-
PDF
- Split View
-
Views
-
Cite
Cite
Ryosuke Otsuji, Nobuhiro Hata, Hidetaka Yamamoto, Daisuke Kuga, Ryusuke Hatae, Yuhei Sangatsuda, Yutaka Fujioka, Naoki Noguchi, Aki Sako, Osamu Togao, Tadamasa Yoshitake, Akira Nakamizo, Masahiro Mizoguchi, Koji Yoshimoto, Hemizygous deletion of cyclin-dependent kinase inhibitor 2A/B with p16 immuno-negative and methylthioadenosine phosphorylase retention predicts poor prognosis in IDH-mutant adult glioma, Neuro-Oncology Advances, Volume 6, Issue 1, January-December 2024, vdae069, https://doi.org/10.1093/noajnl/vdae069
Close - Share Icon Share
Abstract
Homozygous deletion of the tumor suppression genes cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) is a strong adverse prognostic factor in IDH-mutant gliomas, particularly astrocytoma. However, the impact of hemizygous deletion of CDKN2A/B is unknown. Furthermore, the influence of CDKN2A/B status in IDH-mutant and 1p/19q-codeleted oligodendroglioma remains controversial. We examined the impact of CDKN2A/B status classification, including hemizygous deletions, on the prognosis of IDH-mutant gliomas.
We enrolled 101 adults with IDH-mutant glioma between December 2002 and November 2021. CDKN2A/B deletion was evaluated with multiplex ligation-dependent probe amplification (MLPA). Immunohistochemical analysis of p16/MTAP and promoter methylation analysis with methylation-specific MLPA was performed for cases with CDKN2A/B deletion. Kaplan − Meier plots and Cox proportion hazards model analyses were performed to evaluate the impact on overall (OS) and progression-free survival.
Of 101 cases, 12 and 4 were classified as hemizygous and homozygous deletion, respectively. Immunohistochemistry revealed p16-negative and MTAP retention in cases with hemizygous deletion, whereas homozygous deletions had p16-negative and MTAP loss. In astrocytoma, OS was shorter in the order of homozygous deletion, hemizygous deletion, and copy-neutral groups (median OS: 38.5, 59.5, and 93.1 months, respectively). Multivariate analysis revealed hazard ratios of 9.30 (P = .0191) and 2.44 (P = .0943) for homozygous and hemizygous deletions, respectively.
CDKN2A/B hemizygous deletions exerted a negative impact on OS in astrocytoma. Immunohistochemistry of p16/MTAP can be utilized to validate hemizygous or homozygous deletions in combination with conventional molecular diagnosis.
The impact of CDKN2A/B Hemi-del was evaluated in patients with IDH-mutant glioma.
In astrocytoma, OS was shorter in Hemi-del compared with copy-neutral.
IHC clearly detected Hemi-del with p16-negative and MTAP retention.
Homozygous deletion (Homo-del) of cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) is a strong adverse prognostic factor in IDH-mutant gliomas, particularly astrocytoma. However, the impact of hemizygous deletion (Hemi-del) of CDKN2A/B is unknown. Furthermore, the influence of CDKN2A/B status in IDH-mutant and 1p/19q-codeleted oligodendroglioma is controversial. We evaluated CDKN2A/B status in patients with IDH-mutant glioma, using multiplex ligation-dependent probe amplification copy number analysis corrected with IDH-mutant variant allele frequency assessed through digital PCR. In astrocytoma, overall survival (OS) in Hemi-del was between that of Homo-del and copy-neutral. Furthermore, we report the utility of immunohistochemistry (IHC) of p16 and MTAP to distinguish Hemi-del from Homo-del and copy-neutral. We hypothesize that CDKN2A/B Hemi-del exerts a negative impact on OS in astrocytoma, and that the unexpensive method of IHC of p16/MTAP can be used to clearly identify Hemi-del.
Adult-type diffuse gliomas are among the most common primary brain tumors,1 and their molecular characterization is crucial for diagnosis and treatment.2,3 The homozygous deletion (Homo-del) of cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) is a strong adverse prognostic factor in IDH-mutant gliomas.4–9 Astrocytoma, IDH-mutant (hereafter astrocytoma) is stratified according to the 2021 WHO classification of CNS tumors into grades 2, 3, or 4, depending on the presence or absence of a CDKN2A/B Homo-del.3
Previous studies have reported that the chromosome region 9p21, containing the loci encoding p16INK4A, p14ARF, and p15INK4B, is involved in hemizygous deletion (Hemi-del) and Homo-del in gliomas.10–12 CDKN2A encodes p16INK4a and p14ARF by alternative splicing and distinction of reading frames, while CDKN2B exists in their vicinity to encode p15INK4b. CDKN2A/B are tumor suppression genes; p16INK4A and p15INK4B induce a G1 cell cycle arrest by inhibiting the activity of cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) from phosphorylating the RB protein,13–15 while p14ARF activates p5314,15 by binding to and promoting the rapid degradation of MDM2.16 Based on Knudson’s 2-hit hypothesis, oncogenesis may be induced by a second hit of methylation or mutation in the counter allele without deletion of tumor suppression genes.17 CDKN2A promoter methylation occurs frequently, especially in lower-grade gliomas,18–20 and cases with Hemi-del CDKN2A/B accompanied by promoter methylation may, theoretically, present similar to Homo-del of CDKN2A/B.
Although Homo-del of CDKN2A/B is a definitive prognostic factor, the clinical significance of Hemi-del is unclear. Recent studies on the prognostic implications of CDKN2A/B Hemi-del show that it may adversely affect the prognosis of lower-grade gliomas.21,22 However, there is still no consensus on this matter. Furthermore, the influence of CDKN2A/B status in oligodendroglioma, IDH-mutant, and 1p/19q-codeleted (hereafter oligodendroglioma) remains controversial.5,23–26
We evaluated the prognosis of IDH-mutant glioma cases with Hemi-del CDKN2A/B. Furthermore, we performed external verification using The Cancer Genome Atlas (TCGA)-LGG public dataset23 to validate our results. We also examined the promoter methylations of CDKN2A/B, the 2-hit hypothesis for CDKN2A/B, and the potential usefulness of immunohistochemical analyses.
Materials and Methods
Patients
We enrolled 101 patients (> 18 years old) with newly diagnosed IDH-mutant glioma between December 2002 and November 2021 (Supplementary Figure S1). The sample size was not statistically determined prior to the study. The molecular features of all patients were confirmed as described below. All patients received an integrated diagnosis using the WHO 2021 classification based on histopathology and molecular diagnosis. Oligodendroglioma was defined as satisfying both IDH-mutant and chromosome 1p/19q codeletion. One case of IDH-mutant and TERT promoter mutations without 1p/19q codeletion was excluded. The clinical characteristics of the patients are summarized in Table 1. At our facility, the policy for adjuvant treatment was as follows: post-treatment was not performed for grade 2 astrocytoma when complete resection was achieved, while chemotherapy with nimustine (ACNU) and radiotherapy (RT) were performed when less than subtotal resection was achieved. For grade 3, ACNU and RT were performed regardless of the degree of resection.27 For grade 4, synchronous RT with temozolomide was performed, similar to glioblastoma. For oligodendroglioma, only chemotherapy with ACNU was performed, and RT was deferred as much as possible.28,29 This study was approved by the Kyushu University Institutional Review Board for Clinical Research (848 − 00) and conducted in accordance with the 1964 Declaration of Helsinki (as revised in October 2013). Informed consent was obtained from all patients.
| Variable assessed . | All (n = 101) . | Astrocytoma, IDH-mutant (n = 52) . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) . |
|---|---|---|---|
| Age, median (range) | 41 (19 − 78) | 34.5 (19 − 71) | 47 (20 − 78) |
| ≤50 (%) | 28 (27.7) | 44 (84.6) | 29 (59.2) |
| >50 (%) | 73 (72.3) | 8 (15.4) | 20 (40.8) |
| Male sex (%) | 59 (58.4) | 34 (63.0) | 26 (53.1) |
| Tumor size (mm), median (range) | 55 (22 − 144) | 55 (22 − 144) | 50 (25 − 92) |
| KPS | |||
| median (range) | 90 (50 − 100) | 90 (50 − 100) | 90 (60 − 100) |
| >80 (%) | 87 (86.1) | 44 (84.6) | 43 (87.8) |
| ≤80 (%) | 14 (13.9) | 8 (15.4) | 6 (12.2) |
| EOR | |||
| >90% (%) | 61 (60.4) | 28 (53.8) | 33 (67.3) |
| ≤90% (%) | 40 (39.6) | 24 (46.2) | 16 (32.7) |
| Radiation therapy (%) | |||
| (+) | 34 (33.7) | 33 (63.5) | 1 (2.0) |
| (−) | 67 (66.3) | 19 (36.5) | 48 (98.0) |
| Chemotherapy (%) | |||
| (+) | 82 (80.4) | 36 (69.2) | 45 (91.8) |
| (−) | 20 (19.6) | 16 (30.8) | 4 (8.2) |
| WHO grade (%) | |||
| 2 | 54 (53.9) | 28 (53.8) | 26 (53.1) |
| 3 | 44 (43.1) | 21 (40.4) | 23 (46.9) |
| 4 | 3 (2.9) | 3 (5.8) | — |
| IDH-mutant (%) | 101 (100) | 52 (100) | 49 (100) |
| pTERT mutant (%) | 49 (48.5) | 0 (0) | 49 (100) |
| 1p/19q codeletion (%) | 49 (48.5) | 0 (0) | 49 (100) |
| CDKN2A/B copy number (%) | |||
| Homozygous deletion | 4 (4.0) | 3 (5.8) | 1 (2.0) |
| Hemizygous deletion | 12 (11.9) | 9 (17.3) | 3 (6.1) |
| Copy-neutral | 85 (84.2) | 40 (76.9) | 45 (91.8) |
| Variable assessed . | All (n = 101) . | Astrocytoma, IDH-mutant (n = 52) . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) . |
|---|---|---|---|
| Age, median (range) | 41 (19 − 78) | 34.5 (19 − 71) | 47 (20 − 78) |
| ≤50 (%) | 28 (27.7) | 44 (84.6) | 29 (59.2) |
| >50 (%) | 73 (72.3) | 8 (15.4) | 20 (40.8) |
| Male sex (%) | 59 (58.4) | 34 (63.0) | 26 (53.1) |
| Tumor size (mm), median (range) | 55 (22 − 144) | 55 (22 − 144) | 50 (25 − 92) |
| KPS | |||
| median (range) | 90 (50 − 100) | 90 (50 − 100) | 90 (60 − 100) |
| >80 (%) | 87 (86.1) | 44 (84.6) | 43 (87.8) |
| ≤80 (%) | 14 (13.9) | 8 (15.4) | 6 (12.2) |
| EOR | |||
| >90% (%) | 61 (60.4) | 28 (53.8) | 33 (67.3) |
| ≤90% (%) | 40 (39.6) | 24 (46.2) | 16 (32.7) |
| Radiation therapy (%) | |||
| (+) | 34 (33.7) | 33 (63.5) | 1 (2.0) |
| (−) | 67 (66.3) | 19 (36.5) | 48 (98.0) |
| Chemotherapy (%) | |||
| (+) | 82 (80.4) | 36 (69.2) | 45 (91.8) |
| (−) | 20 (19.6) | 16 (30.8) | 4 (8.2) |
| WHO grade (%) | |||
| 2 | 54 (53.9) | 28 (53.8) | 26 (53.1) |
| 3 | 44 (43.1) | 21 (40.4) | 23 (46.9) |
| 4 | 3 (2.9) | 3 (5.8) | — |
| IDH-mutant (%) | 101 (100) | 52 (100) | 49 (100) |
| pTERT mutant (%) | 49 (48.5) | 0 (0) | 49 (100) |
| 1p/19q codeletion (%) | 49 (48.5) | 0 (0) | 49 (100) |
| CDKN2A/B copy number (%) | |||
| Homozygous deletion | 4 (4.0) | 3 (5.8) | 1 (2.0) |
| Hemizygous deletion | 12 (11.9) | 9 (17.3) | 3 (6.1) |
| Copy-neutral | 85 (84.2) | 40 (76.9) | 45 (91.8) |
CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; EOR, extent of resection; IDH, isocitrate dehydrogenase; KPS, Karnofsky Performance Scale; pTERT, promoter of telomerase reverse transcriptase; WHO, World Health Organization.
| Variable assessed . | All (n = 101) . | Astrocytoma, IDH-mutant (n = 52) . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) . |
|---|---|---|---|
| Age, median (range) | 41 (19 − 78) | 34.5 (19 − 71) | 47 (20 − 78) |
| ≤50 (%) | 28 (27.7) | 44 (84.6) | 29 (59.2) |
| >50 (%) | 73 (72.3) | 8 (15.4) | 20 (40.8) |
| Male sex (%) | 59 (58.4) | 34 (63.0) | 26 (53.1) |
| Tumor size (mm), median (range) | 55 (22 − 144) | 55 (22 − 144) | 50 (25 − 92) |
| KPS | |||
| median (range) | 90 (50 − 100) | 90 (50 − 100) | 90 (60 − 100) |
| >80 (%) | 87 (86.1) | 44 (84.6) | 43 (87.8) |
| ≤80 (%) | 14 (13.9) | 8 (15.4) | 6 (12.2) |
| EOR | |||
| >90% (%) | 61 (60.4) | 28 (53.8) | 33 (67.3) |
| ≤90% (%) | 40 (39.6) | 24 (46.2) | 16 (32.7) |
| Radiation therapy (%) | |||
| (+) | 34 (33.7) | 33 (63.5) | 1 (2.0) |
| (−) | 67 (66.3) | 19 (36.5) | 48 (98.0) |
| Chemotherapy (%) | |||
| (+) | 82 (80.4) | 36 (69.2) | 45 (91.8) |
| (−) | 20 (19.6) | 16 (30.8) | 4 (8.2) |
| WHO grade (%) | |||
| 2 | 54 (53.9) | 28 (53.8) | 26 (53.1) |
| 3 | 44 (43.1) | 21 (40.4) | 23 (46.9) |
| 4 | 3 (2.9) | 3 (5.8) | — |
| IDH-mutant (%) | 101 (100) | 52 (100) | 49 (100) |
| pTERT mutant (%) | 49 (48.5) | 0 (0) | 49 (100) |
| 1p/19q codeletion (%) | 49 (48.5) | 0 (0) | 49 (100) |
| CDKN2A/B copy number (%) | |||
| Homozygous deletion | 4 (4.0) | 3 (5.8) | 1 (2.0) |
| Hemizygous deletion | 12 (11.9) | 9 (17.3) | 3 (6.1) |
| Copy-neutral | 85 (84.2) | 40 (76.9) | 45 (91.8) |
| Variable assessed . | All (n = 101) . | Astrocytoma, IDH-mutant (n = 52) . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) . |
|---|---|---|---|
| Age, median (range) | 41 (19 − 78) | 34.5 (19 − 71) | 47 (20 − 78) |
| ≤50 (%) | 28 (27.7) | 44 (84.6) | 29 (59.2) |
| >50 (%) | 73 (72.3) | 8 (15.4) | 20 (40.8) |
| Male sex (%) | 59 (58.4) | 34 (63.0) | 26 (53.1) |
| Tumor size (mm), median (range) | 55 (22 − 144) | 55 (22 − 144) | 50 (25 − 92) |
| KPS | |||
| median (range) | 90 (50 − 100) | 90 (50 − 100) | 90 (60 − 100) |
| >80 (%) | 87 (86.1) | 44 (84.6) | 43 (87.8) |
| ≤80 (%) | 14 (13.9) | 8 (15.4) | 6 (12.2) |
| EOR | |||
| >90% (%) | 61 (60.4) | 28 (53.8) | 33 (67.3) |
| ≤90% (%) | 40 (39.6) | 24 (46.2) | 16 (32.7) |
| Radiation therapy (%) | |||
| (+) | 34 (33.7) | 33 (63.5) | 1 (2.0) |
| (−) | 67 (66.3) | 19 (36.5) | 48 (98.0) |
| Chemotherapy (%) | |||
| (+) | 82 (80.4) | 36 (69.2) | 45 (91.8) |
| (−) | 20 (19.6) | 16 (30.8) | 4 (8.2) |
| WHO grade (%) | |||
| 2 | 54 (53.9) | 28 (53.8) | 26 (53.1) |
| 3 | 44 (43.1) | 21 (40.4) | 23 (46.9) |
| 4 | 3 (2.9) | 3 (5.8) | — |
| IDH-mutant (%) | 101 (100) | 52 (100) | 49 (100) |
| pTERT mutant (%) | 49 (48.5) | 0 (0) | 49 (100) |
| 1p/19q codeletion (%) | 49 (48.5) | 0 (0) | 49 (100) |
| CDKN2A/B copy number (%) | |||
| Homozygous deletion | 4 (4.0) | 3 (5.8) | 1 (2.0) |
| Hemizygous deletion | 12 (11.9) | 9 (17.3) | 3 (6.1) |
| Copy-neutral | 85 (84.2) | 40 (76.9) | 45 (91.8) |
CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; EOR, extent of resection; IDH, isocitrate dehydrogenase; KPS, Karnofsky Performance Scale; pTERT, promoter of telomerase reverse transcriptase; WHO, World Health Organization.
Molecular Diagnosis
DNA was extracted from intraoperative snap-frozen tumor samples using a QIAamp DNA mini kit (Qiagen Science, Germantown, Maryland, USA). Hotspot mutations of IDH1, IDH2, BRAF, and H3F3A were confirmed by Sanger sequencing following high-resolution melt analysis.30–34 TERT promoter mutations were assessed with Sanger sequencing.30,32,33,35 MGMT methylation status was evaluated using methylation-specific PCR.30,32,36 Copy number alterations were detected by multiplex ligation-dependent probe amplification (MLPA) with P088-D1 and P105-D3 probe sets (MRC-Holland, Amsterdam, Netherlands). Following the MLPA reaction, DNA fragment analysis was performed using a 3730 DNA analyzer (Applied Biosystems, Waltham, MA, USA) and analyzed with Coffalyser® software (MRC-Holland).30,37 The 1p/19q status was confirmed by loss of heterozygosity analysis using PCR-based microsatellite markers,32,38–41 and MLPA with P088-D1 probemix. CDKN2A/B deletion was analyzed with both P088-D1 and P105-D3 kits, and other copy number alterations, including those for EGFR, PTEN, PDGFRA, CDK4, and TP53 were confirmed using P105-D3. The thresholds of 0.8 and 1.2 were set for the detection of deletions and gains.30,42 However, since the copy number analysis of MLPA is based on relative quantification, it is affected by the tumor content rate. Therefore, for CDKN2A/B deletion, we set custom thresholds for each case using the following formula (eg, for a tumor content of 80%, the thresholds for Hemi-del and Homo-del were 0.8 and 0.4, respectively).
Tumor cell rate = IDH-mutant VAF × 2
Hemi-del cutoff = 1 − Tumor cell rate × 0.25
Homo-del cutoff = 1 − Tumor cell rate × 0.75
Details of how to derive the formula are described in Supplementary Methods. For the tumor content rate, we used the IDH-mutant variant allele frequency (VAF) from the digital PCR. We also confirmed that the 1p and 19q copy numbers were Hemi-del in oligodendroglioma. CDKN2A/B promoter methylation was assessed using methylation-specific MLPA (MS-MLPA) with ME024-B3 probemix (MRC-Holland), according to the manufacturer’s protocol. Aberrant methylation was identified by the appearance of a signal peak after HhaI enzyme digestion, and methylation was scored when the calculated ratio compared with normal unmethylated loci was more than 15%.43
Digital PCR
Since copy number analysis using MLPA is affected by dilution with genomic DNA derived from non-tumor cells; the ratio of IDH-mutant to IDH-wild type was confirmed by digital PCR using the QuantStudio™ 3D Digital PCR System (ThermoFisher Scientific, Waltham, MA, USA) or QuantStudio™ Absolute Q™ digital PCR system (ThermoFisher Scientific) to confirm the IDH-mutant VAF. Diluted DNA samples (3.3 ng/µL) were used to perform PCR with a custom-made assay (Assay ID: ANDJ4XD, Life Technologies, Carlsbad, CA, USA) for detecting IDH-wild type and IDH-mutant alleles.37,44 VAF of the IDH-mutant was calculated as the ratio of the number of IDH-mutant wells to that of wells containing IDH-mutant and/or IDH-wild type signals, and was analyzed using QuantStudio™ 3D Analysis Suite™ (version 3.1.6-PRC-build18) or QuantStudio™ AbsoluteQ™ Digital PCR Software v6.2.1.
Immunohistochemical Analyses
Specimens were fixed in 10% formalin, gradient-dehydrated with ethanol, and immersed in paraffin. Serial 4-μm-thick sections were cut from the formalin-fixed, paraffin-embedded (FFPE) tissue blocks, and immunohistochemical staining for p16 and methylthioadenosine phosphorylase (MTAP) was performed. Primary antibodies for p16 (mouse monoclonal, E6H4, prediluted, Roche, Heidelberg, Germany) and MTAP (mouse monoclonal, 42-T, × 100, Santa Cruz Biotechnology, Dallas, TX, USA) were used. Pretreatment for heat-induced epitope retrieval was conducted with EDTA-based CC1 buffer (Roche) for p16 and ER2 buffer (Roche) for MTAP. After pretreatment, the primary antibodies were mounted on the tissue sections, and subsequent reactions were conducted with an ultraView universal DAB detection kit (Roche) on a fully automated immunohistochemical staining system (Benchmark ULTRA; Ventana Medical Systems, Tuscan, AZ, USA).
P16 protein expression was classified using a 4-step scale: 0: < 1%, 1: 1% to < 10%, 2: 10% to < 50%, 3: 50% or more. In this study, scales 0 and 1 were evaluated as negative, whereas 2 and 3 were positive. HPV-positive oropharyngeal carcinoma was used as an external control.45 MTAP protein expression was defined as retained if almost all tumor cells and endothelial cells (internal control) exhibited positive staining. If almost all tumor cells demonstrated no immunoreactivity for MTAP with positive staining in endothelial cells, the case was defined as a loss. Immunohistochemical evaluation of p16 and MTAP using direct light microscopy was performed by an experienced pathologist (HY) who was blinded to the molecular features.
Fluorescence In Situ Hybridization for CDKN2A/B deletion
As a supplementary experiment, fluorescence in situ hybridization (FISH) was performed for case 11, in which heterogeneity within the tumor was found. FISH was performed on FFPE sections using the p16/CEN9q Dual Color FISH Probe (Product No. GC002, GSP Lab, Inc., Kanagawa, Japan) to assess the CDKN2A/B copy number. Green and red signals were identical to FITC-labeled CEN9q and Texas-red-stained p16, respectively. FISH signals were evaluated in at least 50 tumor cell nuclei. If green (CEN9q)-only signal patterns with loss of 2 red (p16) signals were observed in > 30% of the cells, the case was defined as Homo-del of CDKN2A/B. If the FISH results did not meet the criteria of Homo-del and the loss of one red (p16) signal pattern was observed in > 30% of the cells, the case was defined as Hemi-del of CDKN2A/B.
The Cancer Genome Atlas dataset
To validate our results, an additional validation cohort from a public dataset from the TCGA-LGG project was analyzed.26 DNA copy numbers and IDH status were retrieved from the publicly available UCSC Xena site (http://xena.ucsc.edu/). Clinical data including survival data were obtained from the NCI Cancer Genomic Data Commons (NCI-GDC: https://gdc.cancer.gov). Of the 538 cases in the dataset, the copy number analysis was unavailable in 11, 5 were without survival data, and 122 cases with IDH-wild type (96 cases) or unknown (26 cases) were excluded. Finally, 400 cases were analyzed. The 1p/19q codeletion was evaluated: 261 cases without the codeletion were classified as astrocytomas, while 139 cases with the codeletion were classified as oligodendrogliomas. Homo-del or Hemi-del CDKN2A/B was defined based on the copy number output generated by the GISTIC 2.0 pipeline.46 Methylation status was evaluated using beta-value, and stratified as hypermethylated (beta > 0.5) or unmethylated (beta ≤ 0.5).
Statistical Analysis
All statistical analyses were performed using JMP Pro version 16.0.0 (SAS Institute Inc., Cary, NC, USA). The distribution of VAF was confirmed by histograms, and outliers were confirmed based on the median and quartiles. Kaplan − Meier analysis was conducted to evaluate overall (OS) and progression-free survival (PFS). The log-rank test was used to compare survival distributions. Univariate and multivariate Cox proportion hazards model analyses were performed to evaluate hazard ratios (HRs) and 95% confidence intervals (CIs) for CDKN2A/B status and other putative prognostic factors. Factors for multivariate analysis included variables with significant or trending differences in univariate analyses or those previously reported to have significant differences. P values were calculated using 2-tailed tests. Statistical significance was set at P < .05.
Results
Evaluation of CDKN2A/B Status and Immunohistochemical Analysis
Copy number analysis using MLPA detected a CDKN2A/B deletion in 16 out of 101 cases. Of those, 12 were astrocytoma and 4 were oligodendroglioma. Based on MLPA results, 2 Homo-del and 10 Hemi-del cases of CDKN2A/B were identified in the astrocytomas, whereas 1 Homo-del and 3 Hemi-del cases were found in the oligodendrogliomas (Tables 1 and 2). MS-MLPA was performed on 12 cases with Hemi-del in MLPA to evaluate the CDKN2A/B promoter methylation. CDKN2A promoter methylation was observed in 8 of the 12 cases (Table 2 and Supplementary Figure S2).
| Case . | Integrated diagnosis (grade) . | Pathological diagnosis . | Molecular diagnosis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histology . | p16 (score) . | MTAP . | IDH1 (VAF, %) . | 1p/19q codel . | pTERT . | CDKN2A/B status . | CDKN2A methylation (M/U) . | Others . | ||
| 1 | Astrocytoma, IDH-mut. (4) | AA | Negative (0) | Loss | R132H (47.0) | − | Wt | Homo | — | |
| 2 | Astrocytoma, IDH-mut. (4) b | DA | Negative (0) | Loss | R132H (22.9) | − | Wt | Homo | — | TP53 hemi |
| 3 | Astrocytoma, IDH-mut. (4) | OD | N/A | N/A | R132H (49.1) | − | Wt | Homo | — | EGFR gain, CDK4 gain |
| 4 | Astrocytoma, IDH-mut. (3) | AA | Negative (0) | Retain | R132H (43.4) | − | Wt | Hemi | M | |
| 5 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (36.7) | − | Wt | Hemi | M | EGFR gain |
| 6 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.3) | − | Wt | Hemi | U | PDGFRA amp |
| 7 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.8) | − | Wt | Hemi | U | |
| 8 | Astrocytoma, IDH-mut. (2) | OA | Negative (1) | Retain | R132H (28.5) | − | Wt | Hemi | U | TP53 hemi |
| 9 | Astrocytoma, IDH-mut. (2) | OA | Negative (0) | Retain | R132H (41.5) | − | Wt | Hemi | M | TP53 hemi |
| 10 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (44.5) | − | Wt | Hemi | M | |
| 11 | Astrocytoma, IDH-mut. (2) | DA | Negative† (1†) | Retain | R132H (40.5) | − | Wt | Hemi | M | |
| 12 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (52.0) | − | Wt | Hemi | M | TP53 homo |
| 13 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (0) | Loss | R132H (32.7) | + | C228T | Homo | — | |
| 14 | Oligodendroglioma, IDH-mut. (3) | AO | N/A | N/A | R132H (44.0) | + | C250T | Hemi | M | EGFR gain |
| 15 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (1) | Retain | R132H (57.8) | + | C228T | Hemi | U | EGFR gain, TP53 hemi |
| 16 | Oligodendroglioma, IDH-mut. (3) | AOA | Negative (1) | Retain | R132H (47.6) | + | C228T | Hemi | M | TP53 hemi |
| Case . | Integrated diagnosis (grade) . | Pathological diagnosis . | Molecular diagnosis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histology . | p16 (score) . | MTAP . | IDH1 (VAF, %) . | 1p/19q codel . | pTERT . | CDKN2A/B status . | CDKN2A methylation (M/U) . | Others . | ||
| 1 | Astrocytoma, IDH-mut. (4) | AA | Negative (0) | Loss | R132H (47.0) | − | Wt | Homo | — | |
| 2 | Astrocytoma, IDH-mut. (4) b | DA | Negative (0) | Loss | R132H (22.9) | − | Wt | Homo | — | TP53 hemi |
| 3 | Astrocytoma, IDH-mut. (4) | OD | N/A | N/A | R132H (49.1) | − | Wt | Homo | — | EGFR gain, CDK4 gain |
| 4 | Astrocytoma, IDH-mut. (3) | AA | Negative (0) | Retain | R132H (43.4) | − | Wt | Hemi | M | |
| 5 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (36.7) | − | Wt | Hemi | M | EGFR gain |
| 6 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.3) | − | Wt | Hemi | U | PDGFRA amp |
| 7 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.8) | − | Wt | Hemi | U | |
| 8 | Astrocytoma, IDH-mut. (2) | OA | Negative (1) | Retain | R132H (28.5) | − | Wt | Hemi | U | TP53 hemi |
| 9 | Astrocytoma, IDH-mut. (2) | OA | Negative (0) | Retain | R132H (41.5) | − | Wt | Hemi | M | TP53 hemi |
| 10 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (44.5) | − | Wt | Hemi | M | |
| 11 | Astrocytoma, IDH-mut. (2) | DA | Negative† (1†) | Retain | R132H (40.5) | − | Wt | Hemi | M | |
| 12 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (52.0) | − | Wt | Hemi | M | TP53 homo |
| 13 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (0) | Loss | R132H (32.7) | + | C228T | Homo | — | |
| 14 | Oligodendroglioma, IDH-mut. (3) | AO | N/A | N/A | R132H (44.0) | + | C250T | Hemi | M | EGFR gain |
| 15 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (1) | Retain | R132H (57.8) | + | C228T | Hemi | U | EGFR gain, TP53 hemi |
| 16 | Oligodendroglioma, IDH-mut. (3) | AOA | Negative (1) | Retain | R132H (47.6) | + | C228T | Hemi | M | TP53 hemi |
AA, anaplastic astrocytoma; amp, amplification; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; CDK4, cyclin-dependent kinase 4; CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; codel, codeletion; DA, diffuse astrocytoma; EGFR, epidermal growth factor receptor; Hemi, hemizygous deletion; Homo, homozygous deletion; IDH, isocitrate dehydrogenase; mut, mutant; N/A, not available; OA, oligoastrocytoma; pTERT, promoter of telomerase reverse transcriptase; TP53, tumor protein P53; VAF, valiant allele frequency; Wt, wild type.
†Heterogeneity within the tumor was found. p16-positive (score 3) and p16-negative (score 1) cells were coexisting. The score of the region with the lowest expression was adopted for the p16 status.
| Case . | Integrated diagnosis (grade) . | Pathological diagnosis . | Molecular diagnosis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histology . | p16 (score) . | MTAP . | IDH1 (VAF, %) . | 1p/19q codel . | pTERT . | CDKN2A/B status . | CDKN2A methylation (M/U) . | Others . | ||
| 1 | Astrocytoma, IDH-mut. (4) | AA | Negative (0) | Loss | R132H (47.0) | − | Wt | Homo | — | |
| 2 | Astrocytoma, IDH-mut. (4) b | DA | Negative (0) | Loss | R132H (22.9) | − | Wt | Homo | — | TP53 hemi |
| 3 | Astrocytoma, IDH-mut. (4) | OD | N/A | N/A | R132H (49.1) | − | Wt | Homo | — | EGFR gain, CDK4 gain |
| 4 | Astrocytoma, IDH-mut. (3) | AA | Negative (0) | Retain | R132H (43.4) | − | Wt | Hemi | M | |
| 5 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (36.7) | − | Wt | Hemi | M | EGFR gain |
| 6 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.3) | − | Wt | Hemi | U | PDGFRA amp |
| 7 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.8) | − | Wt | Hemi | U | |
| 8 | Astrocytoma, IDH-mut. (2) | OA | Negative (1) | Retain | R132H (28.5) | − | Wt | Hemi | U | TP53 hemi |
| 9 | Astrocytoma, IDH-mut. (2) | OA | Negative (0) | Retain | R132H (41.5) | − | Wt | Hemi | M | TP53 hemi |
| 10 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (44.5) | − | Wt | Hemi | M | |
| 11 | Astrocytoma, IDH-mut. (2) | DA | Negative† (1†) | Retain | R132H (40.5) | − | Wt | Hemi | M | |
| 12 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (52.0) | − | Wt | Hemi | M | TP53 homo |
| 13 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (0) | Loss | R132H (32.7) | + | C228T | Homo | — | |
| 14 | Oligodendroglioma, IDH-mut. (3) | AO | N/A | N/A | R132H (44.0) | + | C250T | Hemi | M | EGFR gain |
| 15 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (1) | Retain | R132H (57.8) | + | C228T | Hemi | U | EGFR gain, TP53 hemi |
| 16 | Oligodendroglioma, IDH-mut. (3) | AOA | Negative (1) | Retain | R132H (47.6) | + | C228T | Hemi | M | TP53 hemi |
| Case . | Integrated diagnosis (grade) . | Pathological diagnosis . | Molecular diagnosis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histology . | p16 (score) . | MTAP . | IDH1 (VAF, %) . | 1p/19q codel . | pTERT . | CDKN2A/B status . | CDKN2A methylation (M/U) . | Others . | ||
| 1 | Astrocytoma, IDH-mut. (4) | AA | Negative (0) | Loss | R132H (47.0) | − | Wt | Homo | — | |
| 2 | Astrocytoma, IDH-mut. (4) b | DA | Negative (0) | Loss | R132H (22.9) | − | Wt | Homo | — | TP53 hemi |
| 3 | Astrocytoma, IDH-mut. (4) | OD | N/A | N/A | R132H (49.1) | − | Wt | Homo | — | EGFR gain, CDK4 gain |
| 4 | Astrocytoma, IDH-mut. (3) | AA | Negative (0) | Retain | R132H (43.4) | − | Wt | Hemi | M | |
| 5 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (36.7) | − | Wt | Hemi | M | EGFR gain |
| 6 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.3) | − | Wt | Hemi | U | PDGFRA amp |
| 7 | Astrocytoma, IDH-mut. (3) | AA | Negative (1) | Retain | R132H (42.8) | − | Wt | Hemi | U | |
| 8 | Astrocytoma, IDH-mut. (2) | OA | Negative (1) | Retain | R132H (28.5) | − | Wt | Hemi | U | TP53 hemi |
| 9 | Astrocytoma, IDH-mut. (2) | OA | Negative (0) | Retain | R132H (41.5) | − | Wt | Hemi | M | TP53 hemi |
| 10 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (44.5) | − | Wt | Hemi | M | |
| 11 | Astrocytoma, IDH-mut. (2) | DA | Negative† (1†) | Retain | R132H (40.5) | − | Wt | Hemi | M | |
| 12 | Astrocytoma, IDH-mut. (2) | DA | Negative (1) | Retain | R132H (52.0) | − | Wt | Hemi | M | TP53 homo |
| 13 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (0) | Loss | R132H (32.7) | + | C228T | Homo | — | |
| 14 | Oligodendroglioma, IDH-mut. (3) | AO | N/A | N/A | R132H (44.0) | + | C250T | Hemi | M | EGFR gain |
| 15 | Oligodendroglioma, IDH-mut. (3) | AO | Negative (1) | Retain | R132H (57.8) | + | C228T | Hemi | U | EGFR gain, TP53 hemi |
| 16 | Oligodendroglioma, IDH-mut. (3) | AOA | Negative (1) | Retain | R132H (47.6) | + | C228T | Hemi | M | TP53 hemi |
AA, anaplastic astrocytoma; amp, amplification; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; CDK4, cyclin-dependent kinase 4; CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; codel, codeletion; DA, diffuse astrocytoma; EGFR, epidermal growth factor receptor; Hemi, hemizygous deletion; Homo, homozygous deletion; IDH, isocitrate dehydrogenase; mut, mutant; N/A, not available; OA, oligoastrocytoma; pTERT, promoter of telomerase reverse transcriptase; TP53, tumor protein P53; VAF, valiant allele frequency; Wt, wild type.
†Heterogeneity within the tumor was found. p16-positive (score 3) and p16-negative (score 1) cells were coexisting. The score of the region with the lowest expression was adopted for the p16 status.
Immunohistochemical analysis of p16/MTAP was performed on 16 cases with CDKN2A/B deletion. The results and representative cases are presented in Table 2 and Figure 1, respectively. Immunohistochemical staining revealed both p16/MTAP loss in the Homo-del cases, whereas p16 was lost and MTAP was retained in the Hemi-del cases (Figure 1C). In case 11, heterogeneity with different immunohistochemical results was observed within the tumor tissue, suggesting a cluster of copy-neutral (Neutral) and Hemi-del populations (Table 2 and Supplementary Figure S3). The supplementary FISH evaluation revealed clusters of Neutral and Hemi-del cells, supporting the results of immunohistochemistry.
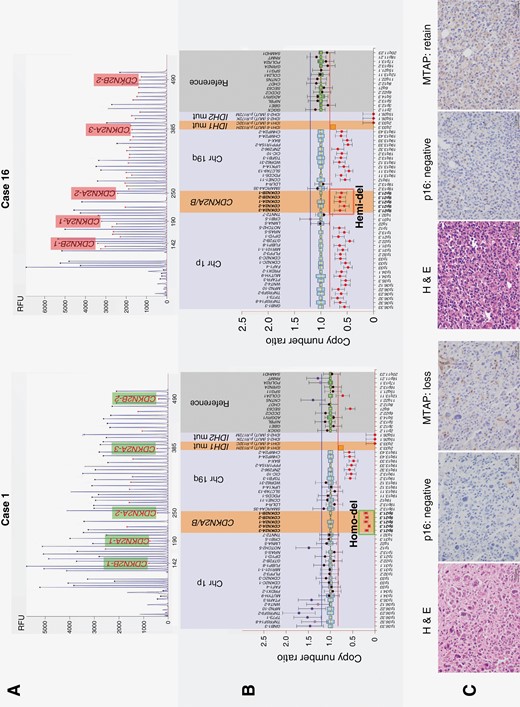
Representative examples of homozygous CDKN2A/B deletion (case 1) and hemizygous deletion (case 16). (A) Electrophoresis of multiplex ligation-dependent probe amplification (MLPA) using P088 probemix. (B) Ratio chart of copy number analysis. Case 1 presents with IDH 1 R132H mutant non 1p/19q-codeleted tumor with CDKN2A/B homozygous deletion. Case 16 presents with IDH 1 R132H mutant and 1p/19q-codeleted tumor with CDKN2A/B hemizygous deletion. (C) Histopathological findings. Hematoxylin and eosin staining and immunohistochemistry of p16 and MTAP are shown from left to right. Histologically, case 1 exhibits high-grade glioma cells with round to polygonal-shaped nuclei and prominent nuclear pleomorphism. Case 16 exhibits cellular proliferation of anaplastic glioma cells with rounded nuclei and perinuclear halo. Expression of p16 in cases 1 and 16 is classified as scale 0 and 1, respectively; both are evaluated as negative. MTAP expression is completely lost in case 1 and retained in case 16. CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; Chr, chromosome; H & E, Hematoxylin and eosin staining; Hemi-del, hemizygous deletion; Homo-del, homozygous deletion; IDH, isocitrate dehydrogenase; MTAP, methylthioadenosine phosphorylase; mut, mutant.
Impact of CDKN2A/B Status on Survival Outcomes
OS and PFS were analyzed by dividing the cases into 3 groups based on CDKN2A/B status: Homo-del, Hemi-del, and Neutral. Kaplan − Meier plots are presented in Figures 2A and B. In astrocytoma, OS was shorter in the order of Homo-del, Hemi-del, and Neutral groups, with a median OS of 37.5, 59.5, and 93.1 months, respectively. Hemi-del tended to have a shorter OS than Neutral (P = .0732). In oligodendroglioma, OS could not be compared because one Homo-del and 3 Hemi-del cases did not reach the endpoint. Regarding PFS, in astrocytoma, the time-to-recurrence tended to be shorter in the order of Homo-del, Hemi-del, and Neutral, with median PFS of 20.9, 38.9, and 53.0 months, respectively. There was no significant difference in PFS between Hemi-del and Neutral in astrocytoma (P = .4030). In oligodendroglioma, Hemi-del and Neutral were compared because one Homo-del case did not reach the endpoint. The recurrence period was significantly shorter in the Hemi-del than in the Neutral group, with median PFS of 33.3 and 113.6 months, respectively (P = .0234).
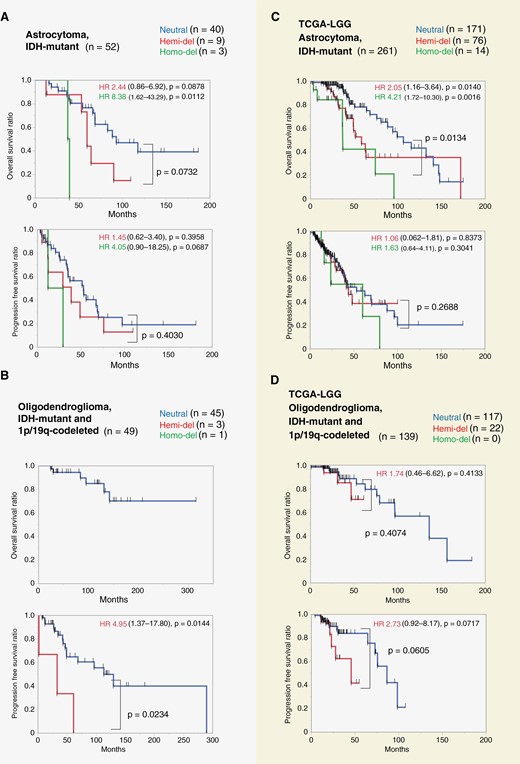
Kaplan–Meier plots of astrocytoma and oligodendroglioma stratified by CDKN2A/B status. A: IDH-mutant astrocytoma cases in our cohort. The duration of OS increased in the order of Homo-del, Hemi-del, and Neutral groups (median OS: 38.5, 59.5, and 93.1 months, respectively). OS was shorter for Hemi-del than for Neutral (P = .0732). B: IDH-mutant and 1p/19q-codeleted oligodendroglioma in our cohort. Hemi-del had a significantly shorter PFS than Neutral (median 33.3 and 113.6 months, respectively, P = .0234). C: IDH-mutant astrocytoma in The Cancer Genome Atlas (TCGA)-LGG data. In this cohort, Hemi-del and Neutral differed significantly (P = .0134). D: IDH-mutant and 1p/19q-codeleted oligodendroglioma in TCGA-LGG data. There was no significant difference in PFS between Hemi-del and Neutral (45.8 and 86.7 months, respectively, P = .0605). Hemi-del, hemizygous deletion of CDKN2A/B; Homo-del, homozygous deletion of CDKN2A/B; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; Neutral, copy-neutral.
The results of Cox proportional hazards model analysis are presented in Table 3. In univariate Cox proportional hazards model for OS for astrocytoma, compared to Neutral, Homo-del, and Hemi-del exhibited HRs of 8.38 (95% CI: 1.62 − 43.29, P = .0112) and 2.33 (95% CI: 0.88 − 6.20, P = .0878), respectively. Multivariate analysis revealed similar results (Homo-del HR: 9.30, 95% CI: 1.44 − 60.04, P = .0191; Hemi-del HR: 2.44, 95% CI: 0.86 − 6.92, P = .0943). No other factors were significant in the multivariate analysis. Regarding PFS for oligodendrogliomas, Hemi-del had HRs of 4.95 (95% CI: 1.37 − 17.80, P = .0144) and 7.43 (95% CI: 1.58 − 34.98, P = .0112) in univariate and multivariate analysis, respectively.
| Variable assessed . | Astrocytoma, IDH-mutant (n = 52) Case (%) . | OS . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | ||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||
| Age > 50 | 8 (15.4) | 1.05 (0.24 − 4.53) | .9455 | 1.55 (0.34 − 7.02) | .5674 | 1.38 (0.48 − 3.97) | .5539 | 1.74 (0.57 − 5.32) | .3330 |
| Male sex | 34 (63.0) | 1.01 (0.39 − 2.60) | .9788 | — | — | 1.09 (0.50 − 2.37) | .8362 | — | — |
| KPS ≤ 80 | 8 (15.4) | 1.97 (0.65 − 5.96) | .2297 | 0.80 (0.20 − 3.20) | .7533 | 0.99 (0.38 − 2.59) | .9810 | 0.61 (0.20 − 1.91) | .4000 |
| EOR ≤ 90% | 24 (46.2) | 1.94 (0.82 − 4.59) | .1298 | 1.96 (0.76 − 5.05) | .1657 | 1.42 (0.69 − 2.90) | .3397 | 1.66 (0.75 − 3.68) | .2158 |
| Radiation therapy (-) | 19 (36.5) | 0.63 (0.25 − 1.63) | .3455 | — | — | 1.35 (0.61 − 3.01) | .4629 | — | — |
| Chemotherapy (-) | 16 (30.8) | 0.50 (0.17 − 1.51) | .2203 | — | — | 1.11 (0.47 − 2.62) | .8169 | — | — |
| WHO grade | |||||||||
| 2 | 28 (53.8) | Ref | — | — | — | Ref | — | — | — |
| 3 | 21 (40.4) | 0.89 (0.35 − 2.24) | .7989. | 0.56 (0.26 − 1.19) | .1332. | ||||
| 4 | 3 (5.8) | 5.09 (1.03 − 25.21) | 0462 | 3.23 (0.71 − 14.80) | 1303 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 40 (76.9) | Ref | — | Ref | — | Ref | — | Ref | — |
| Hemizygous deletion | 9 (17.3) | 2.34 (0.88 − 6.20) | .0878 | 2.44 (0.86 − 6.92) | 0.0943 | 1.45 (0.62 − 3.40) | .3958 | 1.54 (0.65 − 3.70) | 0.3288 |
| Homozygous deletion | 3 (5.8) | 8.38 (1.62 − 43.29) | .0112 | 9.30 (1.44 − 60.04) | 0.0191 | 4.05 (0.90 − 18.25) | .0687 | 5.36 (1.01 − 28.41) | 0.0487 |
| Variable assessed . | Astrocytoma, IDH-mutant (n = 52) Case (%) . | OS . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | ||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||
| Age > 50 | 8 (15.4) | 1.05 (0.24 − 4.53) | .9455 | 1.55 (0.34 − 7.02) | .5674 | 1.38 (0.48 − 3.97) | .5539 | 1.74 (0.57 − 5.32) | .3330 |
| Male sex | 34 (63.0) | 1.01 (0.39 − 2.60) | .9788 | — | — | 1.09 (0.50 − 2.37) | .8362 | — | — |
| KPS ≤ 80 | 8 (15.4) | 1.97 (0.65 − 5.96) | .2297 | 0.80 (0.20 − 3.20) | .7533 | 0.99 (0.38 − 2.59) | .9810 | 0.61 (0.20 − 1.91) | .4000 |
| EOR ≤ 90% | 24 (46.2) | 1.94 (0.82 − 4.59) | .1298 | 1.96 (0.76 − 5.05) | .1657 | 1.42 (0.69 − 2.90) | .3397 | 1.66 (0.75 − 3.68) | .2158 |
| Radiation therapy (-) | 19 (36.5) | 0.63 (0.25 − 1.63) | .3455 | — | — | 1.35 (0.61 − 3.01) | .4629 | — | — |
| Chemotherapy (-) | 16 (30.8) | 0.50 (0.17 − 1.51) | .2203 | — | — | 1.11 (0.47 − 2.62) | .8169 | — | — |
| WHO grade | |||||||||
| 2 | 28 (53.8) | Ref | — | — | — | Ref | — | — | — |
| 3 | 21 (40.4) | 0.89 (0.35 − 2.24) | .7989. | 0.56 (0.26 − 1.19) | .1332. | ||||
| 4 | 3 (5.8) | 5.09 (1.03 − 25.21) | 0462 | 3.23 (0.71 − 14.80) | 1303 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 40 (76.9) | Ref | — | Ref | — | Ref | — | Ref | — |
| Hemizygous deletion | 9 (17.3) | 2.34 (0.88 − 6.20) | .0878 | 2.44 (0.86 − 6.92) | 0.0943 | 1.45 (0.62 − 3.40) | .3958 | 1.54 (0.65 − 3.70) | 0.3288 |
| Homozygous deletion | 3 (5.8) | 8.38 (1.62 − 43.29) | .0112 | 9.30 (1.44 − 60.04) | 0.0191 | 4.05 (0.90 − 18.25) | .0687 | 5.36 (1.01 − 28.41) | 0.0487 |
| Variable assessed . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) Case (%) . | OS† . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||||
| Age > 50 | 20 (40.8) | 2.73 (0.51 − 14.61) | .2415 | 1.40 (0.53 − 3.65) | .4963 | 0.99 (0.33 − 2.93) | .9861 | ||
| Male sex | 26 (53.1) | 0.82 (0.16 − 4.15) | .8111 | 1.27 (0.50 − 3.23) | .6095 | — | — | ||
| KPS ≤ 80 | 6 (12.2) | 1.84 (0.21 − 15.79) | .5772 | 0.94 (0.22 − 4.12) | .9363 | 0.37 (0.07 − 2.05) | .2562 | ||
| EOR ≤ 90% | 16 (32.7) | 2.73 (0.49 − 15.22) | .2530 | 1.85 (0.73 − 4.70) | .1945 | 2.81 (0.96 − 8.26) | .0602 | ||
| Radiation therapy (-) | 48 (98.0) | N/A | N/A | N/A | N/A | — | — | ||
| Chemotherapy (-) | 4 (8.2) | N/A | N/A | 0.41(0.05 − 3.09) | .3860 | — | — | ||
| WHO grade | |||||||||
| 2 | 26 (53.1) | Ref | — | Ref | — | — | — | ||
| 3 | 23 (46.9) | 0.61 (0.11 − 3.34) | .5685 | 1.43 (0.56 − 3.63) | .4542 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 45 (91.8) | Ref | — | Ref | — | Ref | — | ||
| Hemizygous deletion | 3 (6.1) | N/A | N/A | 4.95 (1.37 − 17.80) | .0144 | 7.43 (1.58 − 34.98) | .0112 | ||
| Homozygous deletion | 1 (2.0) | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Variable assessed . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) Case (%) . | OS† . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||||
| Age > 50 | 20 (40.8) | 2.73 (0.51 − 14.61) | .2415 | 1.40 (0.53 − 3.65) | .4963 | 0.99 (0.33 − 2.93) | .9861 | ||
| Male sex | 26 (53.1) | 0.82 (0.16 − 4.15) | .8111 | 1.27 (0.50 − 3.23) | .6095 | — | — | ||
| KPS ≤ 80 | 6 (12.2) | 1.84 (0.21 − 15.79) | .5772 | 0.94 (0.22 − 4.12) | .9363 | 0.37 (0.07 − 2.05) | .2562 | ||
| EOR ≤ 90% | 16 (32.7) | 2.73 (0.49 − 15.22) | .2530 | 1.85 (0.73 − 4.70) | .1945 | 2.81 (0.96 − 8.26) | .0602 | ||
| Radiation therapy (-) | 48 (98.0) | N/A | N/A | N/A | N/A | — | — | ||
| Chemotherapy (-) | 4 (8.2) | N/A | N/A | 0.41(0.05 − 3.09) | .3860 | — | — | ||
| WHO grade | |||||||||
| 2 | 26 (53.1) | Ref | — | Ref | — | — | — | ||
| 3 | 23 (46.9) | 0.61 (0.11 − 3.34) | .5685 | 1.43 (0.56 − 3.63) | .4542 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 45 (91.8) | Ref | — | Ref | — | Ref | — | ||
| Hemizygous deletion | 3 (6.1) | N/A | N/A | 4.95 (1.37 − 17.80) | .0144 | 7.43 (1.58 − 34.98) | .0112 | ||
| Homozygous deletion | 1 (2.0) | N/A | N/A | N/A | N/A | N/A | N/A | ||
CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; CI, confident interval; EOR, extent of resection; HR, hazard ratio; IDH, isocitrate dehydrogenase; KPS, Karnofsky Performance Scale; N/A, not available (the factor could not be analyzed because of unreached endpoint); OS, overall survival; PFS, progression-free survival; Ref, reference; WHO, World Health Organization.
†Only univariate analysis was performed in oligodendrogliomas because analysis of CDKN2A/B hemi/homozygous deletion was unable because of unreached endpoint.
| Variable assessed . | Astrocytoma, IDH-mutant (n = 52) Case (%) . | OS . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | ||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||
| Age > 50 | 8 (15.4) | 1.05 (0.24 − 4.53) | .9455 | 1.55 (0.34 − 7.02) | .5674 | 1.38 (0.48 − 3.97) | .5539 | 1.74 (0.57 − 5.32) | .3330 |
| Male sex | 34 (63.0) | 1.01 (0.39 − 2.60) | .9788 | — | — | 1.09 (0.50 − 2.37) | .8362 | — | — |
| KPS ≤ 80 | 8 (15.4) | 1.97 (0.65 − 5.96) | .2297 | 0.80 (0.20 − 3.20) | .7533 | 0.99 (0.38 − 2.59) | .9810 | 0.61 (0.20 − 1.91) | .4000 |
| EOR ≤ 90% | 24 (46.2) | 1.94 (0.82 − 4.59) | .1298 | 1.96 (0.76 − 5.05) | .1657 | 1.42 (0.69 − 2.90) | .3397 | 1.66 (0.75 − 3.68) | .2158 |
| Radiation therapy (-) | 19 (36.5) | 0.63 (0.25 − 1.63) | .3455 | — | — | 1.35 (0.61 − 3.01) | .4629 | — | — |
| Chemotherapy (-) | 16 (30.8) | 0.50 (0.17 − 1.51) | .2203 | — | — | 1.11 (0.47 − 2.62) | .8169 | — | — |
| WHO grade | |||||||||
| 2 | 28 (53.8) | Ref | — | — | — | Ref | — | — | — |
| 3 | 21 (40.4) | 0.89 (0.35 − 2.24) | .7989. | 0.56 (0.26 − 1.19) | .1332. | ||||
| 4 | 3 (5.8) | 5.09 (1.03 − 25.21) | 0462 | 3.23 (0.71 − 14.80) | 1303 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 40 (76.9) | Ref | — | Ref | — | Ref | — | Ref | — |
| Hemizygous deletion | 9 (17.3) | 2.34 (0.88 − 6.20) | .0878 | 2.44 (0.86 − 6.92) | 0.0943 | 1.45 (0.62 − 3.40) | .3958 | 1.54 (0.65 − 3.70) | 0.3288 |
| Homozygous deletion | 3 (5.8) | 8.38 (1.62 − 43.29) | .0112 | 9.30 (1.44 − 60.04) | 0.0191 | 4.05 (0.90 − 18.25) | .0687 | 5.36 (1.01 − 28.41) | 0.0487 |
| Variable assessed . | Astrocytoma, IDH-mutant (n = 52) Case (%) . | OS . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | ||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||
| Age > 50 | 8 (15.4) | 1.05 (0.24 − 4.53) | .9455 | 1.55 (0.34 − 7.02) | .5674 | 1.38 (0.48 − 3.97) | .5539 | 1.74 (0.57 − 5.32) | .3330 |
| Male sex | 34 (63.0) | 1.01 (0.39 − 2.60) | .9788 | — | — | 1.09 (0.50 − 2.37) | .8362 | — | — |
| KPS ≤ 80 | 8 (15.4) | 1.97 (0.65 − 5.96) | .2297 | 0.80 (0.20 − 3.20) | .7533 | 0.99 (0.38 − 2.59) | .9810 | 0.61 (0.20 − 1.91) | .4000 |
| EOR ≤ 90% | 24 (46.2) | 1.94 (0.82 − 4.59) | .1298 | 1.96 (0.76 − 5.05) | .1657 | 1.42 (0.69 − 2.90) | .3397 | 1.66 (0.75 − 3.68) | .2158 |
| Radiation therapy (-) | 19 (36.5) | 0.63 (0.25 − 1.63) | .3455 | — | — | 1.35 (0.61 − 3.01) | .4629 | — | — |
| Chemotherapy (-) | 16 (30.8) | 0.50 (0.17 − 1.51) | .2203 | — | — | 1.11 (0.47 − 2.62) | .8169 | — | — |
| WHO grade | |||||||||
| 2 | 28 (53.8) | Ref | — | — | — | Ref | — | — | — |
| 3 | 21 (40.4) | 0.89 (0.35 − 2.24) | .7989. | 0.56 (0.26 − 1.19) | .1332. | ||||
| 4 | 3 (5.8) | 5.09 (1.03 − 25.21) | 0462 | 3.23 (0.71 − 14.80) | 1303 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 40 (76.9) | Ref | — | Ref | — | Ref | — | Ref | — |
| Hemizygous deletion | 9 (17.3) | 2.34 (0.88 − 6.20) | .0878 | 2.44 (0.86 − 6.92) | 0.0943 | 1.45 (0.62 − 3.40) | .3958 | 1.54 (0.65 − 3.70) | 0.3288 |
| Homozygous deletion | 3 (5.8) | 8.38 (1.62 − 43.29) | .0112 | 9.30 (1.44 − 60.04) | 0.0191 | 4.05 (0.90 − 18.25) | .0687 | 5.36 (1.01 − 28.41) | 0.0487 |
| Variable assessed . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) Case (%) . | OS† . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||||
| Age > 50 | 20 (40.8) | 2.73 (0.51 − 14.61) | .2415 | 1.40 (0.53 − 3.65) | .4963 | 0.99 (0.33 − 2.93) | .9861 | ||
| Male sex | 26 (53.1) | 0.82 (0.16 − 4.15) | .8111 | 1.27 (0.50 − 3.23) | .6095 | — | — | ||
| KPS ≤ 80 | 6 (12.2) | 1.84 (0.21 − 15.79) | .5772 | 0.94 (0.22 − 4.12) | .9363 | 0.37 (0.07 − 2.05) | .2562 | ||
| EOR ≤ 90% | 16 (32.7) | 2.73 (0.49 − 15.22) | .2530 | 1.85 (0.73 − 4.70) | .1945 | 2.81 (0.96 − 8.26) | .0602 | ||
| Radiation therapy (-) | 48 (98.0) | N/A | N/A | N/A | N/A | — | — | ||
| Chemotherapy (-) | 4 (8.2) | N/A | N/A | 0.41(0.05 − 3.09) | .3860 | — | — | ||
| WHO grade | |||||||||
| 2 | 26 (53.1) | Ref | — | Ref | — | — | — | ||
| 3 | 23 (46.9) | 0.61 (0.11 − 3.34) | .5685 | 1.43 (0.56 − 3.63) | .4542 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 45 (91.8) | Ref | — | Ref | — | Ref | — | ||
| Hemizygous deletion | 3 (6.1) | N/A | N/A | 4.95 (1.37 − 17.80) | .0144 | 7.43 (1.58 − 34.98) | .0112 | ||
| Homozygous deletion | 1 (2.0) | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Variable assessed . | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted (n = 49) Case (%) . | OS† . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||||
| Age > 50 | 20 (40.8) | 2.73 (0.51 − 14.61) | .2415 | 1.40 (0.53 − 3.65) | .4963 | 0.99 (0.33 − 2.93) | .9861 | ||
| Male sex | 26 (53.1) | 0.82 (0.16 − 4.15) | .8111 | 1.27 (0.50 − 3.23) | .6095 | — | — | ||
| KPS ≤ 80 | 6 (12.2) | 1.84 (0.21 − 15.79) | .5772 | 0.94 (0.22 − 4.12) | .9363 | 0.37 (0.07 − 2.05) | .2562 | ||
| EOR ≤ 90% | 16 (32.7) | 2.73 (0.49 − 15.22) | .2530 | 1.85 (0.73 − 4.70) | .1945 | 2.81 (0.96 − 8.26) | .0602 | ||
| Radiation therapy (-) | 48 (98.0) | N/A | N/A | N/A | N/A | — | — | ||
| Chemotherapy (-) | 4 (8.2) | N/A | N/A | 0.41(0.05 − 3.09) | .3860 | — | — | ||
| WHO grade | |||||||||
| 2 | 26 (53.1) | Ref | — | Ref | — | — | — | ||
| 3 | 23 (46.9) | 0.61 (0.11 − 3.34) | .5685 | 1.43 (0.56 − 3.63) | .4542 | ||||
| CDKN2A/B status | |||||||||
| Copy-neutral | 45 (91.8) | Ref | — | Ref | — | Ref | — | ||
| Hemizygous deletion | 3 (6.1) | N/A | N/A | 4.95 (1.37 − 17.80) | .0144 | 7.43 (1.58 − 34.98) | .0112 | ||
| Homozygous deletion | 1 (2.0) | N/A | N/A | N/A | N/A | N/A | N/A | ||
CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; CI, confident interval; EOR, extent of resection; HR, hazard ratio; IDH, isocitrate dehydrogenase; KPS, Karnofsky Performance Scale; N/A, not available (the factor could not be analyzed because of unreached endpoint); OS, overall survival; PFS, progression-free survival; Ref, reference; WHO, World Health Organization.
†Only univariate analysis was performed in oligodendrogliomas because analysis of CDKN2A/B hemi/homozygous deletion was unable because of unreached endpoint.
Correlation Between CDKN2A/B Status and Malignant Transformation in Astrocytomas
We evaluated potential differences in malignant transformation according to CDKN2A/B status in astrocytoma. Of the 52 cases of astrocytoma, 31 recurred during follow-up. Of those, we extracted twenty cases in which surgical resection was performed after recurrence, and evaluated their corresponding pathological findings. Malignant transformation was defined as changes in the histopathological features of a “glioblastoma” according to the WHO 2016 classification, including necrosis and microvascular proliferation. Malignant transformation was confirmed in 1 of the 1 Homo-del, 2 of the 3 Hemi-del, and 5 of the 16 Neutral patients. The time from initial surgery to recurrence with malignant transformation was shortest in Homo-del and longest in Neutral. The median was 26.3 months for Homo-del, 49.0 months for Hemi-del, and not reached for Neutral (Supplementary Figure S4). No significant difference was observed between Hemi-del and Neutral; however, Hemi-del tended to require less time for malignant transformation than Neutral (P = .1112).
TCGA Cohort
For external validation, OS was evaluated based on the CDKN2A/B status of Homo-del, Hemi-del, and Neutral using the TCGA cohort. The allocation results for each group are presented in Supplementary Table S1. Kaplan − Meier plots are presented in Figure 2C and D. Astrocytoma cases exhibited significantly shorter OS in the order of Homo-del, Hemi-del, and Neutral (median OS: 37.3, 58.7, and 106.7 months, respectively; Figure 2C). Hemi-del and Neutral differed significantly (P = .0134). The HRs were 2.05 (95% CI: 1.16 − 3.64, P = .0140) and 4.21 (95% CI: 1.72 − 10.30, P = .0016) for Hemi-del and Homo-del, respectively, with Neutral as a reference. Hemi-del had an intermediate risk between Neutral and Homo-del. The median PFS time for Homo-del, Hemi-del, and Neutral was 60.3, 44.1, and 53.5 months, respectively, with no difference among the groups. Similarly, the effect of CDKN2A/B status was not significant in the Cox proportional hazards model, with HRs of 1.06 (95% CI: 0.62 − 1.81, P = .8373) and 1.63 (95% CI: 0.64 − 4.11, P = .3041) for Hemi-del and Homo-del, respectively.
In oligodendroglioma cases, no Homo-del were identified, and 19 out of 22 cases of Hemi-del did not reach the endpoint. Therefore, we were unable to compare survival outcomes according to CDKN2A/B. There was no significant difference in PFS between Hemi-del and Neutral (median: 45.8 and 86.7 months, respectively, P = .0605; Figure 2D). Hemi-del had an HR of 2.73 (95% CI: 0.92 − 8.17, P = .0717). In addition, we obtained the copy number of MTAP and methylation array data of CDKN2A/B and MTAP to consider the immunohistochemistry results as a supplement. The relationship between CDKN2A and MTAP deletions (Supplementary Figure S5) and methylation of CDKN2B, CDKN2A, and MTAP transcription start sites (Figure 3) are presented.
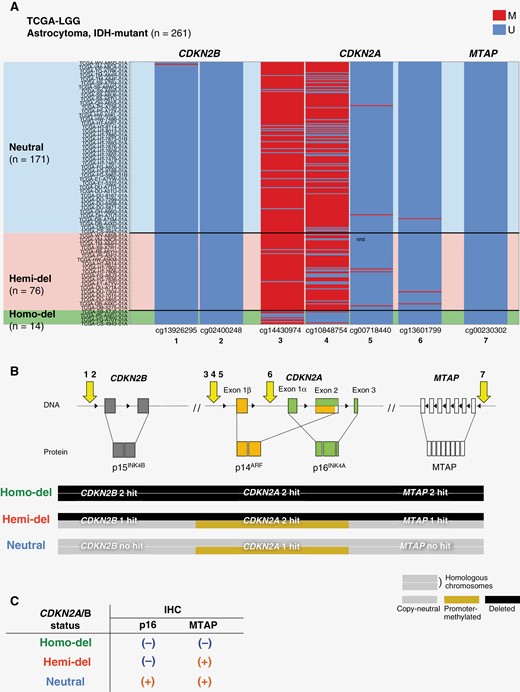
Schematic diagram of CDKNA2A/B and MTAP loci and heatmap showing the methylation status of transcription start sites. (A) Heatmap showing methylation of the transcription start sites at CDKNA2B, CDKN2A, and MTAP loci in cases of The Cancer Genome Atlas-LGG. Cases are aligned by copy number of CDKN2A. CDKN2B and MTAP are unmethylated, whereas CDKN2A has upstream methylations. (B) Schema presents CDKNA2B, CDKN2A, and MTAP loci. The transcription start sites at each locus targeted by the methylation array probes are numbered 1 to 7. The HhaI target of MLPA ME 024 corresponds to CDKN2B (1 − 2) and CDKN2A (3 − 6). In Homo-del, CDKN2A/B and MTAP are deleted in both alleles. In Hemi-del, one allele of CDKN2A is deleted, and the other is silenced by DNA methylation. In Hemi-del, CDKN2A is suppressed by 2 hits but CDKN2B and MTAP are not suppressed, due to a single hit. This explains our IHC results (C) and may explain the gradation of poor prognosis in the order of Homo-del, Hemi-del, and Neutral. CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; Hemi-del, hemizygous deletion; Homo-del, homozygous deletion; M, methylated; MTAP, methylthioadenosine phosphorylase; Neutral, copy-neutral; U, unmethylated.
Discussion
We investigated the impact of Hemi-del CDKN2A/B on the prognosis of IDH-mutant glioma. In our cohort, OS tended to be shorter for Hemi-del of CDKN2A/B in astrocytoma than for Neutral. Also, Hemi-del exhibited an intermediate prognosis between Neutral and Homo-del (Figure 2A). Multivariate analysis suggested that Hemi-del was a negative prognostic factor for OS (Table 3). However, only 9 cases (17.3%) of astrocytoma in our cohort were classified as Hemi-del; therefore, a highly reliable multivariate analysis could not be performed. Whether Hemi-del truly affects the prognosis of astrocytomas needs to be validated with a larger sample size. Thus, an external validation with TCGA-LGG was performed, which revealed that OS was significantly shorter for Hemi-del than for Neutral (Figure 2C). A recent report, in which a survival analysis of Hemi-del CDKN2A/B in IDH-mutant astrocytoma was performed, found a similar negative impact on OS, supporting our results.21,22 These results suggest that the Hemi-del CDKN2A/B may have a poorer prognosis than the Neutral. However, there was no significant difference between Hemi-del and Neutral in both our cohort and the TCGA-LGG cohort regarding their impact on recurrence. When comparing the Kaplan–Meier curves of OS and PFS, the survival period after recurrence was longest in the Neutral status, suggesting that the recurrence pattern could be different. Therefore, we evaluated the time for malignant transformation which was shortest in Homo-del, and longest in Neutral (Supplementary Figure S4). Although a strong selection bias existed and the sample size was small in this analysis, the results may suggest that the Neutral status is prone to recurrence with good prognosis; and recurrence with poor prognosis is more likely to occur in Hemi-del and Homo-del in astrocytoma. Similarly, we investigated the impact of CDKN2A/B status on oligodendroglioma. In some cases, the effect of CDKN2A/B status could not be evaluated because they did not reach the OS endpoint for oligodendroglioma. Hemi-del significantly shortened PFS for oligodendroglioma in our cohort (Figure 2B) and tended to have a shorter PFS than Neutral in TCGA-LGG (Figure 2D). Its clinical significance on oligodendroglioma remains unclear and further investigations are warranted.
Hemi-del CDKN2A/B may affect prognosis, especially in astrocytoma. Thus, the diagnosis of Hemi-del CDKN2A/B is of clinical significance. The present study provides support for the effectiveness of using immunohistochemistry. The Homo-del revealed p16-negative with MTAP loss, while the Hemi-del revealed p16-negative with MTAP retention. In this study, all 16 cases concurred with CDKN2A/B status. Immunostaining is undoubtedly useful, and the effectiveness of MTAP immunostaining as a surrogate marker for CDKN2A/B status has recently been reported.47 However, it should be noted that the Hemi-del CDKN2A/B, which we insist is meaningful, could not be detected by MTAP immunostaining alone. Moreover, cases with Homo-del CDKN2A/B without the loss of MTAP have been reported.47 The study reported that although MTAP is located at a locus adjacent to CDKN2A, several cases did not exhibit a clear correlation between MTAP and CDKN2A. This was confirmed by plotting the copy number correlation between CDKN2A and MTAP in TCGA-LGG (Supplementary Figure S5). Accordingly, for a more robust assessment of CDKN2A/B status, immunohistochemistry combining p16 and MTAP may be superior to MTAP alone. Whereas, when interpreting the results of molecular diagnosis, it is necessary to bear in mind that tumor sampling is affected by dilution when performed at a site with low tumor density. Since the copy number is biased toward neutral due to the contamination of non-tumor-derived DNA, it is necessary to correct the copy number; thus, we determined the tumor content rate by digital PCR. Immunohistochemical analysis, which does not require the use of multiple experiments or calculations to correct for copy numbers, could be a simpler and less expensive alternative. This molecular analysis is a promising method for assessing copy number, especially in settings with limited access to high-throughput sequencing. Furthermore, we believe that immunohistochemical analysis is a promising method to evaluate intra-tumor heterogeneity, especially regarding the extent and rate of CDKN2A/B deletion. In one of the Hemi-del cases (case 11), regions exhibiting CDKN2A/B Hemi-del and Neutral cells coexisted, demonstrating the heterogeneity of the tumor (Supplementary Figure S3). Heterogeneity within a tumor may be overlooked in bulk molecular analysis, especially when the deletion population is small. Immunohistochemistry enables us to approach the currently unclear issue of whether heterogeneity in tumors containing Homo-del clones exists and whether it affects prognosis.
A crucial finding in this study is the discrepancy in p16/MTAP immunostaining in Hemi-del cases. The MTAP locus is located downstream adjacent to the CDKN2A locus encoding p16INK4A. Deletion of CDKN2A/B is accompanied by the deletion of MTAP (Supplementary Figure S5), and this reflects the loss of both p16 and MTAP in Homo-del. In contrast, immunostaining revealed the loss of p16 encoded on CDKN2A and the retention of MTAP. Epigenetic regulation including promoter methylation may be relevant in this regard. In this study, upstream methylation of CDKN2A was confirmed in 8 of the 12 Hemi-del. In the TCGA dataset, methylation was confirmed at sites adjacent to methylation sites observed in our cohort, whereby CDKN2B and MTAP did not have upstream methylation (Figure 3). Promoter methylation on the remaining allele without deletion may affect the loss of p16 expression in Hemi-del. This may constitute a prognostic factor and discrepancy in p16/MTAP. In Hemi-del, only CDKN2A was suppressed by 2 hits, but CDKN2B and MTAP were not suppressed, due to a single hit (Figure 3B). This may explain the gradation of poor prognosis in the order of Homo-del, Hemi-del, and Neutral. Hemi-del with promoter methylation may be caused by G-CIMP accompanied by IDH mutation. However, it remains unclear whether the same mechanism works in all Hemi-del cases, and methylation could not be confirmed in at least 4 cases. Apart from methylation, other potential causes for the downregulation of p16 expression include local deletion of the coding region, mutations, or other silencing mechanisms. Further investigations are required to resolve this issue.
It is necessary to investigate whether the CDKN2A/B Hemi-del group is a homogeneous or heterogeneous population. Immunohistochemical staining revealed that in all cases, the Hemi-del was p16-negative and exhibited retained MTAP; however, this could have been a coincidence because of the small sample size. Dividing the Hemi-del group into 2 groups based on the presence or absence of CDKN2A methylation resulted in survival curves suggesting a difference in prognosis (Supplementary Figure S6). This indicates that it may be necessary to treat Hemi-del with methylation as the CDKN2A loss group in the same way as Homo-del. Conversely, despite the methylation status of transcription start sites at 1500, where methylation was observed in MS-MLPA, and with a similar division into 2 groups, the same trend was not observed in the TCGA-LGG cohort (Supplementary Figure S6). Hemi-del group heterogeneity warrants further exploration.
In astrocytoma, the Hemi-del CDKN2A/B had a greater impact on the prognosis than grade 2 or 3 according to the WHO 2021 classification (Table 3). In our cohort, the evaluation of histological grade 3 did not predict prognosis, such as a reversal of the risk ratio in grade 2/3. Furthermore, Hemi-del was associated with risks in both our and the TCGA-LGG cohorts. Since prognosis prediction by CDKN2A/B status is more impactful than histological findings, we propose that astrocytoma with Hemi-del should be considered as more malignant than copy-neutral (eg, grade 2 astrocytoma with Hemi-del CDKN2A/B should be treated as a more severe degree compared to grade 3).
There are several limitations to our study. First, this study was a single-center retrospective study with a small sample size. These results, including multivariate analysis, should be verified with a larger size sample. Of note, similar results have been reported for large-scale public data, and multicenter studies analyzing a larger population may validate our results. In addition, oligodendroglioma requires long-term survival analysis due to the natural history of the disease. The observation period in our study may have been insufficient, and further accumulation of longitudinal data is necessary. Moreover, studies are needed to determine the mechanism by which CDKN2A/B Hemi-del worsens prognosis, especially regarding the molecular background that contributes in addition to the single allele deletion.
In conclusion, the Hemi-del CDKN2A/B had a negative impact on OS in astrocytoma. Immunohistochemistry of p16/MTAP was useful for detecting Hemi-del or Homo-del, and immunohistochemistry may be combined with molecular diagnostics to validate Hemi-del or Homo-del.
Funding
This work was supported by Japanese Society for the Promotion of Science Grants-in-Aid for Scientific Research (JSPS KAKENHI) Award (Grant No. 20K09392, 20K17972, 21H03044, 22K16690, 23H03021, and 23K08545) and Fukuoka Public Health Promotion Organization Cancer Research Fund.
Acknowledgments
The authors thank Kaori Yasuda, Atsushi Doi, Hiroko Hagiwara, and Cell Innovator Inc. (Fukuoka, Japan) for the useful discussions and advice on in-silico analysis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Authorship statement
Conceived and designed the study: N.H., R.O., H.Y., M.M., D.K., Y.S., Y.F., A.N., and K.Y.. Acquired funding: N.H., M.M., Y.F., Y.S., and K.Y.. Provided study materials: R.O., H.Y., A.S., N.H., M.M., D.K., R.H., Y.S., Y.F., O.T., T.Y., and K.Y.. Collected the data: R.O., H.Y., and N.N.. Performed statistical analysis: R.O.. First draft of the manuscript: R.O.. All authors reviewed and edited the manuscript and approved the final manuscript.




