-
PDF
- Split View
-
Views
-
Cite
Cite
Achiraya Teyateeti, Connie S Geno, Scott S Stafford, Anita Mahajan, Elizabeth S Yan, Kenneth W Merrell, Nadia N Laack, Ian F Parney, Paul D Brown, Krishan R Jethwa, Does the dural resection bed need to be irradiated? Patterns of recurrence and implications for postoperative radiotherapy for temporal lobe gliomas, Neuro-Oncology Practice, Volume 8, Issue 2, April 2021, Pages 190–198, https://doi.org/10.1093/nop/npaa073
Close - Share Icon Share
Abstract
Patterns of recurrence and survival with different surgical and radiotherapy (RT) techniques were evaluated to guide RT target volumes for patients with temporal lobe glioma.
This retrospective cohort study included patients with World Health Organization grades II to IV temporal lobe glioma treated with either partial (PTL) or complete temporal lobectomy (CTL) followed by RT covering both the parenchymal and dural resection bed (whole-cavity radiotherapy [WCRT]) or the parenchymal resection bed only (partial-cavity radiotherapy [PCRT]). Patterns of recurrence, progression-free survival (PFS) and overall survival (OS) were evaluated.
Fifty-one patients were included and 84.3% of patients had high-grade glioma (HGG). CTL and PTL were performed for 11 (21.6%) and 40 (78.4%) patients, respectively. Median RT dose was 60 Gy (range, 40-76 Gy). There were 82.4% and 17.6% of patients who received WCRT and PCRT, respectively. Median follow-up time was 18.4 months (range, 4-161 months). Forty-six patients (90.2%) experienced disease recurrence, most commonly at the parenchymal resection bed (76.5%). No patients experienced an isolated dural recurrence. The median PFS and OS for the PCRT and WCRT cohorts were 8.6 vs 10.8 months (P = .979) and 19.9 vs 18.6 months (P = .859), respectively. PCRT was associated with a lower RT dose to the brainstem, optic, and ocular structures, hippocampus, and pituitary.
We identified no isolated dural recurrence and similar PFS and OS regardless of postoperative RT volume, whereas PCRT was associated with dose reduction to critical structures. Omission of dural RT may be considered a reasonable alternative approach. Further validation with larger comparative studies is warranted.
It is estimated that approximately 87 000 patients were diagnosed with a CNS tumor in 2019, of which 26% were gliomas.1 Standard treatment of glioma includes maximal safe resection, radiotherapy (RT), and chemotherapy based on pathologic and molecular risk stratification.2–9 Owing to the location and associated unique surgical considerations, temporal lobe gliomas may be resected with partial temporal lobectomy (PTL), or when feasible, complete temporal lobectomy (CTL). Postoperative RT target volumes typically include any residual tumor and the temporal resection bed, including the partially evacuated middle cranial fossa and parenchymal resection bed, plus additional brain parenchymal margin to cover potential microscopic disease extension.
Regardless of marginal expansions or RT dose, data suggest that the vast majority of glioma recurrences occur at the site of the original tumor within brain parenchyma and often within the high-dose region of postoperative RT.10–27 The dural resection bed is routinely incorporated into RT treatment volumes, although recurrence in this region is relatively uncommon.28,29 This is an important consideration, particularly for a patient with temporal lobe glioma, because the evacuated middle cranial fossa is in close proximity to multiple critical structures, including the optic nerves, optic chiasm, eyes, pituitary gland, and brainstem, and thus the resultant incidental RT exposure to these organs may increase the risk of treatment-related toxicity.
Based on the aforementioned reports on patterns of failure in temporal lobe glioma, we hypothesized that RT volumes could be safely modified to cover only the parenchymal resection bed while omitting the dural resection bed, thus minimizing RT exposure to nearby critical structures without compromising local disease control.10–27 The purpose of our study was to evaluate the patterns of recurrence and survival in relation to surgical techniques and RT volumes to guide postoperative RT target volume delineation for patients with temporal lobe glioma.
Methods
Patient Selection
Following institutional review board approval, patients diagnosed with World Health Organization (WHO) grades II to IV temporal lobe glioma treated between 1998 and 2018 were retrospectively reviewed. Patients were included if they were age 18 years or older, underwent surgical resection, completed postoperative RT to a total dose of 40 Gy or greater, and had at least 3 months of follow-up.
Treatment
Surgical techniques were either PTL, defined as a wide excision of tumor plus surrounding margin, or CTL, defined as removal of the tumor, temporal cortex, amygdala and hippocampus adjacent to the ambient cistern. Figure 1A demonstrates a representative MRI of a patient following CTL. Extent of the resection was classified as gross total resection (GTR) or subtotal resection (STR) according to the postoperative note and/or MRI performed within 72 hours after surgery. RT typically started within 1 month after surgery, if there were no surgical complications. The clinical target volume (CTV) typically included any residual enhancing tumor, temporal lobe parenchymal resection bed with or without inclusion of the entire resection bed including peripheral dural components, and peritumoral edema plus a 1- to 2-cm margin, at the discretion of the treating physician.
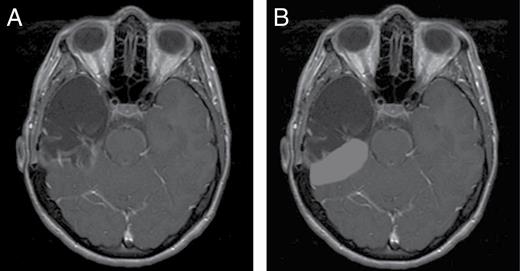
A, Complete temporal lobectomy—remove tumor with temporal cortex, amygdala, and hippocampus adjacent to the ambient cistern. B, Partial cavity radiotherapy—include only the parenchymal resection bed while omitting the dural resection bed.
The parenchymal resection bed was usually included in the high-dose CTV (prescription dose of ≥ 50.4-59.4 Gy). The dural resection bed was either included in the high-dose CTV along with the parenchymal resection bed, included in the low-dose CTV (prescription dose of 45-50.4 Gy), or was omitted from the RT target volumes. Owing to variation in target delineation and the possibility of relatively nonconformal RT treatment delivery with incidental dose to the dural resection bed, the area of interest in the present study, we classified the RT volumes according to the treated volumes accounting for actual RT dose delivered to the dural resection bed. Whole-cavity radiotherapy (WCRT) was defined as a dose of 40 Gy or greater covering both the parenchymal and dural resection bed (ie, a dose of ≥ 40 Gy completely covering the entire temporal resection cavity). Partial-cavity radiotherapy (PCRT) was defined as a dose of 40 Gy or greater covering only the parenchymal resection bed without coverage of the entire dural resection bed. A dose of 40 Gy was chosen because it represents 90% of 45 Gy, which may be considered acceptable coverage of the low-dose CTV both for high-grade glioma (WHO grades III-IV or HGG) and low-grade glioma (WHO grade II or LGG).30,31 For hypofractionated RT of 40 Gy, dose coverage was evaluated at 36 Gy. Figure 1B illustrates an example of PCRT following CTL.
RT treatment techniques included 3-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy, or proton therapy. RT was delivered daily in 1.8- to 2.0-Gy fractions to a dose of 45 Gy or greater or with a hypofractionated regimen of 40 Gy in 15 fractions. An additional boost to the temporal resection bed or residual tumor to greater than 54 Gy was allowed. Chemotherapy use was based on the physician’s and patient’s discretion.
Assessment of Treatment Outcomes and Statistical Analysis
Patients underwent routine oncologic surveillance with physical examination, laboratory evaluation, and MRI brain at 3- to 6-month intervals or as clinically indicated. Patterns of recurrence were reported descriptively. Local recurrence was defined as recurrence within or directly adjacent to the resection bed, whereas distant recurrence was defined as occurring outside the resection bed. Local recurrence was also further categorized as parenchymal, dural, or ventricular. The Kaplan-Meier method was used to estimate overall survival (OS), progression-free survival (PFS), and time-to-recurrence (TTR) from the date of surgery. The differences in OS and PFS between surgical techniques and RT volumes were assessed by log-rank test. Comparisons between WCRT and PCRT cohorts were carried out using the Fisher exact test for categorical data and t test and Mann-Whitney U test for continuous data. All statistical analysis was performed with SPSS, version 21 (IBM Corp) with P less than .05 considered statistically significant.
Results
Between the years 1998 and 2018, 51 patients with WHO grades II to IV temporal lobe glioma were identified. Patient, tumor ,and treatment characteristics are shown in Table 1. The most common diagnosis and treatment strategy was glioblastoma treated with postoperative RT 60 Gy in 30 fractions with concurrent and adjuvant chemotherapy. The rates of gross total resection were comparable between patients undergoing either CTL or PTL (P = .174). There were no statistically significant differences in baseline characteristics between the PCRT and WCRT groups within the entire cohort, nor among the LGG or HGG subgroups (Supplementary Tables A and B).
| Characteristics . | All (n = 51)a . | WCRT (n = 42) . | PCRT (n = 8) . | P . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y, median (range) | 56 (21-76) | 55.5 (22-76) | 58.5 (21-74) | .825 |
| Tumor characteristics | ||||
| Histology, % | ||||
| Oligodendroglioma | 1 (2.0) | 1 (2.4) | – | .099 |
| Oligoastrocytoma | 8 (15.7) | 4 (9.5) | 3 (37.5) | |
| Astrocytoma | 10 (19.6) | 10 (23.8) | – | |
| Glioblastoma | 32 (62.8) | 27 (64.3) | 5 (62.5) | |
| WHO grade, % | ||||
| II | 8 (15.7) | 5 (11.9) | 3 (37.5) | .103 |
| III | 11 (21.6) | 10 (23.8) | – | |
| IV | 32 (62.7) | 27 (64.3) | 5 (62.5) | |
| Treatment characteristics | ||||
| Surgery type, % | ||||
| Complete temporal lobectomy | 11 (21.6)b | 8 (19.0) | 3 (37.5) | .351 |
| Partial temporal lobectomy | 40 (78.4)c | 34 (81.0) | 5 (62.5) | |
| Extent of resection, % | ||||
| Gross total resection | 17 (33.3) | 14 (33.3) | 3 (37.5) | 1.000 |
| Subtotal resection | 34 (66.7) | 28 (66.7) | 5 (62.5) | |
| Systemic treatment, % | ||||
| Concurrent | 38 (74.5) | 31 (73.8) | 7 (87.5) | .661 |
| Adjuvant | 39 (76.5) | 30 (71.4) | 8 (100.0) | .173 |
| Radiotherapy technique, % | ||||
| 3CRT | 29 (56.9) | 25 (59.5) | 3 (37.5) | .050 |
| Intensity-modulated radiotherapy | 21 (41.2) | 17 (40.5) | 4 (50.0) | |
| Proton therapy | 1 (2.0) | – | 1 (12.5) | |
| Radiotherapy dose, Gy, median (range) | 60 (40-76) | 60 (40-76) | 52.2 (40-60) | .093 |
| Characteristics . | All (n = 51)a . | WCRT (n = 42) . | PCRT (n = 8) . | P . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y, median (range) | 56 (21-76) | 55.5 (22-76) | 58.5 (21-74) | .825 |
| Tumor characteristics | ||||
| Histology, % | ||||
| Oligodendroglioma | 1 (2.0) | 1 (2.4) | – | .099 |
| Oligoastrocytoma | 8 (15.7) | 4 (9.5) | 3 (37.5) | |
| Astrocytoma | 10 (19.6) | 10 (23.8) | – | |
| Glioblastoma | 32 (62.8) | 27 (64.3) | 5 (62.5) | |
| WHO grade, % | ||||
| II | 8 (15.7) | 5 (11.9) | 3 (37.5) | .103 |
| III | 11 (21.6) | 10 (23.8) | – | |
| IV | 32 (62.7) | 27 (64.3) | 5 (62.5) | |
| Treatment characteristics | ||||
| Surgery type, % | ||||
| Complete temporal lobectomy | 11 (21.6)b | 8 (19.0) | 3 (37.5) | .351 |
| Partial temporal lobectomy | 40 (78.4)c | 34 (81.0) | 5 (62.5) | |
| Extent of resection, % | ||||
| Gross total resection | 17 (33.3) | 14 (33.3) | 3 (37.5) | 1.000 |
| Subtotal resection | 34 (66.7) | 28 (66.7) | 5 (62.5) | |
| Systemic treatment, % | ||||
| Concurrent | 38 (74.5) | 31 (73.8) | 7 (87.5) | .661 |
| Adjuvant | 39 (76.5) | 30 (71.4) | 8 (100.0) | .173 |
| Radiotherapy technique, % | ||||
| 3CRT | 29 (56.9) | 25 (59.5) | 3 (37.5) | .050 |
| Intensity-modulated radiotherapy | 21 (41.2) | 17 (40.5) | 4 (50.0) | |
| Proton therapy | 1 (2.0) | – | 1 (12.5) | |
| Radiotherapy dose, Gy, median (range) | 60 (40-76) | 60 (40-76) | 52.2 (40-60) | .093 |
Abbreviations: 3CRT, 3-dimensional conformal radiotherapy; PCRT, partial-cavity radiotherapy; WCRT, whole-cavity radiotherapy; WHO, World Health Organization.
aMissing radiotherapy plan in 1 patient.
bIncluding 6 gross total resections and 5 subtotal resections.
cIncluding 11 gross total resections and 29 subtotal resections.
| Characteristics . | All (n = 51)a . | WCRT (n = 42) . | PCRT (n = 8) . | P . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y, median (range) | 56 (21-76) | 55.5 (22-76) | 58.5 (21-74) | .825 |
| Tumor characteristics | ||||
| Histology, % | ||||
| Oligodendroglioma | 1 (2.0) | 1 (2.4) | – | .099 |
| Oligoastrocytoma | 8 (15.7) | 4 (9.5) | 3 (37.5) | |
| Astrocytoma | 10 (19.6) | 10 (23.8) | – | |
| Glioblastoma | 32 (62.8) | 27 (64.3) | 5 (62.5) | |
| WHO grade, % | ||||
| II | 8 (15.7) | 5 (11.9) | 3 (37.5) | .103 |
| III | 11 (21.6) | 10 (23.8) | – | |
| IV | 32 (62.7) | 27 (64.3) | 5 (62.5) | |
| Treatment characteristics | ||||
| Surgery type, % | ||||
| Complete temporal lobectomy | 11 (21.6)b | 8 (19.0) | 3 (37.5) | .351 |
| Partial temporal lobectomy | 40 (78.4)c | 34 (81.0) | 5 (62.5) | |
| Extent of resection, % | ||||
| Gross total resection | 17 (33.3) | 14 (33.3) | 3 (37.5) | 1.000 |
| Subtotal resection | 34 (66.7) | 28 (66.7) | 5 (62.5) | |
| Systemic treatment, % | ||||
| Concurrent | 38 (74.5) | 31 (73.8) | 7 (87.5) | .661 |
| Adjuvant | 39 (76.5) | 30 (71.4) | 8 (100.0) | .173 |
| Radiotherapy technique, % | ||||
| 3CRT | 29 (56.9) | 25 (59.5) | 3 (37.5) | .050 |
| Intensity-modulated radiotherapy | 21 (41.2) | 17 (40.5) | 4 (50.0) | |
| Proton therapy | 1 (2.0) | – | 1 (12.5) | |
| Radiotherapy dose, Gy, median (range) | 60 (40-76) | 60 (40-76) | 52.2 (40-60) | .093 |
| Characteristics . | All (n = 51)a . | WCRT (n = 42) . | PCRT (n = 8) . | P . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y, median (range) | 56 (21-76) | 55.5 (22-76) | 58.5 (21-74) | .825 |
| Tumor characteristics | ||||
| Histology, % | ||||
| Oligodendroglioma | 1 (2.0) | 1 (2.4) | – | .099 |
| Oligoastrocytoma | 8 (15.7) | 4 (9.5) | 3 (37.5) | |
| Astrocytoma | 10 (19.6) | 10 (23.8) | – | |
| Glioblastoma | 32 (62.8) | 27 (64.3) | 5 (62.5) | |
| WHO grade, % | ||||
| II | 8 (15.7) | 5 (11.9) | 3 (37.5) | .103 |
| III | 11 (21.6) | 10 (23.8) | – | |
| IV | 32 (62.7) | 27 (64.3) | 5 (62.5) | |
| Treatment characteristics | ||||
| Surgery type, % | ||||
| Complete temporal lobectomy | 11 (21.6)b | 8 (19.0) | 3 (37.5) | .351 |
| Partial temporal lobectomy | 40 (78.4)c | 34 (81.0) | 5 (62.5) | |
| Extent of resection, % | ||||
| Gross total resection | 17 (33.3) | 14 (33.3) | 3 (37.5) | 1.000 |
| Subtotal resection | 34 (66.7) | 28 (66.7) | 5 (62.5) | |
| Systemic treatment, % | ||||
| Concurrent | 38 (74.5) | 31 (73.8) | 7 (87.5) | .661 |
| Adjuvant | 39 (76.5) | 30 (71.4) | 8 (100.0) | .173 |
| Radiotherapy technique, % | ||||
| 3CRT | 29 (56.9) | 25 (59.5) | 3 (37.5) | .050 |
| Intensity-modulated radiotherapy | 21 (41.2) | 17 (40.5) | 4 (50.0) | |
| Proton therapy | 1 (2.0) | – | 1 (12.5) | |
| Radiotherapy dose, Gy, median (range) | 60 (40-76) | 60 (40-76) | 52.2 (40-60) | .093 |
Abbreviations: 3CRT, 3-dimensional conformal radiotherapy; PCRT, partial-cavity radiotherapy; WCRT, whole-cavity radiotherapy; WHO, World Health Organization.
aMissing radiotherapy plan in 1 patient.
bIncluding 6 gross total resections and 5 subtotal resections.
cIncluding 11 gross total resections and 29 subtotal resections.
By last follow-up, 46 of 51 patients (90.2%) experienced disease recurrence. Of 45 characterizable recurrences, there were 35 local (77.8%), 5 distant (11.1%), and 5 both local and distant (11.1%). Seven of 8 patients (87.5%) with LGG experienced recurrence including 5 local (62.5%), 1 both local and distant (12.5%), and 1 unknown pattern of recurrence in a patient treated with WCRT and PTL (12.5%). Thirty-nine of 43 patients (90.7%) with HGG experienced recurrence—30 local (69.8%), 5 distant (11.6%), and 4 both local and distant (9.3%). Of note, there was an inaccessible RT plan in 1 HGG patient who had recurrence at the parenchyma and dura. Pattern of recurrence in LGG and HGG patients in correlation with RT volumes and surgical techniques are shown in Tables 2 and 3, respectively. The most common site of recurrence was brain parenchymal local recurrence. There were no isolated dural recurrences irrespective of RT volumes and surgical techniques, and no patients with LGG or those who underwent CTL (regardless of tumor grade) experienced a dural recurrence.
Pattern of Failures Stratified by Radiotherapy Volumes and Surgical Techniques in Low-Grade Glioma Patientsa
| . | WCRT (n = 4) . | . | PCRT (n = 3) . | . |
|---|---|---|---|---|
| . | CTL (n = 0) . | PTL (n = 4) . | CTL (n = 1) . | PTL (n = 2) . |
| No recurrence | – | – | – | 1 |
| Parenchyma only | – | 4 | 1 | 1 |
| . | WCRT (n = 4) . | . | PCRT (n = 3) . | . |
|---|---|---|---|---|
| . | CTL (n = 0) . | PTL (n = 4) . | CTL (n = 1) . | PTL (n = 2) . |
| No recurrence | – | – | – | 1 |
| Parenchyma only | – | 4 | 1 | 1 |
Abbreviations: CTL, complete temporal lobectomy; PCRT, partial-cavity radiotherapy; PTL, partial temporal lobectomy; WCRT, whole-cavity radiotherapy.
aUnknown pattern of recurrence in one patient.
Pattern of Failures Stratified by Radiotherapy Volumes and Surgical Techniques in Low-Grade Glioma Patientsa
| . | WCRT (n = 4) . | . | PCRT (n = 3) . | . |
|---|---|---|---|---|
| . | CTL (n = 0) . | PTL (n = 4) . | CTL (n = 1) . | PTL (n = 2) . |
| No recurrence | – | – | – | 1 |
| Parenchyma only | – | 4 | 1 | 1 |
| . | WCRT (n = 4) . | . | PCRT (n = 3) . | . |
|---|---|---|---|---|
| . | CTL (n = 0) . | PTL (n = 4) . | CTL (n = 1) . | PTL (n = 2) . |
| No recurrence | – | – | – | 1 |
| Parenchyma only | – | 4 | 1 | 1 |
Abbreviations: CTL, complete temporal lobectomy; PCRT, partial-cavity radiotherapy; PTL, partial temporal lobectomy; WCRT, whole-cavity radiotherapy.
aUnknown pattern of recurrence in one patient.
Pattern of Failures Stratified by Radiotherapy Volumes and Surgical Techniques in High-Grade Glioma Patientsa
| . | WCRT (n = 37) . | . | PCRT (n = 5) . | . |
|---|---|---|---|---|
| . | CTL (n = 8) . | PTL (n = 29) . | CTL (n = 2) . | PTL (n = 3) . |
| No recurrence | 1 | 3 | – | – |
| Distant recurrence | 2 | 3 | – | – |
| Local recurrence | ||||
| Parenchyma only | 2 | 18 | 2 | 1 |
| Parenchyma and dura | – | 3 | – | 1 |
| Parenchyma and ventricle | 2 | 2 | – | - |
| Parenchyma, dura and ventricle | – | – | – | 1 |
| Ventricle only | 1 | – | – | – |
| . | WCRT (n = 37) . | . | PCRT (n = 5) . | . |
|---|---|---|---|---|
| . | CTL (n = 8) . | PTL (n = 29) . | CTL (n = 2) . | PTL (n = 3) . |
| No recurrence | 1 | 3 | – | – |
| Distant recurrence | 2 | 3 | – | – |
| Local recurrence | ||||
| Parenchyma only | 2 | 18 | 2 | 1 |
| Parenchyma and dura | – | 3 | – | 1 |
| Parenchyma and ventricle | 2 | 2 | – | - |
| Parenchyma, dura and ventricle | – | – | – | 1 |
| Ventricle only | 1 | – | – | – |
Abbreviations: CTL, complete temporal lobectomy; PCRT, partial-cavity radiotherapy; PTL, partial temporal lobectomy; WCRT, whole-cavity radiotherapy.
aMissing radiotherapy plan in one patient.
Pattern of Failures Stratified by Radiotherapy Volumes and Surgical Techniques in High-Grade Glioma Patientsa
| . | WCRT (n = 37) . | . | PCRT (n = 5) . | . |
|---|---|---|---|---|
| . | CTL (n = 8) . | PTL (n = 29) . | CTL (n = 2) . | PTL (n = 3) . |
| No recurrence | 1 | 3 | – | – |
| Distant recurrence | 2 | 3 | – | – |
| Local recurrence | ||||
| Parenchyma only | 2 | 18 | 2 | 1 |
| Parenchyma and dura | – | 3 | – | 1 |
| Parenchyma and ventricle | 2 | 2 | – | - |
| Parenchyma, dura and ventricle | – | – | – | 1 |
| Ventricle only | 1 | – | – | – |
| . | WCRT (n = 37) . | . | PCRT (n = 5) . | . |
|---|---|---|---|---|
| . | CTL (n = 8) . | PTL (n = 29) . | CTL (n = 2) . | PTL (n = 3) . |
| No recurrence | 1 | 3 | – | – |
| Distant recurrence | 2 | 3 | – | – |
| Local recurrence | ||||
| Parenchyma only | 2 | 18 | 2 | 1 |
| Parenchyma and dura | – | 3 | – | 1 |
| Parenchyma and ventricle | 2 | 2 | – | - |
| Parenchyma, dura and ventricle | – | – | – | 1 |
| Ventricle only | 1 | – | – | – |
Abbreviations: CTL, complete temporal lobectomy; PCRT, partial-cavity radiotherapy; PTL, partial temporal lobectomy; WCRT, whole-cavity radiotherapy.
aMissing radiotherapy plan in one patient.
The median follow-up time for the entire cohort was 18.4 months (range, 4-161 months); 58.3 months (range, 12-161 months) for LGG; and 16.6 months (range, 4-86 months). The median TTR among 46 patients with recurrence was 9.2 months (95% CI, 5.3-13.1). The median TTR of local, distant, and both local and distant recurrences were 8.4 (95% CI, 6.9-9.9), 15.5 (95% CI, 9.8-21.1), and 15.6 (95% CI, 0.0-32.1) months, respectively.
The median PFS and OS for entire cohort were 11.9 (95% CI, 5.1-18.7) and 19.5 (95% CI, 14.7-24.3) months, respectively (Supplementary Figures A and B). There were no significant differences in PFS and OS between RT volumes (PCRT vs WCRT) and surgical techniques (CTL vs PTL). Median PFS and OS of PCRT and WCRT cohorts were 8.6 vs 10.8 months (P = .979) (Figure 2A) and 19.9 vs 18.6 months (P = .859) (Figure 2B), respectively. The median PFS and OS for CTL and PTL cohorts were 15.5 vs 10.8 months (P = .627) (Figure 2C) and 19.5 vs 19.0 months (P = .425) (Figure 2D), respectively. Similarly, RT volumes or surgical techniques were not associated with differences in PFS or OS when stratified by LGG and HGG subgroups, as demonstrated in Supplementary Tables C and D.
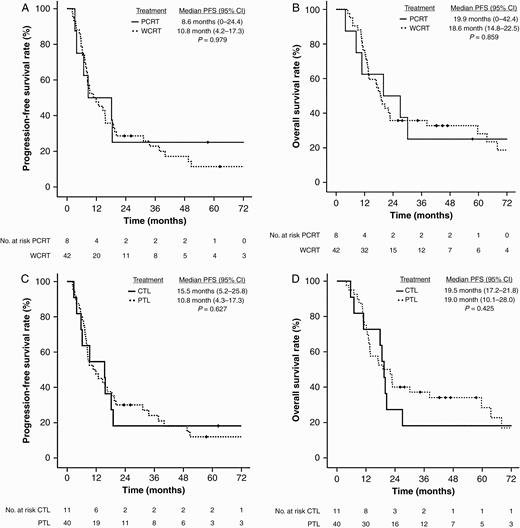
A, Progression-free survival of whole-cavity radiotherapy and partial-cavity radiotherapy. B, Overall survival of whole-cavity radiotherapy and partial-cavity radiotherapy. C, Progression-free survival of complete temporal lobectomy and partial temporal lobectomy. D, Overall survival of complete temporal lobectomy and partial temporal lobectomy.
RT dose delivered to adjacent critical normal structures for patients treated with either WCRT or PCRT are presented in Table 4. PCRT reduced dose in most structures (7.2%-67.5%) with statistically significant mean or maximum dose reductions observed for the brainstem, optic chiasm, contralateral optic nerve, ipsilateral eye, ipsilateral lens, contralateral hippocampus, and pituitary.
| Structures . | . | WCRT, Gy . | PCRT, Gy . | Reduction, % . | P . |
|---|---|---|---|---|---|
| Brain | Mean | 30.91 | 24.47 | 20.8 | .133 |
| Brainstem | Max | 56.52 | 50.83 | 10.1 | .021 |
| Mean | 39.04 | 30.93 | 20.8 | .019 | |
| Optic chiasm | Max | 53.85 | 49.96 | 7.20 | .147 |
| Mean | 44.63 | 36.25 | 18.8 | .048 | |
| Ipsilateral optic nerve | Max | 52.89 | 48.87 | 7.60 | .122 |
| Mean | 39.62 | 33.66 | 15.0 | .178 | |
| Contralateral optic nerve | Max | 32.90 | 22.00 | 33.1 | .040 |
| Mean | 15.56 | 8.11 | 47.9 | .018 | |
| Ipsilateral eye | Max | 25.22 | 13.32 | 47.2 | .004 |
| Mean | 8.47 | 4.06 | 52.0 | .003 | |
| Contralateral eye | Max | 8.08 | 4.86 | 39.8 | .279 |
| Mean | 4.13 | 2.85 | 31.0 | .367 | |
| Ipsilateral lens | Max | 4.63 | 2.79 | 39.8 | .049 |
| Mean | 3.58 | 2.85 | 20.4 | .419 | |
| Contralateral lens | Max | 3.20 | 2.37 | 25.9 | .489 |
| Mean | 2.86 | 3.09 | –8.2 | .920 | |
| Ipsilateral hippocampus | Max | 66.27 | 56.85 | 14.2 | .452 |
| Mean | 62.19 | 55.76 | 10.3 | .615 | |
| Contralateral hippocampus | Max | 36.48 | 26.91 | 26.2 | .447 |
| Mean | 22.19 | 7.22 | 67.5 | .024 | |
| Pituitary | Max | 49.42 | 40.14 | 18.8 | .001 |
| Mean | 41.28 | 24.71 | 40.1 | .003 |
| Structures . | . | WCRT, Gy . | PCRT, Gy . | Reduction, % . | P . |
|---|---|---|---|---|---|
| Brain | Mean | 30.91 | 24.47 | 20.8 | .133 |
| Brainstem | Max | 56.52 | 50.83 | 10.1 | .021 |
| Mean | 39.04 | 30.93 | 20.8 | .019 | |
| Optic chiasm | Max | 53.85 | 49.96 | 7.20 | .147 |
| Mean | 44.63 | 36.25 | 18.8 | .048 | |
| Ipsilateral optic nerve | Max | 52.89 | 48.87 | 7.60 | .122 |
| Mean | 39.62 | 33.66 | 15.0 | .178 | |
| Contralateral optic nerve | Max | 32.90 | 22.00 | 33.1 | .040 |
| Mean | 15.56 | 8.11 | 47.9 | .018 | |
| Ipsilateral eye | Max | 25.22 | 13.32 | 47.2 | .004 |
| Mean | 8.47 | 4.06 | 52.0 | .003 | |
| Contralateral eye | Max | 8.08 | 4.86 | 39.8 | .279 |
| Mean | 4.13 | 2.85 | 31.0 | .367 | |
| Ipsilateral lens | Max | 4.63 | 2.79 | 39.8 | .049 |
| Mean | 3.58 | 2.85 | 20.4 | .419 | |
| Contralateral lens | Max | 3.20 | 2.37 | 25.9 | .489 |
| Mean | 2.86 | 3.09 | –8.2 | .920 | |
| Ipsilateral hippocampus | Max | 66.27 | 56.85 | 14.2 | .452 |
| Mean | 62.19 | 55.76 | 10.3 | .615 | |
| Contralateral hippocampus | Max | 36.48 | 26.91 | 26.2 | .447 |
| Mean | 22.19 | 7.22 | 67.5 | .024 | |
| Pituitary | Max | 49.42 | 40.14 | 18.8 | .001 |
| Mean | 41.28 | 24.71 | 40.1 | .003 |
Abbreviations: Max, maximum; PCRT, partial-cavity radiotherapy; WCRT, whole-cavity radiotherapy.
| Structures . | . | WCRT, Gy . | PCRT, Gy . | Reduction, % . | P . |
|---|---|---|---|---|---|
| Brain | Mean | 30.91 | 24.47 | 20.8 | .133 |
| Brainstem | Max | 56.52 | 50.83 | 10.1 | .021 |
| Mean | 39.04 | 30.93 | 20.8 | .019 | |
| Optic chiasm | Max | 53.85 | 49.96 | 7.20 | .147 |
| Mean | 44.63 | 36.25 | 18.8 | .048 | |
| Ipsilateral optic nerve | Max | 52.89 | 48.87 | 7.60 | .122 |
| Mean | 39.62 | 33.66 | 15.0 | .178 | |
| Contralateral optic nerve | Max | 32.90 | 22.00 | 33.1 | .040 |
| Mean | 15.56 | 8.11 | 47.9 | .018 | |
| Ipsilateral eye | Max | 25.22 | 13.32 | 47.2 | .004 |
| Mean | 8.47 | 4.06 | 52.0 | .003 | |
| Contralateral eye | Max | 8.08 | 4.86 | 39.8 | .279 |
| Mean | 4.13 | 2.85 | 31.0 | .367 | |
| Ipsilateral lens | Max | 4.63 | 2.79 | 39.8 | .049 |
| Mean | 3.58 | 2.85 | 20.4 | .419 | |
| Contralateral lens | Max | 3.20 | 2.37 | 25.9 | .489 |
| Mean | 2.86 | 3.09 | –8.2 | .920 | |
| Ipsilateral hippocampus | Max | 66.27 | 56.85 | 14.2 | .452 |
| Mean | 62.19 | 55.76 | 10.3 | .615 | |
| Contralateral hippocampus | Max | 36.48 | 26.91 | 26.2 | .447 |
| Mean | 22.19 | 7.22 | 67.5 | .024 | |
| Pituitary | Max | 49.42 | 40.14 | 18.8 | .001 |
| Mean | 41.28 | 24.71 | 40.1 | .003 |
| Structures . | . | WCRT, Gy . | PCRT, Gy . | Reduction, % . | P . |
|---|---|---|---|---|---|
| Brain | Mean | 30.91 | 24.47 | 20.8 | .133 |
| Brainstem | Max | 56.52 | 50.83 | 10.1 | .021 |
| Mean | 39.04 | 30.93 | 20.8 | .019 | |
| Optic chiasm | Max | 53.85 | 49.96 | 7.20 | .147 |
| Mean | 44.63 | 36.25 | 18.8 | .048 | |
| Ipsilateral optic nerve | Max | 52.89 | 48.87 | 7.60 | .122 |
| Mean | 39.62 | 33.66 | 15.0 | .178 | |
| Contralateral optic nerve | Max | 32.90 | 22.00 | 33.1 | .040 |
| Mean | 15.56 | 8.11 | 47.9 | .018 | |
| Ipsilateral eye | Max | 25.22 | 13.32 | 47.2 | .004 |
| Mean | 8.47 | 4.06 | 52.0 | .003 | |
| Contralateral eye | Max | 8.08 | 4.86 | 39.8 | .279 |
| Mean | 4.13 | 2.85 | 31.0 | .367 | |
| Ipsilateral lens | Max | 4.63 | 2.79 | 39.8 | .049 |
| Mean | 3.58 | 2.85 | 20.4 | .419 | |
| Contralateral lens | Max | 3.20 | 2.37 | 25.9 | .489 |
| Mean | 2.86 | 3.09 | –8.2 | .920 | |
| Ipsilateral hippocampus | Max | 66.27 | 56.85 | 14.2 | .452 |
| Mean | 62.19 | 55.76 | 10.3 | .615 | |
| Contralateral hippocampus | Max | 36.48 | 26.91 | 26.2 | .447 |
| Mean | 22.19 | 7.22 | 67.5 | .024 | |
| Pituitary | Max | 49.42 | 40.14 | 18.8 | .001 |
| Mean | 41.28 | 24.71 | 40.1 | .003 |
Abbreviations: Max, maximum; PCRT, partial-cavity radiotherapy; WCRT, whole-cavity radiotherapy.
Discussion
We retrospectively assessed patients with temporal lobe glioma to evaluate the potential associations between surgical techniques and postoperative RT volumes with pattern of recurrence and survival. We identified that glioma recurrences are most commonly within or directly adjacent to the parenchymal resection bed, and there were no isolated dural recurrences. Survival analysis demonstrated comparable PFS and OS regardless of whether patients received post-operative WCRT or PCRT. Furthermore, PCRT was associated with a significant reduction in RT dose to multiple critical normal tissues. Based on these data, omission of the dural resection bed from the RT target volume may be considered as an alternative approach that could reduce the risk of RT-associated toxicities without compromising local control in temporal lobe glioma patients.
Postoperative RT target volumes for patients with glioma, as suggested by the Radiation Therapy Oncology Group and European Organization for Research and Treatment of Cancer, typically include the gross tumor volume (GTV) and entire resection bed with an additional 2- to 3-cm margin to cover peritumoral edema and the associated highest risk areas of microscopic disease extension.4–7,30–32 However, even with this generous margin, more than 70% to 80% of recurrences still occur within the irradiated volume and predominantly in or adjacent to the resection bed directly within the high-RT dose region.10–27,33 Furthermore, studies demonstrate that wider marginal expansions from the GTV or the addition of temozolomide have minimal impact on the pattern of recurrence.15,19,23,33–35 Based on these findings, further minimization of marginal expansion from GTV has become an appealing strategy in an effort to reduce toxicity and recently has been endorsed by the Adult Brain Tumor Consortium guidelines, which propose a 5-mm expansion.33
Apart from minimizing marginal expansion into brain parenchyma, omission of the dural resection bed (ie, PCRT) is another approach to further reduce RT volume. One concern of this approach is a potential increased risk of dural resection bed recurrence and its adverse impact on survival. However, data suggest that the dura is an uncommon site for glioma growth and may act as a natural barrier for extracranial extension.28,29 Supporting this hypothesis, we have demonstrated that regardless of RT volume or surgical technique, no patients experienced isolated dural recurrence and no dural recurrences occurred in patients who underwent CTL. These data are most consistent with those reported by Marsh et al, who reported on 5 patients with HGG who underwent CTL and postoperative RT, none of whom experienced dural resection bed recurrence.28 Furthermore, there were no significant differences in PFS or OS among patients in the PCRT and WCRT cohort. Taken together, these data suggest that brain parenchymal recurrence at or adjacent to the site of the initial tumor remains the highest-risk site of recurrence regardless of surgical or RT technique, and omission of the dural resection bed from RT fields is unlikely to be associated with an increased risk of dural recurrence.
The likelihood of RT-related organ injury is directly correlated with the dose delivered to the organ and the volume of the organ irradiated,36–38 and thus the major benefit of PCRT (or other limited RT volume strategy) is a theoretical reduction in the risk of toxicity through a reduction in RT exposure to adjacent critical structures. Marsh et al previously demonstrated that postoperative intensity-modulated radiotherapy targeting PCRT compared to WCRT was associated with a lower mean and maximum RT dose to most adjacent normal structures.28 We similarly identified that treatment with PCRT was associated with a significantly lower mean and maximum RT dose to the brainstem, optic chiasm, contralateral optic nerve, ipsilateral eye, ipsilateral lens, contralateral hippocampus, and pituitary, thus potentially reducing the risk of serious toxicities including vision loss, optic neuropathy, brainstem necrosis, neurocognitive decline, and endocrinopathies.39,40 Despite the majority of patients in our study having received 3DCRT (60%), we surmise that similar dosimetric improvements would have been achievable if patients were treated with more advanced RT techniques such as volumetric modulated arc therapy or intensity-modulated proton therapy.41–44 Thus, PCRT may be considered regardless of chosen RT technique or modality as these incremental improvements could offer further improvement in patient outcomes.
We sought to clarify postoperative target delineation for patients with resected temporal lobe gliomas using conventional surgical, radiographic, and pathologic risk factors. However, an important area of continued investigation involves the use of advanced imaging techniques to identify highest risk regions of tumor recurrence. These techniques include advanced MRI techniques such as DWI, perfusion-weighted imaging, and magnetic resonance spectroscopy or PET with novel radiotracers such as 11C-methionine,18F-fluoroethyltyrosine,18F-fluorodihydroxyphenylalanine,18F-fluciclovine, and 18F-fluoromisonidazole. Theoretically, use of these imaging modalities could facilitate identification of high-risk areas to guide individualized and patient-specific tailoring of target volumes and RT dose while possibly allowing simultaneous reductions in the uniform volumetric expansions into normal brain parenchyma.45,46 Data from these studies have significant potential to improve future postoperative RT for patients with glioma and are eagerly anticipated.
Despite being one of the few studies that has focused on patients with temporal lobe glioma and the correlation of surgical technique and postoperative RT volume with outcomes, our retrospective study does have some important limitations. Patients included in this study received treatment spanning a period of 20 years throughout a time of substantial developments of systemic treatments, surgical approaches, and RT techniques. Additionally, molecular profiles such as MGMT (O6-methylguanine-DNA methyltransferase) promotor methylation, IDH (isocitrate dehydrogenase) mutation, and 1p19q codeletion, which are well documented as important prognostic factors2,4,7,8 were incomplete in our study. Thus, our results may not best reflect treatment outcomes of current practice and may not be applicable to all subgroups. The availability of all electronic RT treatment plans in our current computerized treatment planning system would have facilitated registration of diagnostic scans at tumor recurrence to the RT planning image sets, and thus a more accurate assessment of infield, marginal, and outfield recurrences. Unfortunately, these plans were not available for all patients. Although we did demonstrate a reduction in RT exposure to multiple organs, our findings would have been significantly strengthened if correlated with improved patient adverse event rates or patient-reported outcomes. Finally, our limited patient numbers, particularly among histological and treatment subgroups, prevents the formulation of strong clinical conclusions. Thus, further validation of our findings in the context of another clinical study or prospective trial is warranted.
Conclusion
Patients with temporal lobe glioma who undergo surgery and postoperative RT are most likely to experience disease recurrence within the brain parenchyma adjacent to the tumor resection bed and have a low risk of isolated dural recurrence regardless of surgical or RT technique. These data are hypothesis generating and suggest that omission of portions of the evacuated middle cranial fossa or dural resection bed may theoretically allow for a reduction in risk of RT-associated toxicity to normal tissues without compromising oncologic outcomes, and may be considered as an alternative strategy for selected patients.
Supplementary material
Supplementary material is available online at Neuro-Oncology Practice (http://nop.oxfordjournals.org/).
Acknowledgments
This work was approved by the institutional review board (application No. 18-001601, approval date February 28, 2018).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. I.F.P. reports grants from Merck Pharmaceuticals, outside the submitted work. P.D.B. reports personal fees from UpToDate (contributor), outside the submitted work.




