzed for inclusion in patient charts. Occurrence of last seizure prior to the note was identified by chart review of clinic notes, phone calls, MyChart messages, and other communications within patient charts. This descriptive analysis was repeated for overlapping subgroups of patients who had seizures within 6, 3, and 1 month(s) of the office visit, yielding 82, 65, and 43 patients, respectively.
| Variables Evaluated for Each Patient Note . |
|---|
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note |
| “Seizure/epilepsy/convulsion” used in HPI |
| Number of seizures included in the note |
| Any dates of seizures included in the note |
| Description of seizure included in the note |
| Notes in which patients had focal seizures |
| Seizure symptoms included in the note |
| Lateralizing language used in the note |
| Lüders Terminology used in the note |
| ILAE Terminology used in the note |
| Change in semiology mentioned in the note |
| “Seizure/epilepsy/convulsion” used in assessment/plan |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list |
| AEDs listed in automatically generated medication list |
| AEDs commented on elsewhere in the note |
| No change to AED regimen based on the note |
| Note clearly states no change to AED regimen |
| Patients on prior AEDs |
| Note includes list of prior AEDs |
| Variables Evaluated for Each Patient Note . |
|---|
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note |
| “Seizure/epilepsy/convulsion” used in HPI |
| Number of seizures included in the note |
| Any dates of seizures included in the note |
| Description of seizure included in the note |
| Notes in which patients had focal seizures |
| Seizure symptoms included in the note |
| Lateralizing language used in the note |
| Lüders Terminology used in the note |
| ILAE Terminology used in the note |
| Change in semiology mentioned in the note |
| “Seizure/epilepsy/convulsion” used in assessment/plan |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list |
| AEDs listed in automatically generated medication list |
| AEDs commented on elsewhere in the note |
| No change to AED regimen based on the note |
| Note clearly states no change to AED regimen |
| Patients on prior AEDs |
| Note includes list of prior AEDs |
Abbreviations: AED, antiepileptic drug; HPI, history of present illness; ILAE: International League Against Epilepsy.
| Variables Evaluated for Each Patient Note . |
|---|
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note |
| “Seizure/epilepsy/convulsion” used in HPI |
| Number of seizures included in the note |
| Any dates of seizures included in the note |
| Description of seizure included in the note |
| Notes in which patients had focal seizures |
| Seizure symptoms included in the note |
| Lateralizing language used in the note |
| Lüders Terminology used in the note |
| ILAE Terminology used in the note |
| Change in semiology mentioned in the note |
| “Seizure/epilepsy/convulsion” used in assessment/plan |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list |
| AEDs listed in automatically generated medication list |
| AEDs commented on elsewhere in the note |
| No change to AED regimen based on the note |
| Note clearly states no change to AED regimen |
| Patients on prior AEDs |
| Note includes list of prior AEDs |
| Variables Evaluated for Each Patient Note . |
|---|
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note |
| “Seizure/epilepsy/convulsion” used in HPI |
| Number of seizures included in the note |
| Any dates of seizures included in the note |
| Description of seizure included in the note |
| Notes in which patients had focal seizures |
| Seizure symptoms included in the note |
| Lateralizing language used in the note |
| Lüders Terminology used in the note |
| ILAE Terminology used in the note |
| Change in semiology mentioned in the note |
| “Seizure/epilepsy/convulsion” used in assessment/plan |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list |
| AEDs listed in automatically generated medication list |
| AEDs commented on elsewhere in the note |
| No change to AED regimen based on the note |
| Note clearly states no change to AED regimen |
| Patients on prior AEDs |
| Note includes list of prior AEDs |
Abbreviations: AED, antiepileptic drug; HPI, history of present illness; ILAE: International League Against Epilepsy.
Additionally, patients with seizures within 6 months of their visit were defined as recent seizures, and patients with seizures greater than 6 months prior to their visit were defined as remote seizures. These groups were compared in terms of the variables listed in Table 1. Further, documentation, as related to tumor status, was assessed in this manner, with patients grouped into initial diagnosis or early treatment, stable or regressing tumors, and progressing tumors. Chi-squared tests were used in these comparisons. Data were stored in the Northwestern University Tumor-Related Epilepsy REDCap database (Grant No. UL1TR001422).
Results
A total of 356 patients were seen in neuro-oncology clinic in October 2019. The majority of patients had primary brain tumors (87.9%), while 12.1% of patients had metastatic tumors. Of the full cohort of patients 55.9% had TRE. Patients of all age ranges were seen, with a median age of 55 and the majority of patients were female (Table 2).
| Patient Demographics (N = 356) . | . |
|---|---|
| . | Median [IQR], N (%) . |
| Age | 55 [41-65] |
| Gender | 186 (52.2%) Female |
| Race | |
| White | 284 (79.8%) |
| Black | 18 (5.1%) |
| Asian | 15 (4.2%) |
| American Indian/Alaskan Native | 2 (0.5%) |
| Other | 22 (6.2%) |
| Declined | 15 (4.2%) |
| Ethnicity | |
| Hispanic | 25 (7.0%) |
| Non-Hispanic | 314 (88.2%) |
| Declined | 17 (4.8%) |
| Tumor | |
| Primary CNS tumors | 313 (87.9%) |
| Metastatic tumors | 43 (12.1%) |
| TRE | 199 (55.9%) |
| Tumor status in patients with TRE | |
| Initial diagnosis and early treatment | 70 (35.2%) |
| Stable or regression | 85 (42.7%) |
| Progression | 44 (22.1%) |
| Patient Demographics (N = 356) . | . |
|---|---|
| . | Median [IQR], N (%) . |
| Age | 55 [41-65] |
| Gender | 186 (52.2%) Female |
| Race | |
| White | 284 (79.8%) |
| Black | 18 (5.1%) |
| Asian | 15 (4.2%) |
| American Indian/Alaskan Native | 2 (0.5%) |
| Other | 22 (6.2%) |
| Declined | 15 (4.2%) |
| Ethnicity | |
| Hispanic | 25 (7.0%) |
| Non-Hispanic | 314 (88.2%) |
| Declined | 17 (4.8%) |
| Tumor | |
| Primary CNS tumors | 313 (87.9%) |
| Metastatic tumors | 43 (12.1%) |
| TRE | 199 (55.9%) |
| Tumor status in patients with TRE | |
| Initial diagnosis and early treatment | 70 (35.2%) |
| Stable or regression | 85 (42.7%) |
| Progression | 44 (22.1%) |
Abbreviations: CNS, central nervous system; IQR, interquartile range; TRE: tumor-related epilepsy.
| Patient Demographics (N = 356) . | . |
|---|---|
| . | Median [IQR], N (%) . |
| Age | 55 [41-65] |
| Gender | 186 (52.2%) Female |
| Race | |
| White | 284 (79.8%) |
| Black | 18 (5.1%) |
| Asian | 15 (4.2%) |
| American Indian/Alaskan Native | 2 (0.5%) |
| Other | 22 (6.2%) |
| Declined | 15 (4.2%) |
| Ethnicity | |
| Hispanic | 25 (7.0%) |
| Non-Hispanic | 314 (88.2%) |
| Declined | 17 (4.8%) |
| Tumor | |
| Primary CNS tumors | 313 (87.9%) |
| Metastatic tumors | 43 (12.1%) |
| TRE | 199 (55.9%) |
| Tumor status in patients with TRE | |
| Initial diagnosis and early treatment | 70 (35.2%) |
| Stable or regression | 85 (42.7%) |
| Progression | 44 (22.1%) |
| Patient Demographics (N = 356) . | . |
|---|---|
| . | Median [IQR], N (%) . |
| Age | 55 [41-65] |
| Gender | 186 (52.2%) Female |
| Race | |
| White | 284 (79.8%) |
| Black | 18 (5.1%) |
| Asian | 15 (4.2%) |
| American Indian/Alaskan Native | 2 (0.5%) |
| Other | 22 (6.2%) |
| Declined | 15 (4.2%) |
| Ethnicity | |
| Hispanic | 25 (7.0%) |
| Non-Hispanic | 314 (88.2%) |
| Declined | 17 (4.8%) |
| Tumor | |
| Primary CNS tumors | 313 (87.9%) |
| Metastatic tumors | 43 (12.1%) |
| TRE | 199 (55.9%) |
| Tumor status in patients with TRE | |
| Initial diagnosis and early treatment | 70 (35.2%) |
| Stable or regression | 85 (42.7%) |
| Progression | 44 (22.1%) |
Abbreviations: CNS, central nervous system; IQR, interquartile range; TRE: tumor-related epilepsy.
The most common primary tumor was glioblastoma (GBM) and the most common metastatic tumor was breast cancer. Of all tumor types, anaplastic astrocytomas had the highest percentage of patients with TRE. Patients with grade III and IV tumors were more likely to have seizures (P < .0001 and P = .001, respectively), and patients with metastatic tumors and other lesions were less likely to have seizures (P = .003 and P < .0001, respectively). Detailed information on the distribution of tumor types, grade, and seizure burden can be seen in Table 3.
| Tumor Type . | N . | No Seizures . | Seizures . | P Value . |
|---|---|---|---|---|
| Meningioma | 25 | 11 | 14 | NS |
| Grade II | 29 | 9 | 20 | NS |
| Oligodendroglioma | 15 | 3 | 12 | |
| Diffuse astrocytoma | 8 | 1 | 7 | |
| Ependymoma | 6 | 5 | 1 | |
| Grade III | 49 | 7 | 42 | <.0001 |
| Anaplastic astrocytoma | 32 | 3 | 29 | |
| Anaplastic oligodendroglioma | 15 | 3 | 12 | |
| Ependymoma | 2 | 1 | 1 | |
| Grade IV | 127 | 41 | 86 | .001 |
| Glioblastoma—IDH-wildtype | 108 | 36 | 72 | |
| Glioblastoma—IDH-mutated | 19 | 5 | 14 | |
| Metastatic tumors | 43 | 28 | 15 | .003 |
| Breast cancer | 22 | 15 | 7 | |
| Lung cancer | 9 | 6 | 3 | |
| Melanoma | 7 | 4 | 3 | |
| Thyroid cancer | 1 | 1 | 0 | |
| Prostate cancer | 1 | 1 | 0 | |
| Cystic adenoid cancer | 1 | 1 | 0 | |
| Endometrial cancer | 1 | 0 | 1 | |
| Dural met. of unknown origin | 1 | 0 | 1 | |
| Other | 83 | 63 | 20 | <.0001 |
| Primary CNS lymphoma | 15 | 12 | 3 | |
| Glioma NOS (pathology inconclusive/unavailable) | 13 | 7 | 6 | |
| Systemic hematologic malignancies | 12 | 10 | 2 | |
| Pineal tumors | 5 | 5 | 0 | |
| Cerebellar tumors | 5 | 2 | 3 | |
| Hemangioma | 4 | 2 | 2 | |
| Nerve sheath tumors | 3 | 3 | 0 | |
| Neurosarcoidosis | 3 | 2 | 1 | |
| Pituitary tumors | 3 | 3 | 0 | |
| Schwannomas | 3 | 3 | 0 | |
| Spinal cord lesions | 3 | 3 | 0 | |
| Tuberous sclerosis | 2 | 1 | 1 | |
| Arachnoid cyst | 1 | 1 | 0 | |
| Cavernoma | 1 | 0 | 1 | |
| Germinoma | 1 | 1 | 0 | |
| CNS lesions NOS at time of visit | 9 | 8 | 1 |
| Tumor Type . | N . | No Seizures . | Seizures . | P Value . |
|---|---|---|---|---|
| Meningioma | 25 | 11 | 14 | NS |
| Grade II | 29 | 9 | 20 | NS |
| Oligodendroglioma | 15 | 3 | 12 | |
| Diffuse astrocytoma | 8 | 1 | 7 | |
| Ependymoma | 6 | 5 | 1 | |
| Grade III | 49 | 7 | 42 | <.0001 |
| Anaplastic astrocytoma | 32 | 3 | 29 | |
| Anaplastic oligodendroglioma | 15 | 3 | 12 | |
| Ependymoma | 2 | 1 | 1 | |
| Grade IV | 127 | 41 | 86 | .001 |
| Glioblastoma—IDH-wildtype | 108 | 36 | 72 | |
| Glioblastoma—IDH-mutated | 19 | 5 | 14 | |
| Metastatic tumors | 43 | 28 | 15 | .003 |
| Breast cancer | 22 | 15 | 7 | |
| Lung cancer | 9 | 6 | 3 | |
| Melanoma | 7 | 4 | 3 | |
| Thyroid cancer | 1 | 1 | 0 | |
| Prostate cancer | 1 | 1 | 0 | |
| Cystic adenoid cancer | 1 | 1 | 0 | |
| Endometrial cancer | 1 | 0 | 1 | |
| Dural met. of unknown origin | 1 | 0 | 1 | |
| Other | 83 | 63 | 20 | <.0001 |
| Primary CNS lymphoma | 15 | 12 | 3 | |
| Glioma NOS (pathology inconclusive/unavailable) | 13 | 7 | 6 | |
| Systemic hematologic malignancies | 12 | 10 | 2 | |
| Pineal tumors | 5 | 5 | 0 | |
| Cerebellar tumors | 5 | 2 | 3 | |
| Hemangioma | 4 | 2 | 2 | |
| Nerve sheath tumors | 3 | 3 | 0 | |
| Neurosarcoidosis | 3 | 2 | 1 | |
| Pituitary tumors | 3 | 3 | 0 | |
| Schwannomas | 3 | 3 | 0 | |
| Spinal cord lesions | 3 | 3 | 0 | |
| Tuberous sclerosis | 2 | 1 | 1 | |
| Arachnoid cyst | 1 | 1 | 0 | |
| Cavernoma | 1 | 0 | 1 | |
| Germinoma | 1 | 1 | 0 | |
| CNS lesions NOS at time of visit | 9 | 8 | 1 |
Abbreviations: CNS, central nervous system; IDH, isocitrate dehydrogenase; NOS, not otherwise specified; NS, not significant.
| Tumor Type . | N . | No Seizures . | Seizures . | P Value . |
|---|---|---|---|---|
| Meningioma | 25 | 11 | 14 | NS |
| Grade II | 29 | 9 | 20 | NS |
| Oligodendroglioma | 15 | 3 | 12 | |
| Diffuse astrocytoma | 8 | 1 | 7 | |
| Ependymoma | 6 | 5 | 1 | |
| Grade III | 49 | 7 | 42 | <.0001 |
| Anaplastic astrocytoma | 32 | 3 | 29 | |
| Anaplastic oligodendroglioma | 15 | 3 | 12 | |
| Ependymoma | 2 | 1 | 1 | |
| Grade IV | 127 | 41 | 86 | .001 |
| Glioblastoma—IDH-wildtype | 108 | 36 | 72 | |
| Glioblastoma—IDH-mutated | 19 | 5 | 14 | |
| Metastatic tumors | 43 | 28 | 15 | .003 |
| Breast cancer | 22 | 15 | 7 | |
| Lung cancer | 9 | 6 | 3 | |
| Melanoma | 7 | 4 | 3 | |
| Thyroid cancer | 1 | 1 | 0 | |
| Prostate cancer | 1 | 1 | 0 | |
| Cystic adenoid cancer | 1 | 1 | 0 | |
| Endometrial cancer | 1 | 0 | 1 | |
| Dural met. of unknown origin | 1 | 0 | 1 | |
| Other | 83 | 63 | 20 | <.0001 |
| Primary CNS lymphoma | 15 | 12 | 3 | |
| Glioma NOS (pathology inconclusive/unavailable) | 13 | 7 | 6 | |
| Systemic hematologic malignancies | 12 | 10 | 2 | |
| Pineal tumors | 5 | 5 | 0 | |
| Cerebellar tumors | 5 | 2 | 3 | |
| Hemangioma | 4 | 2 | 2 | |
| Nerve sheath tumors | 3 | 3 | 0 | |
| Neurosarcoidosis | 3 | 2 | 1 | |
| Pituitary tumors | 3 | 3 | 0 | |
| Schwannomas | 3 | 3 | 0 | |
| Spinal cord lesions | 3 | 3 | 0 | |
| Tuberous sclerosis | 2 | 1 | 1 | |
| Arachnoid cyst | 1 | 1 | 0 | |
| Cavernoma | 1 | 0 | 1 | |
| Germinoma | 1 | 1 | 0 | |
| CNS lesions NOS at time of visit | 9 | 8 | 1 |
| Tumor Type . | N . | No Seizures . | Seizures . | P Value . |
|---|---|---|---|---|
| Meningioma | 25 | 11 | 14 | NS |
| Grade II | 29 | 9 | 20 | NS |
| Oligodendroglioma | 15 | 3 | 12 | |
| Diffuse astrocytoma | 8 | 1 | 7 | |
| Ependymoma | 6 | 5 | 1 | |
| Grade III | 49 | 7 | 42 | <.0001 |
| Anaplastic astrocytoma | 32 | 3 | 29 | |
| Anaplastic oligodendroglioma | 15 | 3 | 12 | |
| Ependymoma | 2 | 1 | 1 | |
| Grade IV | 127 | 41 | 86 | .001 |
| Glioblastoma—IDH-wildtype | 108 | 36 | 72 | |
| Glioblastoma—IDH-mutated | 19 | 5 | 14 | |
| Metastatic tumors | 43 | 28 | 15 | .003 |
| Breast cancer | 22 | 15 | 7 | |
| Lung cancer | 9 | 6 | 3 | |
| Melanoma | 7 | 4 | 3 | |
| Thyroid cancer | 1 | 1 | 0 | |
| Prostate cancer | 1 | 1 | 0 | |
| Cystic adenoid cancer | 1 | 1 | 0 | |
| Endometrial cancer | 1 | 0 | 1 | |
| Dural met. of unknown origin | 1 | 0 | 1 | |
| Other | 83 | 63 | 20 | <.0001 |
| Primary CNS lymphoma | 15 | 12 | 3 | |
| Glioma NOS (pathology inconclusive/unavailable) | 13 | 7 | 6 | |
| Systemic hematologic malignancies | 12 | 10 | 2 | |
| Pineal tumors | 5 | 5 | 0 | |
| Cerebellar tumors | 5 | 2 | 3 | |
| Hemangioma | 4 | 2 | 2 | |
| Nerve sheath tumors | 3 | 3 | 0 | |
| Neurosarcoidosis | 3 | 2 | 1 | |
| Pituitary tumors | 3 | 3 | 0 | |
| Schwannomas | 3 | 3 | 0 | |
| Spinal cord lesions | 3 | 3 | 0 | |
| Tuberous sclerosis | 2 | 1 | 1 | |
| Arachnoid cyst | 1 | 1 | 0 | |
| Cavernoma | 1 | 0 | 1 | |
| Germinoma | 1 | 1 | 0 | |
| CNS lesions NOS at time of visit | 9 | 8 | 1 |
Abbreviations: CNS, central nervous system; IDH, isocitrate dehydrogenase; NOS, not otherwise specified; NS, not significant.
The analysis of seizure documentation in patients with TRE revealed that the majority of notes (90.9%) mentioned seizures. Fewer notes (39.6%) provided additional descriptions of the seizures. Inclusion of these descriptive characteristics was less likely as the time between a patient seizure and their clinic visit increased, and those with remote seizures were less likely to include dates of seizures (P = .04), descriptions of seizures (P = .012), and include seizures in the assessment or plan of the note (P = .014). However, notes for patients with remote seizures were more likely to include the number of seizures (P = .015) (Table 4).
Tumor-Related Epilepsy Documentation Based on Time Point of Most Recent Seizure
| . | . | Remote Seizures . | Recent Seizures . | . | . | . |
|---|---|---|---|---|---|---|
| . | Lifetime . | Over 6 Months . | 6 Months . | 3 Months . | 1 Month . | . |
| . | N (%) . | N (%) . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 181 (90.95) | 109 (92.3) | 73 (89.02) | 58 (89.23) | 40 (93.02) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 137 (68.84) | 75 (63.5%) | 62 (75.61) | 51 (78.46) | 35 (81.39) | .013 |
| Number of seizures included in the note | 102 (51.25) | 62 (52.5%) | 40 (48.78) | 32 (49.23) | 21 (48.84) | .015 |
| Any dates of seizures included in the note | 69 (34.67) | 34 (28.8) | 35 (42.68) | 25 (38.46) | 18 (41.86) | .04 |
| Description of seizure included in the note | 79 (39.69) | 36 (30.5) | 43 (52.44) | 39 (60) | 29 (67.44) | .012 |
| Notes in which patients had focal seizures | 49 (24.82) | 19 (16.1) | 30 (36.59) | 27 (41.54) | 21 (48.84) | .002 |
| Seizure symptoms included in the note | 29 (14.57) | 11 (9.3) | 18 (21.95) | 16 (24.62) | 13 (30.23) | NS |
| Lateralizing language used in the note | 21 (10.55) | 8 (6.7) | 13 (15.85) | 11 (16.92) | 8 (18.6) | NS |
| Lüders Terminology used in the note | 5 (2.51) | 3 (2.5) | 2 (2.44) | 0 (0) | 0 (0) | NS |
| ILAE Terminology used in the note | 6 (3.01) | 2 (1.6) | 4 (4.88) | 3 (4.62) | 2 (4.65) | NS |
| Change in semiology mentioned in the note | 8 (4.02) | 3 (2.5) | 5 (6.09) | 3 (4.62) | 2 (4.65) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 142 (71.35) | 79 (66.9) | 64 (78.05) | 51 (78.46) | 36 (83.72) | .014 |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 134 (67.33) | 77 (65.2) | 58 (70.73) | 47 (72.31) | 34 (79.07) | NS |
| AEDs listed in automatically generated medication list | 146 (73.37) | 85 (72.0) | 62 (75.61) | 49 (75.38) | 32 (74.42) | NS |
| AEDs commented on elsewhere in the note | 116 (58.29) | 65 (55.1) | 52 (63.41) | 41 (63.08) | 25 (58.24) | NS |
| No change to AED regimen based on the note | 187 (93.97) | 112 (94.9) | 76 (92.68) | 60 (92.31) | 43 (100) | NS |
| Note clearly states no change to AED regimen | 94 (47.23) | 62 (52.5) | 33 (40.24) | 27 (41.54) | 18 (41.86) | NS |
| Patients on prior AEDs | 47 (23.62) | 33 (27.9) | 15 (18.29) | 12 (18.46) | 7 (16.28) | NS |
| Note includes list of prior AEDs | 9 (4.52) | 6 (5.1) | 3 (6.66) | 3 (4.62) | 1 (2.33) | NS |
| . | . | Remote Seizures . | Recent Seizures . | . | . | . |
|---|---|---|---|---|---|---|
| . | Lifetime . | Over 6 Months . | 6 Months . | 3 Months . | 1 Month . | . |
| . | N (%) . | N (%) . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 181 (90.95) | 109 (92.3) | 73 (89.02) | 58 (89.23) | 40 (93.02) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 137 (68.84) | 75 (63.5%) | 62 (75.61) | 51 (78.46) | 35 (81.39) | .013 |
| Number of seizures included in the note | 102 (51.25) | 62 (52.5%) | 40 (48.78) | 32 (49.23) | 21 (48.84) | .015 |
| Any dates of seizures included in the note | 69 (34.67) | 34 (28.8) | 35 (42.68) | 25 (38.46) | 18 (41.86) | .04 |
| Description of seizure included in the note | 79 (39.69) | 36 (30.5) | 43 (52.44) | 39 (60) | 29 (67.44) | .012 |
| Notes in which patients had focal seizures | 49 (24.82) | 19 (16.1) | 30 (36.59) | 27 (41.54) | 21 (48.84) | .002 |
| Seizure symptoms included in the note | 29 (14.57) | 11 (9.3) | 18 (21.95) | 16 (24.62) | 13 (30.23) | NS |
| Lateralizing language used in the note | 21 (10.55) | 8 (6.7) | 13 (15.85) | 11 (16.92) | 8 (18.6) | NS |
| Lüders Terminology used in the note | 5 (2.51) | 3 (2.5) | 2 (2.44) | 0 (0) | 0 (0) | NS |
| ILAE Terminology used in the note | 6 (3.01) | 2 (1.6) | 4 (4.88) | 3 (4.62) | 2 (4.65) | NS |
| Change in semiology mentioned in the note | 8 (4.02) | 3 (2.5) | 5 (6.09) | 3 (4.62) | 2 (4.65) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 142 (71.35) | 79 (66.9) | 64 (78.05) | 51 (78.46) | 36 (83.72) | .014 |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 134 (67.33) | 77 (65.2) | 58 (70.73) | 47 (72.31) | 34 (79.07) | NS |
| AEDs listed in automatically generated medication list | 146 (73.37) | 85 (72.0) | 62 (75.61) | 49 (75.38) | 32 (74.42) | NS |
| AEDs commented on elsewhere in the note | 116 (58.29) | 65 (55.1) | 52 (63.41) | 41 (63.08) | 25 (58.24) | NS |
| No change to AED regimen based on the note | 187 (93.97) | 112 (94.9) | 76 (92.68) | 60 (92.31) | 43 (100) | NS |
| Note clearly states no change to AED regimen | 94 (47.23) | 62 (52.5) | 33 (40.24) | 27 (41.54) | 18 (41.86) | NS |
| Patients on prior AEDs | 47 (23.62) | 33 (27.9) | 15 (18.29) | 12 (18.46) | 7 (16.28) | NS |
| Note includes list of prior AEDs | 9 (4.52) | 6 (5.1) | 3 (6.66) | 3 (4.62) | 1 (2.33) | NS |
Abbreviations: AED, antiepileptic drug; HPI, history of present illness; ILAE: International League Against Epilepsy; NS, not significant.
Tumor-Related Epilepsy Documentation Based on Time Point of Most Recent Seizure
| . | . | Remote Seizures . | Recent Seizures . | . | . | . |
|---|---|---|---|---|---|---|
| . | Lifetime . | Over 6 Months . | 6 Months . | 3 Months . | 1 Month . | . |
| . | N (%) . | N (%) . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 181 (90.95) | 109 (92.3) | 73 (89.02) | 58 (89.23) | 40 (93.02) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 137 (68.84) | 75 (63.5%) | 62 (75.61) | 51 (78.46) | 35 (81.39) | .013 |
| Number of seizures included in the note | 102 (51.25) | 62 (52.5%) | 40 (48.78) | 32 (49.23) | 21 (48.84) | .015 |
| Any dates of seizures included in the note | 69 (34.67) | 34 (28.8) | 35 (42.68) | 25 (38.46) | 18 (41.86) | .04 |
| Description of seizure included in the note | 79 (39.69) | 36 (30.5) | 43 (52.44) | 39 (60) | 29 (67.44) | .012 |
| Notes in which patients had focal seizures | 49 (24.82) | 19 (16.1) | 30 (36.59) | 27 (41.54) | 21 (48.84) | .002 |
| Seizure symptoms included in the note | 29 (14.57) | 11 (9.3) | 18 (21.95) | 16 (24.62) | 13 (30.23) | NS |
| Lateralizing language used in the note | 21 (10.55) | 8 (6.7) | 13 (15.85) | 11 (16.92) | 8 (18.6) | NS |
| Lüders Terminology used in the note | 5 (2.51) | 3 (2.5) | 2 (2.44) | 0 (0) | 0 (0) | NS |
| ILAE Terminology used in the note | 6 (3.01) | 2 (1.6) | 4 (4.88) | 3 (4.62) | 2 (4.65) | NS |
| Change in semiology mentioned in the note | 8 (4.02) | 3 (2.5) | 5 (6.09) | 3 (4.62) | 2 (4.65) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 142 (71.35) | 79 (66.9) | 64 (78.05) | 51 (78.46) | 36 (83.72) | .014 |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 134 (67.33) | 77 (65.2) | 58 (70.73) | 47 (72.31) | 34 (79.07) | NS |
| AEDs listed in automatically generated medication list | 146 (73.37) | 85 (72.0) | 62 (75.61) | 49 (75.38) | 32 (74.42) | NS |
| AEDs commented on elsewhere in the note | 116 (58.29) | 65 (55.1) | 52 (63.41) | 41 (63.08) | 25 (58.24) | NS |
| No change to AED regimen based on the note | 187 (93.97) | 112 (94.9) | 76 (92.68) | 60 (92.31) | 43 (100) | NS |
| Note clearly states no change to AED regimen | 94 (47.23) | 62 (52.5) | 33 (40.24) | 27 (41.54) | 18 (41.86) | NS |
| Patients on prior AEDs | 47 (23.62) | 33 (27.9) | 15 (18.29) | 12 (18.46) | 7 (16.28) | NS |
| Note includes list of prior AEDs | 9 (4.52) | 6 (5.1) | 3 (6.66) | 3 (4.62) | 1 (2.33) | NS |
| . | . | Remote Seizures . | Recent Seizures . | . | . | . |
|---|---|---|---|---|---|---|
| . | Lifetime . | Over 6 Months . | 6 Months . | 3 Months . | 1 Month . | . |
| . | N (%) . | N (%) . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 181 (90.95) | 109 (92.3) | 73 (89.02) | 58 (89.23) | 40 (93.02) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 137 (68.84) | 75 (63.5%) | 62 (75.61) | 51 (78.46) | 35 (81.39) | .013 |
| Number of seizures included in the note | 102 (51.25) | 62 (52.5%) | 40 (48.78) | 32 (49.23) | 21 (48.84) | .015 |
| Any dates of seizures included in the note | 69 (34.67) | 34 (28.8) | 35 (42.68) | 25 (38.46) | 18 (41.86) | .04 |
| Description of seizure included in the note | 79 (39.69) | 36 (30.5) | 43 (52.44) | 39 (60) | 29 (67.44) | .012 |
| Notes in which patients had focal seizures | 49 (24.82) | 19 (16.1) | 30 (36.59) | 27 (41.54) | 21 (48.84) | .002 |
| Seizure symptoms included in the note | 29 (14.57) | 11 (9.3) | 18 (21.95) | 16 (24.62) | 13 (30.23) | NS |
| Lateralizing language used in the note | 21 (10.55) | 8 (6.7) | 13 (15.85) | 11 (16.92) | 8 (18.6) | NS |
| Lüders Terminology used in the note | 5 (2.51) | 3 (2.5) | 2 (2.44) | 0 (0) | 0 (0) | NS |
| ILAE Terminology used in the note | 6 (3.01) | 2 (1.6) | 4 (4.88) | 3 (4.62) | 2 (4.65) | NS |
| Change in semiology mentioned in the note | 8 (4.02) | 3 (2.5) | 5 (6.09) | 3 (4.62) | 2 (4.65) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 142 (71.35) | 79 (66.9) | 64 (78.05) | 51 (78.46) | 36 (83.72) | .014 |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 134 (67.33) | 77 (65.2) | 58 (70.73) | 47 (72.31) | 34 (79.07) | NS |
| AEDs listed in automatically generated medication list | 146 (73.37) | 85 (72.0) | 62 (75.61) | 49 (75.38) | 32 (74.42) | NS |
| AEDs commented on elsewhere in the note | 116 (58.29) | 65 (55.1) | 52 (63.41) | 41 (63.08) | 25 (58.24) | NS |
| No change to AED regimen based on the note | 187 (93.97) | 112 (94.9) | 76 (92.68) | 60 (92.31) | 43 (100) | NS |
| Note clearly states no change to AED regimen | 94 (47.23) | 62 (52.5) | 33 (40.24) | 27 (41.54) | 18 (41.86) | NS |
| Patients on prior AEDs | 47 (23.62) | 33 (27.9) | 15 (18.29) | 12 (18.46) | 7 (16.28) | NS |
| Note includes list of prior AEDs | 9 (4.52) | 6 (5.1) | 3 (6.66) | 3 (4.62) | 1 (2.33) | NS |
Abbreviations: AED, antiepileptic drug; HPI, history of present illness; ILAE: International League Against Epilepsy; NS, not significant.
The frequency with which antiepileptic medications were mentioned in the note remained relatively stable in each follow-up note after the first seizure was documented. The majority of notes (93.9%) did not describe a change in AEDs in medication lists. However, fewer notes (47.2%) directly stated that there was no change in AED in typed portions of the note. Similarly, mention of prior AED regimens was reported in a minority (23.6%) of notes, with a list of prior AEDs was rarely (4.5%) included. Documentation regarding AEDs was not found to differ in those with remote vs recent seizures (Table 4).
Seizure semiology, terminology as recommended by Lüders et al in 1998, and classification proposed by the International League Against Epilepsy in 2017 were rarely included in patient notes at any time point (Table 4).24,25
Tumor status was largely not found to relate to seizure documentation. Only the number of seizures documented within notes was found to vary based on tumor status, with notes for patients with stable or regressing tumors including the number 61.1% of the time, while notes for patients with tumors that were recently diagnosed or in early treatment and progressing tumors included the number of seizures 47.1% and 36.3% of the time, respectively (P = .037). No other differences in documentation were noted (Table 5).
| . | Initial Treatment . | Stable/Regression . | Progression . | . |
|---|---|---|---|---|
| . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 63 (90) | 79 (92.9) | 39 (88.6) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 47 (67.1) | 62 (72.9) | 27 (61.3) | NS |
| Number of seizures included in the note | 33 (47.1) | 52 (61.1) | 16 (36.3) | .037 |
| Any dates of seizures included in the note | 26 (37.1) | 32 (37.6) | 11 (25) | NS |
| Description of seizure included in the note | 29 (41.4) | 37 (43.5) | 12 (27.2) | NS |
| Notes in which patients had focal seizures | 17 (24.2) | 24 (28.2) | 7 (15.9) | NS |
| Seizure symptoms included in the note | 10 (14.2) | 14 (16.4) | 5 (11.3) | NS |
| Lateralizing language used in the note | 8 (11.4) | 10 (11.7) | 3 (6.8) | NS |
| Lüders Terminology used in the note | 2 (2.8) | 3 (3.5) | 0 (0) | NS |
| ILAE Terminology used in the note | 3 (4.2) | 3 (3.5) | 0 (0) | NS |
| Change in semiology mentioned in the note | 1 (1.4) | 4 (4.7) | 3 (6.8) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 50 (71.4) | 64 (75.2) | 29 (65.9) | NS |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 49 (70) | 60 (70.5) | 26 (59.1) | NS |
| AEDs listed in automatically generated medication list | 52 (74.2) | 60 (70.5) | 34 (77.2) | NS |
| AEDs commented on elsewhere in the note | 44 (62.8) | 49 (57.6) | 24 (54.5) | NS |
| No change to AED regimen based on the note | 67 (95.7) | 78 (91.7) | 42 (95.4) | NS |
| Note clearly states no change to AED regimen | 35 (50) | 40 (47.1) | 20 (45.4) | NS |
| Patients on prior AEDs | 16 (22.8) | 25 (29.4) | 7 (15.9) | NS |
| Note includes list of prior AEDs | 5 (7.1) | 4 (4.7) | 0 (0) | NS |
| . | Initial Treatment . | Stable/Regression . | Progression . | . |
|---|---|---|---|---|
| . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 63 (90) | 79 (92.9) | 39 (88.6) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 47 (67.1) | 62 (72.9) | 27 (61.3) | NS |
| Number of seizures included in the note | 33 (47.1) | 52 (61.1) | 16 (36.3) | .037 |
| Any dates of seizures included in the note | 26 (37.1) | 32 (37.6) | 11 (25) | NS |
| Description of seizure included in the note | 29 (41.4) | 37 (43.5) | 12 (27.2) | NS |
| Notes in which patients had focal seizures | 17 (24.2) | 24 (28.2) | 7 (15.9) | NS |
| Seizure symptoms included in the note | 10 (14.2) | 14 (16.4) | 5 (11.3) | NS |
| Lateralizing language used in the note | 8 (11.4) | 10 (11.7) | 3 (6.8) | NS |
| Lüders Terminology used in the note | 2 (2.8) | 3 (3.5) | 0 (0) | NS |
| ILAE Terminology used in the note | 3 (4.2) | 3 (3.5) | 0 (0) | NS |
| Change in semiology mentioned in the note | 1 (1.4) | 4 (4.7) | 3 (6.8) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 50 (71.4) | 64 (75.2) | 29 (65.9) | NS |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 49 (70) | 60 (70.5) | 26 (59.1) | NS |
| AEDs listed in automatically generated medication list | 52 (74.2) | 60 (70.5) | 34 (77.2) | NS |
| AEDs commented on elsewhere in the note | 44 (62.8) | 49 (57.6) | 24 (54.5) | NS |
| No change to AED regimen based on the note | 67 (95.7) | 78 (91.7) | 42 (95.4) | NS |
| Note clearly states no change to AED regimen | 35 (50) | 40 (47.1) | 20 (45.4) | NS |
| Patients on prior AEDs | 16 (22.8) | 25 (29.4) | 7 (15.9) | NS |
| Note includes list of prior AEDs | 5 (7.1) | 4 (4.7) | 0 (0) | NS |
Abbreviations: AED, antiepileptic drug; HPI, history of present illness; ILAE: International League Against Epilepsy; NS, not significant.
| . | Initial Treatment . | Stable/Regression . | Progression . | . |
|---|---|---|---|---|
| . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 63 (90) | 79 (92.9) | 39 (88.6) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 47 (67.1) | 62 (72.9) | 27 (61.3) | NS |
| Number of seizures included in the note | 33 (47.1) | 52 (61.1) | 16 (36.3) | .037 |
| Any dates of seizures included in the note | 26 (37.1) | 32 (37.6) | 11 (25) | NS |
| Description of seizure included in the note | 29 (41.4) | 37 (43.5) | 12 (27.2) | NS |
| Notes in which patients had focal seizures | 17 (24.2) | 24 (28.2) | 7 (15.9) | NS |
| Seizure symptoms included in the note | 10 (14.2) | 14 (16.4) | 5 (11.3) | NS |
| Lateralizing language used in the note | 8 (11.4) | 10 (11.7) | 3 (6.8) | NS |
| Lüders Terminology used in the note | 2 (2.8) | 3 (3.5) | 0 (0) | NS |
| ILAE Terminology used in the note | 3 (4.2) | 3 (3.5) | 0 (0) | NS |
| Change in semiology mentioned in the note | 1 (1.4) | 4 (4.7) | 3 (6.8) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 50 (71.4) | 64 (75.2) | 29 (65.9) | NS |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 49 (70) | 60 (70.5) | 26 (59.1) | NS |
| AEDs listed in automatically generated medication list | 52 (74.2) | 60 (70.5) | 34 (77.2) | NS |
| AEDs commented on elsewhere in the note | 44 (62.8) | 49 (57.6) | 24 (54.5) | NS |
| No change to AED regimen based on the note | 67 (95.7) | 78 (91.7) | 42 (95.4) | NS |
| Note clearly states no change to AED regimen | 35 (50) | 40 (47.1) | 20 (45.4) | NS |
| Patients on prior AEDs | 16 (22.8) | 25 (29.4) | 7 (15.9) | NS |
| Note includes list of prior AEDs | 5 (7.1) | 4 (4.7) | 0 (0) | NS |
| . | Initial Treatment . | Stable/Regression . | Progression . | . |
|---|---|---|---|---|
| . | N (%) . | N (%) . | N (%) . | P Value . |
| “Seizure/epilepsy/convulsion” mentioned anywhere in the note | 63 (90) | 79 (92.9) | 39 (88.6) | NS |
| “Seizure/epilepsy/convulsion” used in HPI | 47 (67.1) | 62 (72.9) | 27 (61.3) | NS |
| Number of seizures included in the note | 33 (47.1) | 52 (61.1) | 16 (36.3) | .037 |
| Any dates of seizures included in the note | 26 (37.1) | 32 (37.6) | 11 (25) | NS |
| Description of seizure included in the note | 29 (41.4) | 37 (43.5) | 12 (27.2) | NS |
| Notes in which patients had focal seizures | 17 (24.2) | 24 (28.2) | 7 (15.9) | NS |
| Seizure symptoms included in the note | 10 (14.2) | 14 (16.4) | 5 (11.3) | NS |
| Lateralizing language used in the note | 8 (11.4) | 10 (11.7) | 3 (6.8) | NS |
| Lüders Terminology used in the note | 2 (2.8) | 3 (3.5) | 0 (0) | NS |
| ILAE Terminology used in the note | 3 (4.2) | 3 (3.5) | 0 (0) | NS |
| Change in semiology mentioned in the note | 1 (1.4) | 4 (4.7) | 3 (6.8) | NS |
| “Seizure/epilepsy/convulsion” used in assessment/plan | 50 (71.4) | 64 (75.2) | 29 (65.9) | NS |
| “Seizure/epilepsy/convulsion” mentioned as a separate heading in problem list | 49 (70) | 60 (70.5) | 26 (59.1) | NS |
| AEDs listed in automatically generated medication list | 52 (74.2) | 60 (70.5) | 34 (77.2) | NS |
| AEDs commented on elsewhere in the note | 44 (62.8) | 49 (57.6) | 24 (54.5) | NS |
| No change to AED regimen based on the note | 67 (95.7) | 78 (91.7) | 42 (95.4) | NS |
| Note clearly states no change to AED regimen | 35 (50) | 40 (47.1) | 20 (45.4) | NS |
| Patients on prior AEDs | 16 (22.8) | 25 (29.4) | 7 (15.9) | NS |
| Note includes list of prior AEDs | 5 (7.1) | 4 (4.7) | 0 (0) | NS |
Abbreviations: AED, antiepileptic drug; HPI, history of present illness; ILAE: International League Against Epilepsy; NS, not significant.
Discussion
Results of a descriptive study of TRE documentation in a neuro-oncology clinic are presented. It is in concordance with previously published data in terms of neuro-oncology clinic patient tumor type frequency, with GBM being the most frequent primary tumor and metastatic breast and lung cancer and melanoma being the most common metastatic tumor types.26,27 It is also in line with current literature demonstrating significant seizure burden in lower-grade gliomas, and that IDH-mutated GBM has a higher TRE incidence when compared to IDH-wildtype GBM.
Our study is one of the first to define the proportion of patients with TRE in a neuro-oncology clinic setting. Fifty-six percent of patients seen in our cohort had TRE, with 36.7% having had a seizure within 6 months of their clinic visit. As such, we demonstrate a significant proportion of neuro-oncology patients requiring active seizure surveillance and management. This underscores the centrality of collaboration across specialties, and specifically between neuro-oncologists and epileptologists, to care of patients with TRE.
The majority of notes for all patients seen in the neuro-oncology clinic do include the words “seizures,” “epilepsy,” or “convulsion.” However, details regarding seizure semiology and AED regimen are missing in the majority of documentation. Patients with seizures within 6 months of their clinic visit did show some improved seizure documentation and included more seizure descriptors, however, documentation across all timepoints may be improved (see Table 4). Similarly, documentation in patients with any tumor status may be improved, as well.
Changes in frequency and seizure semiology have been described in association with tumor progression.19–23,28 This has led to calls for systematic evaluation of seizures in those with TRE and further study into correlating seizure activity with treatment response.20,21 However, without improvement and standardization of seizure documentation, successful utilization of seizures as an outcome measure will not be possible. Similarly, without adequate and detailed baseline information on seizure aura, frequency, focality, and lateralization, changes from baseline cannot be accurately assessed.
There are significant gaps in our understanding of TRE, including mechanisms, role of seizures in the oncologic course, and relationship of seizures to prognosis. Furthermore, recommendations on management, both medical and surgical, for TRE exist, but are limited by the current, incomplete state of understanding of TRE.1–9 A clear understanding of the current frequency of seizures and seizure semiology is beneficial for future improvements in the utility of seizures as a clinical trial endpoint and potentially more importantly the complex interactions between tumors and their surrounding neuronal environments.
Notably, standardized tools to improve seizure documentation exist; however, these tools are meant for epilepsy specialists.18,29 Although TRE is significant and an important potential outcome measure of tumor management, epilepsy is often not the primary concern of the neuro-oncologist, and existing tools are thus not appropriate in scope. There is a clear need for a standard tool for seizure documentation that notes appropriate details about frequency and semiology, but that is also readily usable and able to be incorporated in the limited time available during neuro-oncology visits. Such a tool could also be generalized to non-epilepsy specialties in which seizures are commonly seen, such as poststroke epilepsy, and may also be of value to general practitioners who care for patients with known seizure disorders. A tool designed to quickly and accurately characterize a patient’s seizures would be beneficial for neuro-oncologists’ evaluation of a patient’s tumor progression without requiring the use of additional imaging.
This study has some limitations. First, the retrospective use of electronic medical records may not be reflective of the care these patients receive. For example, auto-populated portions of notes may overestimate care given, while it is also possible written portions underestimate care delivery. Furthermore, if the presence of seizures was not accurately documented, the number of patients deemed as having TRE was inaccurately low. However, although an imperfect reflection of care, documentation still remains important for continued research within TRE. Further, as this was conducted in a single institution, generalizability to other hospitals and clinics may be limited. The limited duration of assessment also may not reflect the clinical picture over the course of the year. Finally, the excluded notes for patients who had multiple visits during October 2019 may differ in the thoroughness of documentation.
As this study focused on office notes from the visits in question, other forms of communication, including telephone encounters or MyChart messages where providers may have entered more specific information regarding patients’ seizures, were not accounted for in this study. Also, as with any study on chart documentation for multiple physicians, note styles differ from physician to physician, making classification of location within a note not completely objective when some physicians’ notes are not separated in a classic format. In addition, we did not differentiate between written and copy-forwarded portions of notes, which may also differ between providers.
Conclusion
This study defines a representative cohort of patients seen in neuro-oncology clinic and characterizes their tumor types and seizure incidence. Among patients with TRE, our study also analyzes seizure documentation within neuro-oncology clinic notes. Without a standardized documentation practice, our study shows that documentation can be improved, which would facilitate further analysis of impact on patient care as well as future research within TRE. Future studies in larger cohorts, investigating the change in seizure documentation between patient visits, and investigating ancillary communications are warranted.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
REDCap is supported at Feinberg School of Medicine by the Northwestern University Clinical and Translational Science (NUCATS) Institute, Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422.
Conflict of interest statement. The authors declare that they have no conflicts of interest.




