-
PDF
- Split View
-
Views
-
Cite
Cite
Louis Larrouquere, Christelle Dufour, Cécile Faure-Conter, Claire Alapetite, David Meyronet, Stéphanie Bolle, Alice Bonneville-Levard, Marie-Pierre Sunyach, Valérie Laurence, Didier Frappaz, Intensive pediatric chemotherapy regimen (PNET HR+5) in adult high-risk medulloblastoma and pineoblastoma patients, Neuro-Oncology Advances, Volume 6, Issue 1, January-December 2024, vdae141, https://doi.org/10.1093/noajnl/vdae141
Close - Share Icon Share
Abstract
High-risk medulloblastoma (HRMB) is rare in adults. The 5-year overall survival rate is less than 60%. We present here a retrospective analysis of adults treated with an intensive pediatric chemo-radiotherapy regimen PNET HR + 5: NCT00936156.
Eighteen patients over the age of 20 (range, 20–33 years) with HRMB (n = 13), pinealoblastoma (n = 4), and central nervous system embryonal tumor (n = 1) were treated with 2 courses of carboplatin-etoposide followed by 2 courses of high-dose thiotepa (HDT) with autologous hematopoietic stem-cell rescue. A craniospinal irradiation (CSI; 36 Gy craniospinal axis then a boost of 18 Gy to the primary tumor site) was then initiated within 150 days of surgery, completed with 6 cycles of temozolomide; the axis irradiation was not mandatory for non-metastatic pinealoblastoma.
We observed no progression under chemotherapy and no toxic death. Four patients received only 1 HDT. Two non-metastatic pinaloblastomas received only focal irradiation. One medulloblastoma received only 25 Gy on the axis. 56% (10/18) received 6 cycles of temozolomide. No long-term toxicity was recorded. The median time between surgery and CSI was 175 days (range, 115–250). With a median follow-up of 6.0 years (range, 2.6–9), the progression-free survival and overall survival rates for medulloblastoma were respectively 65% (95% CI: 31%–86%) and 76% (95% CI: 42%–91%) at 5 years.
The PNET HR + 5 regimen showed promising results in an adult population, with a meaningful improvement in progression-free survival and overall survival in patients with HRMB.
PNET HR + 5 regimen used in adult high-risk medulloblastoma is feasible with minor regimen adaptation.
PNET HR + 5 regimen resulted in 65% progression-free survival and 76% overall survival of HRMB patients after 5 years.
Adults with high-risk medulloblastoma (HRMB) have a 5-year overall survival (OS) rate of less than 60%. This brain tumor mainly arises in children and adolescents, and the chemotherapy and radiation therapy regimens reported in adults have thus largely been adapted from pediatric standard-of-care. High-dose thiotepa (HDT) with autologous hematopoietic stem-cell rescue has shown promising results in recurrent, refractory, or poor prognosis brain tumors. A recently published phase II study (PNET HR + 5 trial, NCT00936156), used 2 cycles of HDT for children and adolescents/young adults until 20 years with HRMB in addition to standard chemotherapy and craniospinal irradiation. Patient outcome was promising with a 5-year OS rate of 76% (63–86). Here, retrospective analyses of a PNET HR + 5 regimen administered to adults and their outcome, revealed similar to the pediatric population with a 5-year OS rate of 76% (42–91). No progression under treatment, no toxic death, and no long-term toxicity were observed.
Medulloblastoma is the most frequent brain cancer in children and young adults and rarely appears later in adulthood.1 Its physiopathology, genetics, prognosis, treatment toxicity, and treatment sequelae differ greatly between children and adults.2
Prognosis depends on the stage of the disease3 and on histo-molecular prognostic factors, including the histological presence of large cell anaplasia and the amplification of the MYCN/MYC oncogene.4 Medulloblastoma is divided into 4 molecular subgroups according to the latest WHO classification published in 20215: (1) activation of WNT pathway (WNT+), (2) activation of the sonic hedgehog pathway without TP53 mutation (SHH + TP53wt), (3) SHH + with TP53 mutation (SHH + TP53mut), and (4) absence of activation of the WNT and the sonic hedgehog pathways (non-WNT/non-SHH).5 The distribution of these subgroups is different across age groups, with SHH + TP53wt being the most frequent subgroup in adults.6 In addition, the prognostic impact of these subtypes varies between adults and children; for instance, the WNT + subgroup is associated with a favorable prognosis in children, whereas it resembles that of the SHH + TP53wt subgroup in adults.6–8 Beyond these molecular subgroups, there are three grades of the disease in children: Low risk, standard risk, and high risk of relapse,9 and only 2 in adults: standard risk and high risk of relapse.10 High-risk is currently characterized in both groups by the presence of metastases and/or cerebrospinal fluid (CSF) invasion and/or the presence of a postoperative residue >1.5 cm² and/or the presence of MYC/MYCN amplification and/or histological presence of large cell anaplasia. The prognostic impact of postoperative residual disease >1.5 cm² alone is contested in pediatric patients due to inconsistent data, while it continues to be considered a high-risk factor in adult patients.11 Central nervous system (CNS) embryonal tumor and pinealoblastoma, though differing in origin, are usually grouped with high-risk medulloblastomas for pragmatic reasons.
Historically, the treatment of medulloblastoma in children began with surgery and then radiotherapy. The development of chemotherapy then aimed at reinforcing the treatment in the group considered at high risk of relapse with the use of high-dose chemotherapy with autologous hematopoietic stem-cell rescue (ASCR).12
The results of the French phase II study PNET HR + 5 (NCT0093615) were recently published for a prospective cohort of children, adolescents, and young adults (AYA) aged 5 to 20 years with high-risk medulloblastoma (HRMB) and pinealoblastoma.12 After initial surgery or biopsy, patients received 2 cycles of carboplatin and etoposide (CE) followed by tandem intensification chemotherapy with high-dose thiotepa (HDT) and ASCR. Craniospinal irradiation with a focal boost on the primitive tumor bed was then delivered followed by maintenance with temozolomide for 6 months. The HRMB 3- and 5-year progression-free survival (PFS) rates with 95% confidence intervals (95% CI) were 78% (65–88) and 76% (63–86), and the 3- and 5-year OS rates were 84% (72–92) and 76% (63–86), respectively.12 As this study showed one of the best survival rates available in pediatric literature for HRMB, the treatment regimen was proposed to adults with HRMB aged 20 and over in the framework of the French national weekly multidisciplinary virtual meeting dedicated to AYA medulloblastomas.13
We present here a retrospective study of adult HRMB, pinealoblastoma, and CNS embryonal tumors treated with PNET HR + 5.
Methods
Patients and Data Collection
After institutional review board approval, we retrospectively reviewed charts of patients diagnosed between 2007 and 2018 with high-risk medulloblastoma, pinaloblastoma, or CNS embryonal tumor, over the age of 20 and treated according to the PNET HR + 5 regimen at the Center Léon Bérard (CLB), Lyon, France; Gustave Roussy Cancer Center (GR), Villejuif, France and Institut Curie (IC), Paris, France. Patients were identified through a CLB, GR, and IC database search. Patient records were retrospectively analyzed. We created a data collection database and recorded patient demographics, clinical characteristics, and data regarding treatments and toxicity management, vital status, date of death, or last follow-up. Medulloblastoma subgroups were identified from paraffin-embedded tumor samples using routine methods with primary antibodies (beta-catenin, YAP1, Filamin A, GAB1, P53). MYC and MYCN statuses were assessed by fluorescence in situ hybridization or by array-CGH using Agilent arrays. The central review was performed under the framework of the national RENOCLIP-LOC network. High-risk medulloblastoma was defined by cerebrospinal fluid (CSF) invasion, and/or the presence of CNS metastases, and/or the presence of a postoperative residue >1.5 cm², and/or the presence of MYC/MYCN amplification or histological presence of large cell anaplasia. Tumor response was defined as follows: Progressive disease was defined as a ≥25% increase, partial response (PR) was defined as a ≥50% decrease, complete response (CR) was defined as the complete disappearance of all enhancing, measurable, and non-measurable disease, and no malignant cell in CSF analysis and stable disease (SD) did not qualify for CR, PR, or progression. The overall response rate (ORR) was defined by adding the CR and PR. Toxicity was reported using CTCAE v5.0. Modified Chang staging3 was used and is defined as follows: M0 indicates no evidence of gross residual tumor or metastasis, M1 is the presence of microscopic tumor cells in the cerebrospinal fluid, M2 is gross nodular seeding within the CNS other than the spinal space, M3 is gross nodular seeding in the spinal subarachnoid space, and M4 is metastasis outside the cerebrospinal axis. This study was approved by the CLB institutional review board according to the French Reference Methodology MR-004 (Commission Nationale Informatique et Libertés CNIL, reference number 2211136).
Treatment Regimen
After primary tumor biopsy or resection, the treatment consisted of 2 cycles of induction chemotherapy with carboplatin (160 mg/m²/day D1-D2-D3-D4-D5) and etoposide (100 mg/m²/day D1-D2-D3-D4-D5) (CE) every 21 days, followed by tumor resection if indicated. First CE treatment was initiated as soon as possible (between 2 and 4 weeks after surgery). ASCR were harvested according to the institutional procedures during induction chemotherapy. Induction chemotherapy was followed by 2 cycles of HDT (200 mg/m2/day, D1-D2-D3) with ASCR infused at D4, every 21 days if hematologic parameters were sufficient (transfusion independent with platelets ≥100 giga/L, leukocytes ≥1.5 giga/L and neutrophils ≥0.8 giga/L). Thereafter, photon craniospinal irradiation (CSI) consisting of 36 Gy (1.8 Gy, 5 days/week) of the craniospinal axis (for metastatic and/or infratentorial tumors only) and an additional dose of 18 Gy (1.8 Gy, 5 days/week) on the bed of the primary tumor. For post-chemotherapy residual lesions (primary tumor and/or metastases) before radiation therapy, an additional dose of 9 or 18 Gy could be administered depending on the site of residual lesions (1.8 Gy, 5 days/week). For M0 medulloblastoma with postoperative residual tumor and CR before radiotherapy, radiation therapy could be decreased to 23.4 Gy on the craniospinal axis. It was recommended to start radiation therapy no later than 45 days after the last ASCR. Non-metastatic pinealoblastoma received only 1.8 Gy, 5 days/week, 54 Gy to the primary tumor bed. Proton therapy could be used instead of photon therapy for the additional dose on the primitive/metastasis bed and for the additional dose on residual tumors. The maximum recommended time frame was 150 days from the initial surgery to the start of CSI. Finally, one month after and no more than 3 months after the end of CSI, 6 cycles of temozolomide (150 mg/m²/day, D1-D2-D3-D4-D5) every 28 days were delivered, provided hematologic parameters were sufficient (platelets ≥ 100 giga/L, leukocytes ≥ 1.5 giga/L, and neutrophils ≥ 0.8 giga/L). The treatment regimen is extensively described in the prospective clinical trial PNET HR + 5 published by Dufour et al.12
Statistical Analysis
Descriptive statistics, including frequency distributions, medians, means, and ranges, were calculated for variables of interest. Overall survival was calculated from medulloblastoma diagnosis until death (event) or last follow-up (censored). Progression-free survival was calculated from diagnosis until first tumor progression, death, or last follow-up (censored). Statistical analyses were performed using Prism (GraphPad Software, San Diego, CA, USA). Figures were constructed with Prism (GraphPad Software, San Diego, CA, USA).
Results
Patient Characteristics
Tables 1 and 2 show the characteristics of the 18 patients. The median age at diagnosis was 23.5 years (range, 20–33 years). The primary diagnosis was medulloblastoma for 13 patients, cerebellar CNS embryonal tumor for 1 patient and pinealoblastoma for 4 patients. The status of high-risk medulloblastoma was attributed to patients displaying a >1.5 cm² postoperative residue (2/13, 15.3%), invasion of CSF (2/13, 15.3%), CNS metastasis (7/13, 54%), and/or the histological presence of large cell anaplasia (2/13, 15.3%). At diagnosis, the modified Chang’s stage,3 was M0 for 4 (31%), M1 for 2 (15%), M2 for 5 (39%), and M3 for 2 (15%) medulloblastomas. Three (23%) medulloblastomas were SHH + TP53wt, 2 (15%) were SHH + TP53mut, 4 (31%) were non-WNT/non-SHH and molecular data was not available for 4 patients (diagnosis before 2015 and technique not available locally). No germline mutations were recorded in this cohort.
| Clinical Characteristics (n = 18) . | Treatment feasibility . | ||
|---|---|---|---|
| Sex | Carboplatine etoposide | ||
| Male | 10 (66%) | 2 cycles | 18 |
| Female | 8 (44%) | High-dose Thiotepa | |
| Age at diagnosis (years) | 0 cycle | 0 | |
| Median | 23.5 | 1 cycle | 4 |
| Range | 20-33 | 2 cycles | 14 |
| ECOG PS after primary surgery | Craniospinal irradiation (n = 16) | ||
| 0–1 | 12 (67%) | Yes | 16 |
| 2 | 5 (28%) | No | 0 |
| 3 | 1 (5%) | Proton therapy boost | |
| Histology | Yes | 12 | |
| Medulloblastoma | 13 (72%) | No | 6 |
| Pinealoblastoma | 4 (22%) | Temozolomide | |
| CNS embryonal tumor | 1 (6%) | None | 7 |
| High-risk status for medulloblastoma (n = 13) | 3 cycles | 1 | |
| Postoperative residue >1.5cm² | 2 (15.3%) | 6 cycles | 10 |
| CSF positive | 2 (15.3%) | Protocol completion (PNET HR + 5) | |
| Metastasis | 7 (54%) | Yes | 9 |
| Anaplasia | 2 (15.3%) | No | 9 |
| Modified Chang’s staging (n = 13) | |||
| 0 | 4 (31%) | ||
| 1 | 2 (15%) | ||
| 2 | 5 (39%) | ||
| 3 | 2 (15%) | ||
| 4 | 0 (0%) | ||
| Molecular subtype (n = 13) | |||
| WNT+ | 0 (0%) | ||
| SHH + TP53wt | 3 (23%) | ||
| SHH + TP53mut | 2 (15%) | ||
| non-WNT/non-SHH | 4 (31%) | ||
| NE | 4 (31%) | ||
| Clinical Characteristics (n = 18) . | Treatment feasibility . | ||
|---|---|---|---|
| Sex | Carboplatine etoposide | ||
| Male | 10 (66%) | 2 cycles | 18 |
| Female | 8 (44%) | High-dose Thiotepa | |
| Age at diagnosis (years) | 0 cycle | 0 | |
| Median | 23.5 | 1 cycle | 4 |
| Range | 20-33 | 2 cycles | 14 |
| ECOG PS after primary surgery | Craniospinal irradiation (n = 16) | ||
| 0–1 | 12 (67%) | Yes | 16 |
| 2 | 5 (28%) | No | 0 |
| 3 | 1 (5%) | Proton therapy boost | |
| Histology | Yes | 12 | |
| Medulloblastoma | 13 (72%) | No | 6 |
| Pinealoblastoma | 4 (22%) | Temozolomide | |
| CNS embryonal tumor | 1 (6%) | None | 7 |
| High-risk status for medulloblastoma (n = 13) | 3 cycles | 1 | |
| Postoperative residue >1.5cm² | 2 (15.3%) | 6 cycles | 10 |
| CSF positive | 2 (15.3%) | Protocol completion (PNET HR + 5) | |
| Metastasis | 7 (54%) | Yes | 9 |
| Anaplasia | 2 (15.3%) | No | 9 |
| Modified Chang’s staging (n = 13) | |||
| 0 | 4 (31%) | ||
| 1 | 2 (15%) | ||
| 2 | 5 (39%) | ||
| 3 | 2 (15%) | ||
| 4 | 0 (0%) | ||
| Molecular subtype (n = 13) | |||
| WNT+ | 0 (0%) | ||
| SHH + TP53wt | 3 (23%) | ||
| SHH + TP53mut | 2 (15%) | ||
| non-WNT/non-SHH | 4 (31%) | ||
| NE | 4 (31%) | ||
ECOG PS, eastern cooperative oncology group performance status; CNS, central nervous system; CSF, cerebrospinal fluid; WNT+, activation of WNT pathway; SHH+, activation of the sonic hedgehog pathway; TP53wt, TP53 wild type; TP53mut, TP53 mutation; non-WNT/non-SHH, absence of activation of the WNT and the sonic hedgehog pathway, NE, not evaluable.
| Clinical Characteristics (n = 18) . | Treatment feasibility . | ||
|---|---|---|---|
| Sex | Carboplatine etoposide | ||
| Male | 10 (66%) | 2 cycles | 18 |
| Female | 8 (44%) | High-dose Thiotepa | |
| Age at diagnosis (years) | 0 cycle | 0 | |
| Median | 23.5 | 1 cycle | 4 |
| Range | 20-33 | 2 cycles | 14 |
| ECOG PS after primary surgery | Craniospinal irradiation (n = 16) | ||
| 0–1 | 12 (67%) | Yes | 16 |
| 2 | 5 (28%) | No | 0 |
| 3 | 1 (5%) | Proton therapy boost | |
| Histology | Yes | 12 | |
| Medulloblastoma | 13 (72%) | No | 6 |
| Pinealoblastoma | 4 (22%) | Temozolomide | |
| CNS embryonal tumor | 1 (6%) | None | 7 |
| High-risk status for medulloblastoma (n = 13) | 3 cycles | 1 | |
| Postoperative residue >1.5cm² | 2 (15.3%) | 6 cycles | 10 |
| CSF positive | 2 (15.3%) | Protocol completion (PNET HR + 5) | |
| Metastasis | 7 (54%) | Yes | 9 |
| Anaplasia | 2 (15.3%) | No | 9 |
| Modified Chang’s staging (n = 13) | |||
| 0 | 4 (31%) | ||
| 1 | 2 (15%) | ||
| 2 | 5 (39%) | ||
| 3 | 2 (15%) | ||
| 4 | 0 (0%) | ||
| Molecular subtype (n = 13) | |||
| WNT+ | 0 (0%) | ||
| SHH + TP53wt | 3 (23%) | ||
| SHH + TP53mut | 2 (15%) | ||
| non-WNT/non-SHH | 4 (31%) | ||
| NE | 4 (31%) | ||
| Clinical Characteristics (n = 18) . | Treatment feasibility . | ||
|---|---|---|---|
| Sex | Carboplatine etoposide | ||
| Male | 10 (66%) | 2 cycles | 18 |
| Female | 8 (44%) | High-dose Thiotepa | |
| Age at diagnosis (years) | 0 cycle | 0 | |
| Median | 23.5 | 1 cycle | 4 |
| Range | 20-33 | 2 cycles | 14 |
| ECOG PS after primary surgery | Craniospinal irradiation (n = 16) | ||
| 0–1 | 12 (67%) | Yes | 16 |
| 2 | 5 (28%) | No | 0 |
| 3 | 1 (5%) | Proton therapy boost | |
| Histology | Yes | 12 | |
| Medulloblastoma | 13 (72%) | No | 6 |
| Pinealoblastoma | 4 (22%) | Temozolomide | |
| CNS embryonal tumor | 1 (6%) | None | 7 |
| High-risk status for medulloblastoma (n = 13) | 3 cycles | 1 | |
| Postoperative residue >1.5cm² | 2 (15.3%) | 6 cycles | 10 |
| CSF positive | 2 (15.3%) | Protocol completion (PNET HR + 5) | |
| Metastasis | 7 (54%) | Yes | 9 |
| Anaplasia | 2 (15.3%) | No | 9 |
| Modified Chang’s staging (n = 13) | |||
| 0 | 4 (31%) | ||
| 1 | 2 (15%) | ||
| 2 | 5 (39%) | ||
| 3 | 2 (15%) | ||
| 4 | 0 (0%) | ||
| Molecular subtype (n = 13) | |||
| WNT+ | 0 (0%) | ||
| SHH + TP53wt | 3 (23%) | ||
| SHH + TP53mut | 2 (15%) | ||
| non-WNT/non-SHH | 4 (31%) | ||
| NE | 4 (31%) | ||
ECOG PS, eastern cooperative oncology group performance status; CNS, central nervous system; CSF, cerebrospinal fluid; WNT+, activation of WNT pathway; SHH+, activation of the sonic hedgehog pathway; TP53wt, TP53 wild type; TP53mut, TP53 mutation; non-WNT/non-SHH, absence of activation of the WNT and the sonic hedgehog pathway, NE, not evaluable.
Individual Clinical Characteristics and Individual Treatment Sequence, Disease Response, and Treatment Outcomes
| . | Patient No. . | Age at diagnosis . | Sex . | Diagnosis—subtype . | Anaplasia/MYC/ MYCN . | CSF positive . | Surgery type . | Locally extended/metastasis . | Modified chang’s staging . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual clinical characteristics | 1 | 20 | M | Medulloblastoma—NE | None | No | Partial | Cerebellum | 0 | |||
| 2 | 21 | M | Medulloblastoma—SHH + TP53wt | None | No | Partial | Cerebellum, suprasellar area | 2 | ||||
| 3 | 21 | F | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 4 | 22 | M | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 5 | 23 | M | Medulloblastoma—SHH + TP53mut | Anaplasia | No | Complete | — | 0 | ||||
| 6 | 23 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Cerebellum, ventricules | 2 | ||||
| 7 | 24 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Spinal cord | 3 | ||||
| 8 | 24 | F | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Infundibulum | 2 | ||||
| 9 | 26 | F | Medulloblastoma—SHH + TP53mut | None | Yes | Partial | Spinal cord | 3 | ||||
| 10 | 27 | F | Medulloblastoma—NE | None | Yes | Partial | Cerebellum, ventricules | 2 | ||||
| 11 | 28 | M | Medulloblastoma—non-WNT/non-SHH | Anaplasia | No | Partial | Cerebellum | 0 | ||||
| 12 | 29 | F | Medulloblastoma—SHH + TP53wt | None | No | Biopsy | Cerebellum, Midbrain | 0 | ||||
| 13 | 33 | F | Medulloblastoma—SHH + TP53wt | None | Yes | Partial | Cerebellum, leptomeninges | 2 | ||||
| 14 | 30 | F | CNS embryonal tumor—NE | None | No | Biopsy | Cerebellum, bifocal | NE | ||||
| 15 | 20 | M | Pinealoblastoma—NE | NE | No | Partial | Spinal cord | NE | ||||
| 16 | 20 | M | Pinealoblastoma—NE | NE | No | Biopsy | Infundibulum, Ventricules | NE | ||||
| 17 | 23 | F | Pinealoblastoma—NE | NE | No | Partial | Cerebellum, Supratentorial regions | NE | ||||
| 18 | 29 | H | Pinealoblastoma—NE | NE | No | Partial | Pineal | NE | ||||
| Individual treatment sequence and disease response . | Patient No. . | Number of CE cycle . | Disease response after 2 cycles of CE . | Number of HDT cycle . | Disease response after HDT . | Radiation therapy . | Proton therapy Boost . | Disease response after radiation therapy . | 6 cycles of temozolomide . | Disease response after end of treatment . | ||
| 1 | 2 | PR | 2 | CR | Craniospinal 25 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 2 | 2 | PR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 3 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 4 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 5 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 6 | 2 | PR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 7 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | No | CR | |||
| 8 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 9 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | No | PR | |||
| 10 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54Gy | No | SD | No | SD | |||
| 11 | 2 | SD | 2 | PR | Craniospinal 36 Gy Locally 54Gy | Yes | PR | Yes | CR | |||
| 12 | 2 | SD | 2 | SD | Craniospinal 36 Gy Locally 54Gy | Yes | CR | No | CR | |||
| 13 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 14 | 2 | SD | 1 | SD | Craniospinal 36 Gy Locally 54 Gy | Yes | SD | Yes | PR | |||
| 15 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | Yes | PR | |||
| 16 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | PR | No | PR | |||
| 17 | 2 | SD | 2 | SD | Locally 54 Gy | Yes | CR | Yes | CR | |||
| 18 | 2 | CR | 2 | CR | Locally 54 Gy | Yes | CR | Yes | CR | |||
| Individual time between treatments and treatment outcomes . | Patient No. . | Number of CE cycle . | Time between treatments (days) . | Outcome . | PFS (years) . | OS (years) . | ||||||
| Surgery to First CE . | First CE to first HDT/resurgery . | First HDT to radiation therapy . | First CE to CSI . | Surgery to CSI . | ||||||||
| 1 | 2 | 42 | 51 | 78 | 141 | 183 | FOD | 9.0 | + | 9.0 | + | |
| 2 | 2 | 15 | 49 | 51 | 100 | 115 | FOD | 2.6 | + | 2.6 | + | |
| 3 | 2 | 27 | 57 | 89 | 146 | 173 | DOD | 6.9 | 7.3 | |||
| 4 | 2 | 35 | 53 | 72 | 125 | 160 | FOD | 6.6 | + | 6.6 | + | |
| 5 | 2 | 40 | 56 | 52 | 108 | 148 | DOD | 0.7 | 1.3 | |||
| 6 | 2 | 25 | 53 | 72 | 125 | 150 | FOD | 5.4 | + | 5.4 | + | |
| 7 | 2 | 42 | 75 | 71 | 146 | 188 | DOD | 3.5 | 3.7 | |||
| 8 | 2 | 31 | 59 | 85 | 144 | 175 | FOD | 3.8 | + | 3.8 | + | |
| 9 | 2 | 19 | 52 | 81 | 133 | 152 | DOD | 2.1 | 3.6 | |||
| 10 | 2 | 82 | 59 | 66 | 125 | 207 | DOD | 4.4 | 6.9 | |||
| 11 | 2 | 32 | 50 | 93 | 143 | 175 | FOD | 6.0 | + | 6.0 | + | |
| 12 | 2 | 64 | 68 | 118 | 186 | 250 | FOD | 4.3 | + | 4.3 | + | |
| 13 | 2 | 22 | 56 | 76 | 132 | 154 | FOD | 5.1 | + | 5.1 | + | |
| 14 | 2 | 56 | 57 | 48 | 105 | 161 | FOD | 4.4 | + | 4.4 | + | |
| 15 | 2 | 64 | 52 | 72 | 124 | 188 | DOD | 3.2 | 5.2 | |||
| 16 | 2 | 27 | 62 | 104 | 166 | 193 | FOD | 3.6 | + | 3.6 | + | |
| 17 | 2 | 70 | 57 | 89 | 155 | 225 | FOD | 5.2 | + | 5.2 | + | |
| 18 | 2 | 69 | 59 | 68 | 127 | 196 | FOD | 6.8 | + | 6.8 | + | |
| . | Patient No. . | Age at diagnosis . | Sex . | Diagnosis—subtype . | Anaplasia/MYC/ MYCN . | CSF positive . | Surgery type . | Locally extended/metastasis . | Modified chang’s staging . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual clinical characteristics | 1 | 20 | M | Medulloblastoma—NE | None | No | Partial | Cerebellum | 0 | |||
| 2 | 21 | M | Medulloblastoma—SHH + TP53wt | None | No | Partial | Cerebellum, suprasellar area | 2 | ||||
| 3 | 21 | F | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 4 | 22 | M | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 5 | 23 | M | Medulloblastoma—SHH + TP53mut | Anaplasia | No | Complete | — | 0 | ||||
| 6 | 23 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Cerebellum, ventricules | 2 | ||||
| 7 | 24 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Spinal cord | 3 | ||||
| 8 | 24 | F | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Infundibulum | 2 | ||||
| 9 | 26 | F | Medulloblastoma—SHH + TP53mut | None | Yes | Partial | Spinal cord | 3 | ||||
| 10 | 27 | F | Medulloblastoma—NE | None | Yes | Partial | Cerebellum, ventricules | 2 | ||||
| 11 | 28 | M | Medulloblastoma—non-WNT/non-SHH | Anaplasia | No | Partial | Cerebellum | 0 | ||||
| 12 | 29 | F | Medulloblastoma—SHH + TP53wt | None | No | Biopsy | Cerebellum, Midbrain | 0 | ||||
| 13 | 33 | F | Medulloblastoma—SHH + TP53wt | None | Yes | Partial | Cerebellum, leptomeninges | 2 | ||||
| 14 | 30 | F | CNS embryonal tumor—NE | None | No | Biopsy | Cerebellum, bifocal | NE | ||||
| 15 | 20 | M | Pinealoblastoma—NE | NE | No | Partial | Spinal cord | NE | ||||
| 16 | 20 | M | Pinealoblastoma—NE | NE | No | Biopsy | Infundibulum, Ventricules | NE | ||||
| 17 | 23 | F | Pinealoblastoma—NE | NE | No | Partial | Cerebellum, Supratentorial regions | NE | ||||
| 18 | 29 | H | Pinealoblastoma—NE | NE | No | Partial | Pineal | NE | ||||
| Individual treatment sequence and disease response . | Patient No. . | Number of CE cycle . | Disease response after 2 cycles of CE . | Number of HDT cycle . | Disease response after HDT . | Radiation therapy . | Proton therapy Boost . | Disease response after radiation therapy . | 6 cycles of temozolomide . | Disease response after end of treatment . | ||
| 1 | 2 | PR | 2 | CR | Craniospinal 25 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 2 | 2 | PR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 3 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 4 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 5 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 6 | 2 | PR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 7 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | No | CR | |||
| 8 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 9 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | No | PR | |||
| 10 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54Gy | No | SD | No | SD | |||
| 11 | 2 | SD | 2 | PR | Craniospinal 36 Gy Locally 54Gy | Yes | PR | Yes | CR | |||
| 12 | 2 | SD | 2 | SD | Craniospinal 36 Gy Locally 54Gy | Yes | CR | No | CR | |||
| 13 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 14 | 2 | SD | 1 | SD | Craniospinal 36 Gy Locally 54 Gy | Yes | SD | Yes | PR | |||
| 15 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | Yes | PR | |||
| 16 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | PR | No | PR | |||
| 17 | 2 | SD | 2 | SD | Locally 54 Gy | Yes | CR | Yes | CR | |||
| 18 | 2 | CR | 2 | CR | Locally 54 Gy | Yes | CR | Yes | CR | |||
| Individual time between treatments and treatment outcomes . | Patient No. . | Number of CE cycle . | Time between treatments (days) . | Outcome . | PFS (years) . | OS (years) . | ||||||
| Surgery to First CE . | First CE to first HDT/resurgery . | First HDT to radiation therapy . | First CE to CSI . | Surgery to CSI . | ||||||||
| 1 | 2 | 42 | 51 | 78 | 141 | 183 | FOD | 9.0 | + | 9.0 | + | |
| 2 | 2 | 15 | 49 | 51 | 100 | 115 | FOD | 2.6 | + | 2.6 | + | |
| 3 | 2 | 27 | 57 | 89 | 146 | 173 | DOD | 6.9 | 7.3 | |||
| 4 | 2 | 35 | 53 | 72 | 125 | 160 | FOD | 6.6 | + | 6.6 | + | |
| 5 | 2 | 40 | 56 | 52 | 108 | 148 | DOD | 0.7 | 1.3 | |||
| 6 | 2 | 25 | 53 | 72 | 125 | 150 | FOD | 5.4 | + | 5.4 | + | |
| 7 | 2 | 42 | 75 | 71 | 146 | 188 | DOD | 3.5 | 3.7 | |||
| 8 | 2 | 31 | 59 | 85 | 144 | 175 | FOD | 3.8 | + | 3.8 | + | |
| 9 | 2 | 19 | 52 | 81 | 133 | 152 | DOD | 2.1 | 3.6 | |||
| 10 | 2 | 82 | 59 | 66 | 125 | 207 | DOD | 4.4 | 6.9 | |||
| 11 | 2 | 32 | 50 | 93 | 143 | 175 | FOD | 6.0 | + | 6.0 | + | |
| 12 | 2 | 64 | 68 | 118 | 186 | 250 | FOD | 4.3 | + | 4.3 | + | |
| 13 | 2 | 22 | 56 | 76 | 132 | 154 | FOD | 5.1 | + | 5.1 | + | |
| 14 | 2 | 56 | 57 | 48 | 105 | 161 | FOD | 4.4 | + | 4.4 | + | |
| 15 | 2 | 64 | 52 | 72 | 124 | 188 | DOD | 3.2 | 5.2 | |||
| 16 | 2 | 27 | 62 | 104 | 166 | 193 | FOD | 3.6 | + | 3.6 | + | |
| 17 | 2 | 70 | 57 | 89 | 155 | 225 | FOD | 5.2 | + | 5.2 | + | |
| 18 | 2 | 69 | 59 | 68 | 127 | 196 | FOD | 6.8 | + | 6.8 | + | |
PS, performance status; CE, carboplatin etoposide; HDT, high-dose thiotepa; CSI, craniospinal irradiation; CNS, central nervous system; WNT+, activation of WNT pathway; SHH+, activation of the sonic hedgehog pathway; TP53wt, TP53 wild type; TP53mut, TP53 mutation; non-WNT/non-SHH, absence of activation of the WNT and the sonic hedgehog pathway; NE, not evaluable; CSF, cerebrospinal fluid; CR, complete remission; PR, partial response; SD, stable disease; DOD, dead of disease; FOD, free of disease; +, alive at last follow up.
Individual Clinical Characteristics and Individual Treatment Sequence, Disease Response, and Treatment Outcomes
| . | Patient No. . | Age at diagnosis . | Sex . | Diagnosis—subtype . | Anaplasia/MYC/ MYCN . | CSF positive . | Surgery type . | Locally extended/metastasis . | Modified chang’s staging . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual clinical characteristics | 1 | 20 | M | Medulloblastoma—NE | None | No | Partial | Cerebellum | 0 | |||
| 2 | 21 | M | Medulloblastoma—SHH + TP53wt | None | No | Partial | Cerebellum, suprasellar area | 2 | ||||
| 3 | 21 | F | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 4 | 22 | M | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 5 | 23 | M | Medulloblastoma—SHH + TP53mut | Anaplasia | No | Complete | — | 0 | ||||
| 6 | 23 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Cerebellum, ventricules | 2 | ||||
| 7 | 24 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Spinal cord | 3 | ||||
| 8 | 24 | F | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Infundibulum | 2 | ||||
| 9 | 26 | F | Medulloblastoma—SHH + TP53mut | None | Yes | Partial | Spinal cord | 3 | ||||
| 10 | 27 | F | Medulloblastoma—NE | None | Yes | Partial | Cerebellum, ventricules | 2 | ||||
| 11 | 28 | M | Medulloblastoma—non-WNT/non-SHH | Anaplasia | No | Partial | Cerebellum | 0 | ||||
| 12 | 29 | F | Medulloblastoma—SHH + TP53wt | None | No | Biopsy | Cerebellum, Midbrain | 0 | ||||
| 13 | 33 | F | Medulloblastoma—SHH + TP53wt | None | Yes | Partial | Cerebellum, leptomeninges | 2 | ||||
| 14 | 30 | F | CNS embryonal tumor—NE | None | No | Biopsy | Cerebellum, bifocal | NE | ||||
| 15 | 20 | M | Pinealoblastoma—NE | NE | No | Partial | Spinal cord | NE | ||||
| 16 | 20 | M | Pinealoblastoma—NE | NE | No | Biopsy | Infundibulum, Ventricules | NE | ||||
| 17 | 23 | F | Pinealoblastoma—NE | NE | No | Partial | Cerebellum, Supratentorial regions | NE | ||||
| 18 | 29 | H | Pinealoblastoma—NE | NE | No | Partial | Pineal | NE | ||||
| Individual treatment sequence and disease response . | Patient No. . | Number of CE cycle . | Disease response after 2 cycles of CE . | Number of HDT cycle . | Disease response after HDT . | Radiation therapy . | Proton therapy Boost . | Disease response after radiation therapy . | 6 cycles of temozolomide . | Disease response after end of treatment . | ||
| 1 | 2 | PR | 2 | CR | Craniospinal 25 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 2 | 2 | PR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 3 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 4 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 5 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 6 | 2 | PR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 7 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | No | CR | |||
| 8 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 9 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | No | PR | |||
| 10 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54Gy | No | SD | No | SD | |||
| 11 | 2 | SD | 2 | PR | Craniospinal 36 Gy Locally 54Gy | Yes | PR | Yes | CR | |||
| 12 | 2 | SD | 2 | SD | Craniospinal 36 Gy Locally 54Gy | Yes | CR | No | CR | |||
| 13 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 14 | 2 | SD | 1 | SD | Craniospinal 36 Gy Locally 54 Gy | Yes | SD | Yes | PR | |||
| 15 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | Yes | PR | |||
| 16 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | PR | No | PR | |||
| 17 | 2 | SD | 2 | SD | Locally 54 Gy | Yes | CR | Yes | CR | |||
| 18 | 2 | CR | 2 | CR | Locally 54 Gy | Yes | CR | Yes | CR | |||
| Individual time between treatments and treatment outcomes . | Patient No. . | Number of CE cycle . | Time between treatments (days) . | Outcome . | PFS (years) . | OS (years) . | ||||||
| Surgery to First CE . | First CE to first HDT/resurgery . | First HDT to radiation therapy . | First CE to CSI . | Surgery to CSI . | ||||||||
| 1 | 2 | 42 | 51 | 78 | 141 | 183 | FOD | 9.0 | + | 9.0 | + | |
| 2 | 2 | 15 | 49 | 51 | 100 | 115 | FOD | 2.6 | + | 2.6 | + | |
| 3 | 2 | 27 | 57 | 89 | 146 | 173 | DOD | 6.9 | 7.3 | |||
| 4 | 2 | 35 | 53 | 72 | 125 | 160 | FOD | 6.6 | + | 6.6 | + | |
| 5 | 2 | 40 | 56 | 52 | 108 | 148 | DOD | 0.7 | 1.3 | |||
| 6 | 2 | 25 | 53 | 72 | 125 | 150 | FOD | 5.4 | + | 5.4 | + | |
| 7 | 2 | 42 | 75 | 71 | 146 | 188 | DOD | 3.5 | 3.7 | |||
| 8 | 2 | 31 | 59 | 85 | 144 | 175 | FOD | 3.8 | + | 3.8 | + | |
| 9 | 2 | 19 | 52 | 81 | 133 | 152 | DOD | 2.1 | 3.6 | |||
| 10 | 2 | 82 | 59 | 66 | 125 | 207 | DOD | 4.4 | 6.9 | |||
| 11 | 2 | 32 | 50 | 93 | 143 | 175 | FOD | 6.0 | + | 6.0 | + | |
| 12 | 2 | 64 | 68 | 118 | 186 | 250 | FOD | 4.3 | + | 4.3 | + | |
| 13 | 2 | 22 | 56 | 76 | 132 | 154 | FOD | 5.1 | + | 5.1 | + | |
| 14 | 2 | 56 | 57 | 48 | 105 | 161 | FOD | 4.4 | + | 4.4 | + | |
| 15 | 2 | 64 | 52 | 72 | 124 | 188 | DOD | 3.2 | 5.2 | |||
| 16 | 2 | 27 | 62 | 104 | 166 | 193 | FOD | 3.6 | + | 3.6 | + | |
| 17 | 2 | 70 | 57 | 89 | 155 | 225 | FOD | 5.2 | + | 5.2 | + | |
| 18 | 2 | 69 | 59 | 68 | 127 | 196 | FOD | 6.8 | + | 6.8 | + | |
| . | Patient No. . | Age at diagnosis . | Sex . | Diagnosis—subtype . | Anaplasia/MYC/ MYCN . | CSF positive . | Surgery type . | Locally extended/metastasis . | Modified chang’s staging . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual clinical characteristics | 1 | 20 | M | Medulloblastoma—NE | None | No | Partial | Cerebellum | 0 | |||
| 2 | 21 | M | Medulloblastoma—SHH + TP53wt | None | No | Partial | Cerebellum, suprasellar area | 2 | ||||
| 3 | 21 | F | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 4 | 22 | M | Medulloblastoma—NE | None | Yes | Complete | — | 1 | ||||
| 5 | 23 | M | Medulloblastoma—SHH + TP53mut | Anaplasia | No | Complete | — | 0 | ||||
| 6 | 23 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Cerebellum, ventricules | 2 | ||||
| 7 | 24 | M | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Spinal cord | 3 | ||||
| 8 | 24 | F | Medulloblastoma—non-WNT/non-SHH | None | No | Partial | Infundibulum | 2 | ||||
| 9 | 26 | F | Medulloblastoma—SHH + TP53mut | None | Yes | Partial | Spinal cord | 3 | ||||
| 10 | 27 | F | Medulloblastoma—NE | None | Yes | Partial | Cerebellum, ventricules | 2 | ||||
| 11 | 28 | M | Medulloblastoma—non-WNT/non-SHH | Anaplasia | No | Partial | Cerebellum | 0 | ||||
| 12 | 29 | F | Medulloblastoma—SHH + TP53wt | None | No | Biopsy | Cerebellum, Midbrain | 0 | ||||
| 13 | 33 | F | Medulloblastoma—SHH + TP53wt | None | Yes | Partial | Cerebellum, leptomeninges | 2 | ||||
| 14 | 30 | F | CNS embryonal tumor—NE | None | No | Biopsy | Cerebellum, bifocal | NE | ||||
| 15 | 20 | M | Pinealoblastoma—NE | NE | No | Partial | Spinal cord | NE | ||||
| 16 | 20 | M | Pinealoblastoma—NE | NE | No | Biopsy | Infundibulum, Ventricules | NE | ||||
| 17 | 23 | F | Pinealoblastoma—NE | NE | No | Partial | Cerebellum, Supratentorial regions | NE | ||||
| 18 | 29 | H | Pinealoblastoma—NE | NE | No | Partial | Pineal | NE | ||||
| Individual treatment sequence and disease response . | Patient No. . | Number of CE cycle . | Disease response after 2 cycles of CE . | Number of HDT cycle . | Disease response after HDT . | Radiation therapy . | Proton therapy Boost . | Disease response after radiation therapy . | 6 cycles of temozolomide . | Disease response after end of treatment . | ||
| 1 | 2 | PR | 2 | CR | Craniospinal 25 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 2 | 2 | PR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 3 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | Yes | CR | |||
| 4 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 5 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 6 | 2 | PR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | No | CR | |||
| 7 | 2 | CR | 1 | CR | Craniospinal 36 Gy Locally 54 Gy | No | CR | No | CR | |||
| 8 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 9 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | No | PR | |||
| 10 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54Gy | No | SD | No | SD | |||
| 11 | 2 | SD | 2 | PR | Craniospinal 36 Gy Locally 54Gy | Yes | PR | Yes | CR | |||
| 12 | 2 | SD | 2 | SD | Craniospinal 36 Gy Locally 54Gy | Yes | CR | No | CR | |||
| 13 | 2 | CR | 2 | CR | Craniospinal 36 Gy Locally 54 Gy | Yes | CR | Yes | CR | |||
| 14 | 2 | SD | 1 | SD | Craniospinal 36 Gy Locally 54 Gy | Yes | SD | Yes | PR | |||
| 15 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | No | PR | Yes | PR | |||
| 16 | 2 | PR | 2 | PR | Craniospinal 36 Gy Locally 54 Gy | Yes | PR | No | PR | |||
| 17 | 2 | SD | 2 | SD | Locally 54 Gy | Yes | CR | Yes | CR | |||
| 18 | 2 | CR | 2 | CR | Locally 54 Gy | Yes | CR | Yes | CR | |||
| Individual time between treatments and treatment outcomes . | Patient No. . | Number of CE cycle . | Time between treatments (days) . | Outcome . | PFS (years) . | OS (years) . | ||||||
| Surgery to First CE . | First CE to first HDT/resurgery . | First HDT to radiation therapy . | First CE to CSI . | Surgery to CSI . | ||||||||
| 1 | 2 | 42 | 51 | 78 | 141 | 183 | FOD | 9.0 | + | 9.0 | + | |
| 2 | 2 | 15 | 49 | 51 | 100 | 115 | FOD | 2.6 | + | 2.6 | + | |
| 3 | 2 | 27 | 57 | 89 | 146 | 173 | DOD | 6.9 | 7.3 | |||
| 4 | 2 | 35 | 53 | 72 | 125 | 160 | FOD | 6.6 | + | 6.6 | + | |
| 5 | 2 | 40 | 56 | 52 | 108 | 148 | DOD | 0.7 | 1.3 | |||
| 6 | 2 | 25 | 53 | 72 | 125 | 150 | FOD | 5.4 | + | 5.4 | + | |
| 7 | 2 | 42 | 75 | 71 | 146 | 188 | DOD | 3.5 | 3.7 | |||
| 8 | 2 | 31 | 59 | 85 | 144 | 175 | FOD | 3.8 | + | 3.8 | + | |
| 9 | 2 | 19 | 52 | 81 | 133 | 152 | DOD | 2.1 | 3.6 | |||
| 10 | 2 | 82 | 59 | 66 | 125 | 207 | DOD | 4.4 | 6.9 | |||
| 11 | 2 | 32 | 50 | 93 | 143 | 175 | FOD | 6.0 | + | 6.0 | + | |
| 12 | 2 | 64 | 68 | 118 | 186 | 250 | FOD | 4.3 | + | 4.3 | + | |
| 13 | 2 | 22 | 56 | 76 | 132 | 154 | FOD | 5.1 | + | 5.1 | + | |
| 14 | 2 | 56 | 57 | 48 | 105 | 161 | FOD | 4.4 | + | 4.4 | + | |
| 15 | 2 | 64 | 52 | 72 | 124 | 188 | DOD | 3.2 | 5.2 | |||
| 16 | 2 | 27 | 62 | 104 | 166 | 193 | FOD | 3.6 | + | 3.6 | + | |
| 17 | 2 | 70 | 57 | 89 | 155 | 225 | FOD | 5.2 | + | 5.2 | + | |
| 18 | 2 | 69 | 59 | 68 | 127 | 196 | FOD | 6.8 | + | 6.8 | + | |
PS, performance status; CE, carboplatin etoposide; HDT, high-dose thiotepa; CSI, craniospinal irradiation; CNS, central nervous system; WNT+, activation of WNT pathway; SHH+, activation of the sonic hedgehog pathway; TP53wt, TP53 wild type; TP53mut, TP53 mutation; non-WNT/non-SHH, absence of activation of the WNT and the sonic hedgehog pathway; NE, not evaluable; CSF, cerebrospinal fluid; CR, complete remission; PR, partial response; SD, stable disease; DOD, dead of disease; FOD, free of disease; +, alive at last follow up.
Treatment Feasibility, Delays, and Completion
Tables 1, 2, and 3 show treatment feasibility, delays, and completion. All patients received 2 cycles of CE with a median time of completion of 56.5 days (range, 49–75). Fourteen patients received 2 cycles of HDT with a median time of completion of 74 days (range, 48–118). The remaining 4 patients received only 1 cycle of HDT for the following reasons: Persistent aplasia (n = 2), lack of tumor response after 1 cycle of HDT (n = 1), and the medical decision to switch to radiation therapy without any additional delay (n = 1). Fifteen patients received radiation therapy with 54 Gy on the primitive tumor/metastasis bed and 36 Gy on the craniospinal axis. One patient with an M0 medulloblastoma in CR after 2 HDT received 25 Gy on the craniospinal axis, and 2 patients with non-metastatic pinealoblastoma received focal irradiation. Proton therapy was performed for the additional dose on the primitive/metastasis bed and for the additional dose on residual tumors for 67% (12/18) of patients. After CSI, 7 patients did not receive temozolomide for unrecovered hematologic parameters and 1 patient received only 3 cycles due to hematologic toxicities. 56% (10/18) completed the maintenance treatment (6 cycles). Median delays as compared with the treatment plan, were 9.5 days (range, 0–54) between surgery and first CE, 14.5 days (range,7–33) between first CE and first HDT, 32 days (range, 6–76) between first HDT and initiation of CSI (Figure 1). Altogether, the median delay between surgery and CSI compared to the recommended delay was 25 days (range, 0–100). The main reason for the delay was treatment toxicity and in particular hematological toxicity (thrombocytopenia and neutropenia).
Carboplatin Etoposide, High-Dose Thiotepa and Craniospinal Irradiation Toxicity and Delays
| Carboplatin etoposide (n = 36) . | Time between treatments . | ||
|---|---|---|---|
| Neutropenia grade 3/4 | 47% | Surgery to first CE (recommended timing <28 days) | |
| Thrombocytopenia grade 3/4 | 75% | Median time | 37.5 |
| Platelet Transfusions needed | 58% | Range | 15–82 |
| Blood Transfusions needed | 19% | Median delay | 9.5 |
| High-dose Thiotepa (n = 32) | First CE to first HDT/resurgery (recommended timing <42 days) | ||
| Neutropenia <0.5 giga/L duration | Median time | 56.5 | |
| Median | 7 | Range | 49–75 |
| Range | 3–9 | Median delay | 14.5 |
| Platelet transfusions/cycle | First HDT to CSI (recommended timing <42 days) | ||
| Median | 2 | Median time | 74 |
| Range | 0–5 | Range | 48–118 |
| Blood transfusions/cycle | Median delay | 32 | |
| Median | 2 | First CE to CSI (recommended timing <112 days) | |
| Range | 0–6 | Median time | 132.5 |
| Non hematological toxicity G3/4 | Range | 100–186 | |
| Nausea | 28% | Median delay | 20.5 |
| Anorexia | 22% | Surgery to CSI (recommended timing <150 days) | |
| Mucositis | 16% | Median time | 175 |
| Hepatitis | 6% | Range | 115–250 |
| Craniospinal irradiation (n = 16) | Median delay | 25 | |
| Prolonged neutropenia <0.5 giga/L (>21days) | 25% | ||
| Prolonged cytopenia (>90 days) | 45% | ||
| Carboplatin etoposide (n = 36) . | Time between treatments . | ||
|---|---|---|---|
| Neutropenia grade 3/4 | 47% | Surgery to first CE (recommended timing <28 days) | |
| Thrombocytopenia grade 3/4 | 75% | Median time | 37.5 |
| Platelet Transfusions needed | 58% | Range | 15–82 |
| Blood Transfusions needed | 19% | Median delay | 9.5 |
| High-dose Thiotepa (n = 32) | First CE to first HDT/resurgery (recommended timing <42 days) | ||
| Neutropenia <0.5 giga/L duration | Median time | 56.5 | |
| Median | 7 | Range | 49–75 |
| Range | 3–9 | Median delay | 14.5 |
| Platelet transfusions/cycle | First HDT to CSI (recommended timing <42 days) | ||
| Median | 2 | Median time | 74 |
| Range | 0–5 | Range | 48–118 |
| Blood transfusions/cycle | Median delay | 32 | |
| Median | 2 | First CE to CSI (recommended timing <112 days) | |
| Range | 0–6 | Median time | 132.5 |
| Non hematological toxicity G3/4 | Range | 100–186 | |
| Nausea | 28% | Median delay | 20.5 |
| Anorexia | 22% | Surgery to CSI (recommended timing <150 days) | |
| Mucositis | 16% | Median time | 175 |
| Hepatitis | 6% | Range | 115–250 |
| Craniospinal irradiation (n = 16) | Median delay | 25 | |
| Prolonged neutropenia <0.5 giga/L (>21days) | 25% | ||
| Prolonged cytopenia (>90 days) | 45% | ||
Carboplatin Etoposide, High-Dose Thiotepa and Craniospinal Irradiation Toxicity and Delays
| Carboplatin etoposide (n = 36) . | Time between treatments . | ||
|---|---|---|---|
| Neutropenia grade 3/4 | 47% | Surgery to first CE (recommended timing <28 days) | |
| Thrombocytopenia grade 3/4 | 75% | Median time | 37.5 |
| Platelet Transfusions needed | 58% | Range | 15–82 |
| Blood Transfusions needed | 19% | Median delay | 9.5 |
| High-dose Thiotepa (n = 32) | First CE to first HDT/resurgery (recommended timing <42 days) | ||
| Neutropenia <0.5 giga/L duration | Median time | 56.5 | |
| Median | 7 | Range | 49–75 |
| Range | 3–9 | Median delay | 14.5 |
| Platelet transfusions/cycle | First HDT to CSI (recommended timing <42 days) | ||
| Median | 2 | Median time | 74 |
| Range | 0–5 | Range | 48–118 |
| Blood transfusions/cycle | Median delay | 32 | |
| Median | 2 | First CE to CSI (recommended timing <112 days) | |
| Range | 0–6 | Median time | 132.5 |
| Non hematological toxicity G3/4 | Range | 100–186 | |
| Nausea | 28% | Median delay | 20.5 |
| Anorexia | 22% | Surgery to CSI (recommended timing <150 days) | |
| Mucositis | 16% | Median time | 175 |
| Hepatitis | 6% | Range | 115–250 |
| Craniospinal irradiation (n = 16) | Median delay | 25 | |
| Prolonged neutropenia <0.5 giga/L (>21days) | 25% | ||
| Prolonged cytopenia (>90 days) | 45% | ||
| Carboplatin etoposide (n = 36) . | Time between treatments . | ||
|---|---|---|---|
| Neutropenia grade 3/4 | 47% | Surgery to first CE (recommended timing <28 days) | |
| Thrombocytopenia grade 3/4 | 75% | Median time | 37.5 |
| Platelet Transfusions needed | 58% | Range | 15–82 |
| Blood Transfusions needed | 19% | Median delay | 9.5 |
| High-dose Thiotepa (n = 32) | First CE to first HDT/resurgery (recommended timing <42 days) | ||
| Neutropenia <0.5 giga/L duration | Median time | 56.5 | |
| Median | 7 | Range | 49–75 |
| Range | 3–9 | Median delay | 14.5 |
| Platelet transfusions/cycle | First HDT to CSI (recommended timing <42 days) | ||
| Median | 2 | Median time | 74 |
| Range | 0–5 | Range | 48–118 |
| Blood transfusions/cycle | Median delay | 32 | |
| Median | 2 | First CE to CSI (recommended timing <112 days) | |
| Range | 0–6 | Median time | 132.5 |
| Non hematological toxicity G3/4 | Range | 100–186 | |
| Nausea | 28% | Median delay | 20.5 |
| Anorexia | 22% | Surgery to CSI (recommended timing <150 days) | |
| Mucositis | 16% | Median time | 175 |
| Hepatitis | 6% | Range | 115–250 |
| Craniospinal irradiation (n = 16) | Median delay | 25 | |
| Prolonged neutropenia <0.5 giga/L (>21days) | 25% | ||
| Prolonged cytopenia (>90 days) | 45% | ||
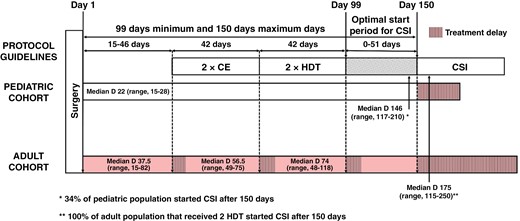
Schematic diagram of treatment regimen administration delays in the pediatric and adult medulloblastoma cohorts compared to protocol administration recommendations. CE, carboplatin etoposide; HDT, high-dose thiotepa; CSI, craniospinal irradiation, Median D, Median time of completion in days.
Carboplatin Etoposide, HDT, and Craniospinal Irradiation Toxicity
Table 3 shows treatment toxicity. There was no toxic death. Among the 36 cycles of CE, grade 3/4 neutropenia occurred in 47% and grade 3/4 thrombocytopenia in 75% of cases. Platelet transfusions and blood transfusions were required in 58% and 19% of cycles, respectively. Out of 32 cycles of HDT, the median duration of neutropenia <0.5 giga/L was 7 days (range, 3–9 days). Non-hematological toxicity of HDT was manageable with 28% of grades 3/4 nausea, 22% of grades 3/4 anorexia, 16% of grades 3/4 mucositis, and 6% of grades 3/4 hepatitis. Among the 16 patients who received CSI after HDT, 25% of patients had prolonged neutropenia <0.5 giga/L (>21 days) and 45% had prolonged cytopenia (platelet < 100 giga/L and/or neutropenia < 1 giga/L for >90 days). No long-term toxicity was recorded (with the exception of toxicity on fertility, which was not analyzed in this cohort).
Carboplatin Etoposide, HDT, and Craniospinal Irradiation Disease Responses
Table 2 shows the disease response to treatment. There was no progression during treatment. After surgery, only 1 patient was considered to display a CR. After 2 cycles of CE, ORR was 78% with 33% of CR, and 45% of PR. 22% of patients had an SD. After HDT, ORR was 83% with 50% of CR and 33% of PR. 17% of patients had an SD. After radiation therapy, responses were 67% of CR, 22% of PR. 11% of patients had an SD. After temozolomide (10/18 patients received 6 cycles of temozolomide), ORR was 94 % with 72% of CR and 22% of PR. 6% of patients had an SD.
Outcomes
Table 2 and Figure 2 show treatment outcomes. The median follow-up was 6.0 years (range, 2.6–9). For medulloblastomas, PFS was 85% (95% CI: 51%–96%) at 3 years and 65% (95% CI: 31%–86%) at 5 years and OS was 92% (95% CI: 57%–99%) at 3 years and 76% (95% CI: 42%–91%) at 5 years.
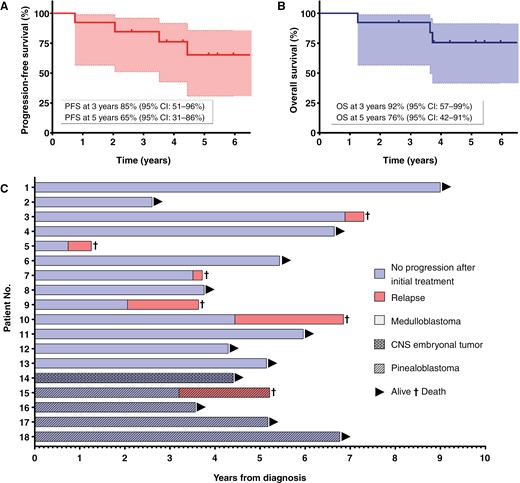
Kaplan–Meier curve of treatment outcome for adult medulloblastoma patients (n = 13): progression-free survival (PFS) (A) and overall survival (OS) (B) at 3 and 5 years. Individual outcomes of patients (n = 18) with medulloblastoma, pinealoblastoma, and central nervous system (CNS) embryonal tumor showing progression-free survival (PFS), overall survival (OS), and follow-up (C). No., number.
Discussion
The current study shows the feasibility and the limits of transposing a pediatric protocol to adults. High rates of PFS and OS were observed with 65% (95% CI: 31%–86%) and 76% (95% CI: 42%–91%) at 5 years, respectively. These results are comparable with those of the prospective study that validated the efficacy of the PNET HR + 5 regimen in a pediatric cohort (aged 5 to 20) with HRMB. Indeed, PFS and OS were 76% (95% CI: 63%–86%) at 5 years.12
It is widely recognized that timing is essential for optimal treatment. CE was administered on average for 4 weeks instead of 3 weeks, as planned. This results in a median delay of 14.5 days, which has an impact on dose intensity, the start of HDT and, in particular, the start of the radiation therapy. Hematological toxicity was high and may be explained by the dose of carboplatin administered in mg/m² instead of AUC according to Calvert’s formula.14 Indeed, for an adult patient with an average body surface of 1.73 m², a carboplatin dose of 1400 mg (which corresponds to the cumulative dose of one CE cycle) converted in AUC is 9.5. Carboplatin has been used in monotherapy in seminoma at a maximum dose of AUC 10,15,16 resulting in similar hematological toxicity. In adults, the highest dose of carboplatin used in the polychemotherapy regimen is AUC 6. Carboplatin AUC 6 at D1 and etoposide 100 mg/m2 at D1-D2-D3 with cycles of 21 days were used for lung cancer with acceptable toxicity.17 It may be worth using this schedule in the PNET HR + 5 regimen in adults in order to limit the delay between surgery and the start of radiation therapy. The originality of the PNET HR + 5 regimen is the addition of tandem HDT with ACSR. The concept of high-dose chemotherapy in pediatric brain tumors originated from the desire to reduce or replace radiotherapy in order to limit its long-term toxicity18 but also as a salvage treatment after a first relapse or to increase the response of brain tumors at a high risk of recurrence.19 HDT has shown impressive response rates in some brain tumor types including medulloblastomas and pinealoblastomas with manageable toxicity.19–21 No toxic deaths were recorded, and recovery from aplasia was achieved between 10 and 14 days after HDT. In adults, recurrent or progressive brain tumors were treated with 2 cycles of HDT (200 mg/m2/day, D1-D2-D3 with ASCR infused at D6, every 21 days) in a US cohort20: 12 patients received 2 cycles of HDT and none experienced toxic death, and recovery from aplasia was achieved on average 11 days after HDT.
Other high-dose chemotherapy regimens have been used in adults with brain cancer but resulted in higher toxicity and even toxic death.21,22 For example, the high-dose carboplatin, thiotepa, and etoposide regimen with ASCR led to a rate of toxic death of 12% in an adult cohort of recurrent medulloblastoma.22 Thus, tandem HDT appears feasible in adults (no toxic deaths19–21) and may result in significantly improved outcomes when added to the standard of care that combines chemotherapy and CSI for medulloblastoma.
The literature suggests that a prolonged delay in the delivery of radiation therapy may have an impact on the survival of medulloblastoma.23,24 In the current study there was a significant delay in the initiation of radiotherapy. Indeed, the median delay between surgery and radiation was 175 days (range, 115–250) whereas the protocol recommended the start of radiation within 90 to 150 days. All the patients who received 2 HDT, received radiation more than 150 days after the primary surgery. Conversely, for pediatric patients treated with the PNET HR + 5 regimen, the delay between surgery and radiation was 146 days (range, 117–210) and was greater than 150 days in only 34% of children (Figure 1). Whether or not this cutoff at day 150 remains crucial or not in the current context of effective chemotherapy remains a matter of debate. This delay was due in part to the median time from first surgery to first EC, which was 37.5 days (range, 15–82) in adults compared to 22 days (range, 15–27.5) in pediatric patients. This delay may be explained by the rarity of the disease in adults and the need to refer to an expert center for initial staging and then for therapeutic management. Indeed, there was an additional delay when the initial management (surgery) was not performed in an expert center because of the time lost from changing the team managing the patient and from waiting for histological and complementary examinations not performed initially.
The feasibility of full-dose CSI after tandem HDT in the adult population was a challenge. Tolerance of CSI is well documented and results in almost no hematological grades 3/4 when it is used alone or prior to standard chemotherapy and less than 20% of hematological grades 3/4 toxicity when it is used after standard chemotherapy.25,26 In our adult cohort, 50% (8/16) did not receive 6 cycles of temozolomide after CSI due to hematologic recovery beyond 3 months after completion of CSI or hematologic toxicity of temozolomide versus 21% (12/57) in children treated with the PNET HR + 5 regimen. In the group of children who did not receive temozolomide adjuvant, none relapsed,27 compared with 50% relapse in the same group in our adult cohort. It is important to highlight that there was no long-term toxicity recorded in this study except for the risk of infertility, which has not been evaluated here. Other protocols without high-dose chemotherapy used in adult medulloblastoma may cause more long-term toxicity. For instance, the combination of CSI followed by HIT 2000 chemotherapy regimen (8 courses of lomustine, vincristine, and cisplatin)28 used in this German cohort of non-metastatic adult medulloblastomas resulted in significant toxicity. Among 70 patients, the toxicity profile was high with 74% of grade 2/3 peripheral neuropathy, 55% of grade 3/4 hematotoxicity, and 32% of grade 2/3 ototoxicity. Only 24% of patients could receive at least 4 cycles of the full treatment regimen. Ototoxicity and neuropathy can be irreversible toxicities that can severely affect quality of life. More recently, 26 German patients (mixed standard and HRMB) included in the NOA-07 study29 were treated with CSI in combination with vincristine followed by a maximum of 8 six-weekly cycles of cisplatin, lomustine, and vincristine regimen. 30% of patients received less than 4 cycles because of toxicity and all patients needed dose modification. There was major toxicity limiting the regimen: 67% of grade 3/4 leukopenia, 33% of grade 3/4 thrombocytopenia. In addition, patients had 20% of grade 3/4 polyneuropathy and 20% of grade 3/4 ototoxicity which are known to be long-term toxicities that can affect posttreatment quality of life. Although not all patients were at high risk, the 3-year rate of PFS and OS were 66.6% and 70.0%, respectively. This regimen is currently one of the arms proposed in a new European clinical trial to treat adult medulloblastoma and in particular M1 medulloblastoma.30
Undoubtedly, the median survival of adults treated in Europe and America for an HRMB is poor despite a full-dose radiotherapy and chemotherapy regimen. High-risk adult medulloblastoma from the Torino group31 received 2 cycles of pre-radiation chemotherapy regimen and then 4 cycles of maintenance chemotherapy by either a MOPP-like regimen (nitrogen mustard, vincristine, procarbazine, and a steroid) or courses of cisplatin, etoposide, cyclophosphamide. In patients with metastatic medulloblastoma, 5-year PFS and OS were 45% and 52%, respectively. The Milano group32 reported 8 patients with HRMB treated with pre-radiation intensive chemotherapy followed by CSI and maintenance chemotherapy (vincristine, lomustine). The 5-year disease-free survival and OS were 65 ± 11% and 73 ± 10%. Another intensive regimen was used in 23 German adults with metastatic medulloblastomas who were treated according to the HIT2000 protocol,33 either with 2 cycles of HIT-SKK regimen (intravenous cyclophosphamide, vincristine, methotrexate, carboplatin, and etoposide and concomitant intraventricular methotrexate) then CSI and 3–8 cycles of maintenance chemotherapy (cisplatin, lomustine and vincristine) or CSI then 3–8 cycles of maintenance chemotherapy. The 4-year event-free survival and OS were 52% ± 12% and 91% ± 6%, respectively. The ECOG-ACRIN Cancer Research Group reported 11 adults with HRMB treated with 3 cycles of cisplatin, etoposide, cyclophosphamide, and vincristine followed by CSI.34 Their 5-year PFS and OS were 27% and 55%, respectively. No better outcome was reported in a large retrospective North American study, as the 5-year rate of PFS and OS were 62% and 39%, respectively35 in adults with HRMB treated with CSI and standard chemotherapy (various regimens). Furthermore, it is important to remember that our cohort contains only high-risk tumors including 69% (9/13) metastatic medulloblastoma with 54% (7/13) of patients with ≥2 Modified Chang’s staging.
Knowledge of non-medulloblastoma embryonal tumors is even more limited in adults. Patients are usually treated with a medulloblastoma-like pediatric regimen. In the study published by Friedrich et al,36 7 PNET and 10 pinealoblastomas were treated according to the HIT 2000 protocol. The estimated rates for 3-year PFS and OS were 68% ± 12% and 66% ± 13%, respectively. Here we report 4 additional adult patients with pinealoblastoma.
The limitations of our study are the retrospective nature of the analyses, the small number of patients treated with PNET HR + 5 regimen (n = 18), the small number of HRMB (n = 13), and missing data for molecular subtypes for 4 medulloblastomas. The average age of our patients remains low because it was felt that intensive treatment should not be proposed for adults over 35 years of age based on the probability of toxicity in an older population. Consequently, outcomes and toxicity data should be interpreted with caution, especially when compared to other studies that included older patients. It should be remembered that very late relapse may specifically occur in the adult population of medulloblastoma, and therefore longer follow-up may be required in this population. Regarding toxicity, 2 patients with non-metastatic pinealoblastoma received only focal radiation and 1 patient with non-metastatic medulloblastoma received only 25 Gy of radiation on the axis, interpretation of toxicity after radiotherapy may thus be biased. Moreover, 2 patients did not receive 2 cycles of HDT to limit delays between surgery and radiotherapy, which limits the interpretation of toxicity and actual delays if a second cycle of HDT was given. In essence, the toxicity of these patients may have been underestimated because they did not receive the full protocol.
Our retrospective study reports encouraging results for adult HRMB that deserve further investigations in a large international clinical trial. There is a need for clinical trials in adult medulloblastoma to better define chemotherapy regimens and transpose recent discoveries in terms of prognostic factors and treatment options. The EORTC 1634-BTG/NOA-23 trial will randomize adults with standard-risk medulloblastomas between standard dose vs reduced-dosed craniospinal radiotherapy and SHH + patient subgroups in order to administer either the SMO inhibitor sonidegib in addition to standard radio-chemotherapy or standard radio-chemotherapy alone.30 The next question for an international clinical trial is the place of high-dose chemotherapy in a large clinical trial of adults with HRMB. Due to the rarity of HRMB in adults, it will likely be necessary to design a non-randomized trial that includes both Europe and the United States to ensure an adequate number of inclusions.
New prognostic factors are emerging from the molecular analyses of medulloblastoma, and studies are necessary to better define the adult populations at risk of relapse in the future.6,8 There is an absolute need to better coordinate treatment practices in Europe and to merge data on orphan diseases. The EURACAN network was designed to address this urgent need.37
In conclusion, the PNET HR + 5 regimen is feasible in an adult population and despite significant toxicity and delay in treatment compared with the pediatric population, it has resulted in meaningful improvement in OS and PFS in adult HRMB patients.
Funding
None.
Acknowledgments
We thank Brigitte Manship for editing the article. We thank the RENOCLIP LOC network (Réseau national de neuro-oncologie clinico Pathologique pour les cancers rares du système nerveux central) for its centralized review of medulloblastomas and the French National Registry of Pineal Tumors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Authorship statement
Data collection: L.L.. Data analysis and interpretation: L.L., D.F., C.F.C., and C.D.. Report writing: L.L., D.F., C.D., C.F.C., V.L., A.B.L., M.P.S., D.M., S.B., and D.M.. Proofreading and approval: L.L., D.F., C.D., C.F.C., V.L., A.B.L., M.P.S., D.M., S.B., and D.M..
Data availability
The raw data supporting the findings of this study are available from the corresponding author upon request.




