-
PDF
- Split View
-
Views
-
Cite
Cite
Ashish Wadekar, Hemlata Jangir, Saumya Sahu, Charli Roy, Sumanta Das, Ashish Suri, Mehar Chand Sharma, Chitra Sarkar, Vaishali Suri, Evaluating the efficacy of Hip1R, Vimentin, and H3K27me3 as surrogate markers for 1p/19q co-deletion in oligodendrogliomas, Neuro-Oncology Advances, Volume 7, Issue 1, January-December 2025, vdaf060, https://doi.org/10.1093/noajnl/vdaf060
Close - Share Icon Share
Abstract
Assessment of the chromosomal 1p/19q status is essential to distinguish between IDH-mutant astrocytoma and oligodendroglioma. Genetic analyses, however, are expensive, time-consuming, and not widely accessible. Immunohistochemical loss of ATRX is currently the only established surrogate marker for a non-1p/19q co-deleted genotype. To find cost-effective approaches and improve risk stratification, we aimed to assess the immunohistochemical expression HIP1R, Vimentin, and H3K27me3 as surrogate markers in predicting 1p/19q status for oligodendrogliomas.
A total of 182 adult-type-diffuse gliomas were analyzed for IDH1 R132H, ATRX, P 53, MIB-1-LI, HIP1R, Vimentin, and H3K27me3 expression using immunohistochemistry. 1p/19q co-deletion was assessed by fluorescence in situ hybridization (FISH) assay. IDH sequencing was performed in IDH1 R132H negative cases. Histomorphological and molecular classification of these gliomas was performed according to World Health Organization (WHO) 2021 CNS5 classification.
In this study, 102 IDH-mutant oligodendrogliomas, 44 IDH-mutant astrocytomas, and 36 IDH-wild-type glioblastomas exhibited distinct patterns of the IHC markers. In oligodendrogliomas, HIP1R showed either homogeneous or homogenous with mosaic staining, Vimentin was negative and H3K27me3 was lost in all cases. In Astrocytoma and glioblastomas, HIP1R was predominantly mosaic, Vimentin was widely positive, and H3K27me3 was variable. Combining these markers, especially the positivity of HIP1R, negative Vimentin, and complete loss of H3K27me3, achieved perfect diagnostic accuracy, making them highly reliable for differentiating oligodendroglioma from astrocytoma, and glioblastomas.
The study demonstrates that the combinations of the three immunohistochemical markers HIP1R, Vimentin, and H3K27me3 can accurately predict 1p/19q co-deletion status in IDH-mutant gliomas. This method offers a reliable, robust, cost-effective alternative to complex techniques like FISH.
Lay Summary
Gliomas are a type of aggressive brain tumor. One specific type of glioma is missing a part of its DNA called 1p/19q. Testing for this missing DNA be expensive and may not be available everywhere. The authors of this study wanted to find simpler ways to identify gliomas with this missing DNA. To do this, they looked for proteins in the tumor tissue that can be easily tested. They found that a combination of three proteins that can easily be tested for might indicate whether the 1p/19q section of DNA is missing or present.
Cost-Effective Diagnostic Approach: The use of HIP1R, vimentin, and H3K27me3 immunohistochemical markers reduces diagnostic costs by over 60% compared to traditional genetic tests, offering a more affordable and accessible option for diagnosing 1p/19q co-deletion in oligodendrogliomas, especially in low- and middle-income countries (LMICs).
Rapid and Reliable Results: This panel provides a cost-effective diagnostic approach with the advantage of rapid results, enhancing glioma diagnosis in settings with limited access to advanced molecular techniques and ensuring timely and accurate patient management.
This study highlights the importance of using HIP1R, vimentin, and H3K27me3 immunohistochemistry for diagnosing 1p/19q co-deletion in oligodendrogliomas. Compared to traditional methods like FISH, which is expensive, equipment-intensive, and time-onsuming, this three-marker panel offers a highly accurate, cost-effective, and rapid diagnostic alternative. While FISH assay and other advanced genetic tests are effective, their limited availability and high costs in resource-constrained settings pose challenges. The integrated expressions of HIP1R, vimentin, and H3K27me3 provide comparable accuracy and enhance accessibility, making it particularly beneficial for low and middle-income countries. This study highlights a practical solution to improve glioma diagnosis and patient management where advanced molecular techniques are less accessible.
Oligodendrogliomas are characterized by a distinctive molecular feature, the co-deletion of chromosomal arms 1p and 19q (1p/19q co-deletion). This genetic alteration not only plays a crucial role in the diagnosis of oligodendrogliomas but also serves as a pivotal prognostic biomarker, influencing therapeutic strategies and patient outcomes.1 The gold standard for detecting 1p/19q co-deletion has long been fluorescence in situ hybridization (FISH), recognized for its high sensitivity and specificity. However, the widespread implementation of FISH is hindered by substantial costs, variability in cutoff thresholds, and limited accessibility in many healthcare settings, particularly in low- and middle-income countries (LMICs).1,2
FISH, while sensitive, has inherent limitations that impact its diagnostic accuracy, especially in the context of detecting whole-arm losses such as 1p/19q co-deletion. The technique’s reliance on probe hybridization can sometimes lead to interpretation errors, including false negative or positive interpretations, particularly when assessing complex chromosomal alterations.2 Further, FISH in formalin-fixed paraffin-embedded (FFPE) samples faces challenges such as truncation artifacts, where DNA degradation during fixation can lead to incomplete probe hybridization. This can obscure true genetic status, especially in detecting complex chromosomal alterations like 1p/19q co-deletion. FFPE samples also exhibit variable DNA quality, affecting probe binding efficiency and result reliability. Moreover, FISH assays require specialized equipment and skilled personnel, making them challenging to implement uniformly across diverse healthcare settings, especially those with limited resources.3 Other molecular methods, such as PCR-based LOH analysis, have higher specificity but are more labor-intensive and require a non-neoplastic control.4 In addition, SNP array and inferred copy number variation calling from methylation profiling represent more advanced molecular techniques with the ability to assess 1p/19q status. These methods, however, remain less accessible in resource-constrained environments.5,6
In response to these limitations, few recent studies have explored immunohistochemical (IHC) markers as potential surrogate indicators of 1p/19q co-deletion in oligodendrogliomas. Notably, markers such as Huntingtin-interacting protein 1-related (HIP1R), Vimentin, and histone H3 lysine 27 tri-methylation (H3K27Me3) have shown promise in reflecting the underlying genetic alterations characteristic of these tumors.7 Leveraging established pathology infrastructure and expertise, IHC techniques offer a feasible alternative that could potentially enhance diagnostic capabilities and mitigate disparities in diagnostic access across different regions.
The present study aims to assess the feasibility and reliability of Hip1R, Vimentin, and H3K27Me3 as surrogate markers for 1p/19q co-deletion in oligodendrogliomas and evaluate their utility in LMICs, where alternative, cost-effective diagnostic tools are urgently needed. By comparing these markers with traditional FISH assays, this study seeks to contribute insights into their clinical potential and applicability in resource-constrained healthcare environments.
Materials and Methods
Clinical Data
This ambispective study included 182 cases of diffuse adult-type gliomas reviewed between January 2022 and December 2023 at the neuropathology laboratory of All India Institute of Medical Sciences, New Delhi. Demographic details were collected from the medical records available. Hematoxylin and Eosin (H&E) slides were re-evaluated by 3 pathologists (AW, HJ and VS), who have a special interest in neuropathology. The diagnosis was reconfirmed following the recent World Health Organization (WHO) classification. All experiments using human samples were approved by the ethical committee of our institution.
Immunohistochemistry
Immunostaining was performed on 5 μm thick FFPE tumor sections using an automated immunostainer (Benchmark XT, Ventana, Tucson, AZ, USA). The standard protocols were followed, including pretreatment with cell conditioning 1 buffer (Ventana) for 52 minutes and standard Ventana signal amplification. Diaminobenzidine was used as a chromogen.
IHC was performed using the following antibodies: OLIG2 (IgG1 goat polyclonal antibody H11 clone; R&D systems, 1:150), Isocitrate dehydrogenase 1 (IDH1 R132H, Dianova, mouse monoclonal, 1:50), alpha thalassemia/mental retardation syndrome X-linked (ATRX, Sigma-Aldrich, St. Louis, MO, USA dilution 1:400), P53 (Santa Cruz Biotechnology, Inc., CA, USA; 1:200), MIB-1 ((DAKO, Denmark, 1:200), H3K27Me3 (H3K27M, Millipore, Billerica, MA, USA, Dilution 1: 1000)), Vimentin (VIM (GA63061-2, DAKO, Jena, Germany, 1:300), HIP1R (ab140608, Abcam, Cambridge, UK, 1:200). For IDH, combined cytoplasmic and nuclear staining was deemed immuno-positive. ATRX status was evaluated based on the complete absence of nuclear staining while ensuring retained expression in endothelial cells for internal controls, defining it as negative. Strong nuclear staining of p53 in more than 30% of tumor cells indicated positivity.8 IDH1 R132H IHC-negative cases underwent Sanger sequencing for IDH1 and IDH2 genes using ABI 3500xL from Applied Biosystems. IHC staining for HIP1R, Vimentin, and H3K27me3 was evaluated based on the extent and pattern of staining in tumor cells. HIP1R and Vimentin staining were considered positive if there was cytoplasmic and membranous positivity. HIP1R staining patterns are classified into four categories based on distribution and intensity. Homogeneous (H) staining appears uniform and strong across all tumor cells. Homogeneous with mosaic (H + M) staining is predominantly uniform but includes focal patchy areas. Mosaic staining is patchy, with irregular cytoplasmic positivity. Sparse staining is characterized by weak and scattered positivity in only a limited number of tumor cells. Staining for vimentin was categorized as negative (no detectable staining), focal (staining in less than 75% of tumor cells), or diffuse (staining in 75% or more of tumor cells). Vimentin positivity in blood vessels served as an internal control, while immune-positivity in mini-gemistocytes and reactive astrocytes was not considered in the evaluation. For H3K27me3, positivity in endothelial cells served as a control. Staining was classified as complete loss (CL) if it was absent in 95% or more of tumor cells, partial loss if it was absent in 10-95% of tumor cells, and retained if staining was present in more than 95% of tumor cells. Only tumor fragments were assessed for staining, while cortical infiltrating parts and fragments of white matter were excluded from the assessment for these markers.7
Fluorescence In Situ Hybridization (FISH)
Fluorochrome labeled probes from Vysis Inc., Abbott Laboratories SA, included locus-specific probes for 1p36 and 19q13 paired with reference probes for 1q25 and 19p13, used for 1p/19q co-deletion analysis. The evaluation involved analyzing signals in at least 200 intact nuclei, with non-neoplastic cortical tissue sections from epilepsy surgery serving as controls. Criteria for 1p/19q co-deletion required a minimum of 40% of nuclei to display a 1:2 ratio of test to reference probe signals, as per established protocols.9
Sanger Sequencing (SS)
DNA was isolated from FFPE tissue, using the Promega ReliaPrep™ _FFPE gDNA Miniprep System (Cat. No. A2352 Promega, Hilden, Germany) as per the manufacturer’s instructions. Nucleic acids were quantified by UV spectrophotometric analysis using Qubit 2.0 Fluorometer (Thermo Fisher Scientific).
For IDH1 sequencing, 129-bp fragment encompassing the catalytic domain of IDH1, including codon 132 was amplified using the HotStar Hi-Fidelity DNA polymerase kit (Qiagen) by following the manufacturer’s instructions. The IDH1 primer set included the following sequences: 5′AATGAGCTCTATATGCCATCACTG 3′ for the forward primer and 5′ TTCATACCTTGCTTAATGGGTGT 3′ for the reverse primer. PCR amplification was conducted in a 10μL volume reaction containing 0.5μM of each forward and reverse primers, 2.5 units HotStarTaq DNA polymerase, 10mM of each deoxy-nucleotides, and 100ng DNA. The reaction mixture underwent an initial enzyme activation at 95 °C for 15 minutes, followed by 40 amplification cycles, each including a denaturation step at 95 °C for 30 seconds, an annealing step at 55 °C for 35seconds, and extension at 72 °C for 40seconds. The final extension step was executed at 72 °C for 10 minutes. Subsequent confirmation via agarose gel electrophoresis affirmed the accurate amplification of the PCR product at the desired size. PCR products were purified using the ExoSAP-IT (Thermo Fisher Scientific/Applied Biosystem). Sequencing reactions were performed using the Terminator v3.1 Cycle Sequencing Kit (Applied Biosystem) (Component: 5X Dilution Buffer and Big Dye) and analysis was carried out on Applied Biosystems 3500 XL Genetic Analyzer.
Statistical Analysis
Statistical analyses were conducted using STATA version 16.0. Continuous variables were summarized using descriptive statistics for calculating the mean values. Sensitivity, specificity, and diagnostic accuracy were assessed to evaluate the performance of the immunohistochemical panel in accurately classifying tumor subtypes and providing insights into its diagnostic efficiency. Kaplan–Meier survival analysis was performed to compare the tumor groups.
Results
The study comprised 182 adult patients diagnosed with diffuse infiltrating gliomas. This cohort included 102 cases of IDH-mutant, 1p/19q co-deleted oligodendrogliomas. Additionally, there were 44 cases of Astrocytoma, IDH-mutant, and 36 cases of IDH-wild-type glioblastomas. The mean age of patients is 40.37 (range; 18-77) years. Among these patients, there is a male predominance including 127 male and 54 female with male-to-female ratio of 2.35:1.
Oligodendroglioma, IDH-mutant 1/p19q co-deleted
The study included 102 oligodendrogliomas constituting 72 cases of grade 2 and 30 cases of grade 3 tumors, with a mean age of 38.42 (range; 19-72) years. All cases were IDH mutated, 97 tumors were confirmed positive for IDH1 R132H by IHC while 5 cases exhibited non-canonical mutations identified by Sanger sequencing, all of which were IDH2 R172K. ATRX expression was retained and P53 was negative in all cases. Importantly, all cases showed 1p/19q co-deletion, with 1p deleted in 65-90% of cells, and a similar range was observed for 19q deletion in all cases. The mean MIB-1 labeling index (LI) for grade 2 tumors was 1.5% (range; 1-5%), while 21.6% (range; 2-45%) for grade 3 (Figure 1). The mean overall survival was 21.23 months (range: 1.2–107.9 months) (Table 1). Of the patients, 86 (84.3%) were still alive at the time of the conclusion of the study, while 16 (15.7%) had either passed away or were lost to follow-up (Figure 3).
Immunohistochemistry Was Performed For IDH, ATRX, P53, MIB, HIP 1 R, Vimentin, and H3K27me3 on Tumor Samples From Oligodendroglioma (Grades 2 and 3), Astrocytoma (Grades 2, 3, and 4), and Glioblastoma.
| Tumors . | n . | IDH R 132 H . | ATRX . | P53 . | Hip1R . | Vimentin . | H3K27Me3 . | 1p/19q co-deletion (FISH assay) . | Mean survival . |
|---|---|---|---|---|---|---|---|---|---|
| Oligodendroglioma (ODG) Validating Cohort | 102 24 | +ve (IHC): 97 (98.5%) +ve (SS): 5 (1.5%) +ve (IHC): 23 (95.8%) +ve (SS): 1 (4.2%) | Retained: 102 (100%) Retained: 24 (100%) | −ve: 102 (100%) −ve: 24 (100%) | H: 86 (84.3%) H + M: 16 (15.7%) H: 21 (87.5%) H + M: 3 (12.5%) | −ve: 93 (91.2%) FP: 9 (8.8%) −ve: 18 (75%) FP: 6 (25%) | CL: 102 (100%) CL: 23 (95.8%) PL: 1 (4.2%) | All cases All Cases | 21.23 months (range: 1.2–103.4 months) |
| Astrocytoma Validating Cohort | 44 22 | +ve (IHC): 40 (90.9%) +ve (SS): 4 (9.1%) +ve (IHC): 24 (100%) | Loss: 34 (77.3%) Retained: 10 (22.7%) Loss: 19 (86.4%) Retained: 3 (13.6%) | +ve: 33 (75%) −ve: 11 (25%) +ve: 21 (95.5%) −ve: 1 (4.5%) | M: 36 (81.8%) Sparse: 5 (11.4%) −ve: 3 (6.8%) M: 17 (77.2%) Sparse: 1 (4.5%) −ve: 4 (18.3%) | DP: 36 (81.8%) FP: 8 (18.2%) DP: 19 (86.4%) FP: 3 (13.6%) | CL: 33 (75%) PL: 9 (20.5%) Retained: 2 (4.5%) CL: 11 (50%) PL: 11 (50%) | None None | 18.25 months (range: 2–81.4 months) |
| GBM Validating Cohort | 36 27 | −ve: 36(100%) −ve: 27 (100%) | Retained: 36 (100%) Retained: 27 (100%) | +ve: 10 (27.7%)−ve: 26 (72.3%) +ve: 6 (22.2%) −ve: 21 (77.8%) | M: 28 (77.8%) H + M: 2 (5.6%) Sparse: 3 (8.3%) −ve: 3 (8.3%) M: 19 (70.4%) H + M: 1 (3.7%) Sparse: 3 (11.1%) −ve: 4 (14.8%) | DP: 25 (69.4%) FP: 11 (30.6%) DP: 17 (62.9%) FP: 9 (33.3%) −ve: 1 (3.7%) | PL: 19 (52.7%) CL: 10 (27.7%) Retained: 7 (19.6%) PL: 12 (44.4%) CL: 9 (33.3%) Retained: 6 (22.3%) | None None | 10.93 months (range: 0.2–82.6 months) |
| P value ODG vs Astro ODG vs GBM | – | 0.19 <0.001 | <0.001 0.17 | <0.001 0.20 | <0.001 0.02 | <0.05 0.045 | 0.15 0.007 | – | <0.001 |
| Tumors . | n . | IDH R 132 H . | ATRX . | P53 . | Hip1R . | Vimentin . | H3K27Me3 . | 1p/19q co-deletion (FISH assay) . | Mean survival . |
|---|---|---|---|---|---|---|---|---|---|
| Oligodendroglioma (ODG) Validating Cohort | 102 24 | +ve (IHC): 97 (98.5%) +ve (SS): 5 (1.5%) +ve (IHC): 23 (95.8%) +ve (SS): 1 (4.2%) | Retained: 102 (100%) Retained: 24 (100%) | −ve: 102 (100%) −ve: 24 (100%) | H: 86 (84.3%) H + M: 16 (15.7%) H: 21 (87.5%) H + M: 3 (12.5%) | −ve: 93 (91.2%) FP: 9 (8.8%) −ve: 18 (75%) FP: 6 (25%) | CL: 102 (100%) CL: 23 (95.8%) PL: 1 (4.2%) | All cases All Cases | 21.23 months (range: 1.2–103.4 months) |
| Astrocytoma Validating Cohort | 44 22 | +ve (IHC): 40 (90.9%) +ve (SS): 4 (9.1%) +ve (IHC): 24 (100%) | Loss: 34 (77.3%) Retained: 10 (22.7%) Loss: 19 (86.4%) Retained: 3 (13.6%) | +ve: 33 (75%) −ve: 11 (25%) +ve: 21 (95.5%) −ve: 1 (4.5%) | M: 36 (81.8%) Sparse: 5 (11.4%) −ve: 3 (6.8%) M: 17 (77.2%) Sparse: 1 (4.5%) −ve: 4 (18.3%) | DP: 36 (81.8%) FP: 8 (18.2%) DP: 19 (86.4%) FP: 3 (13.6%) | CL: 33 (75%) PL: 9 (20.5%) Retained: 2 (4.5%) CL: 11 (50%) PL: 11 (50%) | None None | 18.25 months (range: 2–81.4 months) |
| GBM Validating Cohort | 36 27 | −ve: 36(100%) −ve: 27 (100%) | Retained: 36 (100%) Retained: 27 (100%) | +ve: 10 (27.7%)−ve: 26 (72.3%) +ve: 6 (22.2%) −ve: 21 (77.8%) | M: 28 (77.8%) H + M: 2 (5.6%) Sparse: 3 (8.3%) −ve: 3 (8.3%) M: 19 (70.4%) H + M: 1 (3.7%) Sparse: 3 (11.1%) −ve: 4 (14.8%) | DP: 25 (69.4%) FP: 11 (30.6%) DP: 17 (62.9%) FP: 9 (33.3%) −ve: 1 (3.7%) | PL: 19 (52.7%) CL: 10 (27.7%) Retained: 7 (19.6%) PL: 12 (44.4%) CL: 9 (33.3%) Retained: 6 (22.3%) | None None | 10.93 months (range: 0.2–82.6 months) |
| P value ODG vs Astro ODG vs GBM | – | 0.19 <0.001 | <0.001 0.17 | <0.001 0.20 | <0.001 0.02 | <0.05 0.045 | 0.15 0.007 | – | <0.001 |
The table shows the number of cases, their expression patterns, percentages, and statistical correlation. In addition, Sanger sequencing (SS) was performed in IDH-negative cases.
Abbreviations: CL = complete loss, DP = diffuse positive, H = homogenous, H + M = homogenous + mosaic, FP = focal positive, PL = partial loss, +ve = positive, −ve = negative.
Immunohistochemistry Was Performed For IDH, ATRX, P53, MIB, HIP 1 R, Vimentin, and H3K27me3 on Tumor Samples From Oligodendroglioma (Grades 2 and 3), Astrocytoma (Grades 2, 3, and 4), and Glioblastoma.
| Tumors . | n . | IDH R 132 H . | ATRX . | P53 . | Hip1R . | Vimentin . | H3K27Me3 . | 1p/19q co-deletion (FISH assay) . | Mean survival . |
|---|---|---|---|---|---|---|---|---|---|
| Oligodendroglioma (ODG) Validating Cohort | 102 24 | +ve (IHC): 97 (98.5%) +ve (SS): 5 (1.5%) +ve (IHC): 23 (95.8%) +ve (SS): 1 (4.2%) | Retained: 102 (100%) Retained: 24 (100%) | −ve: 102 (100%) −ve: 24 (100%) | H: 86 (84.3%) H + M: 16 (15.7%) H: 21 (87.5%) H + M: 3 (12.5%) | −ve: 93 (91.2%) FP: 9 (8.8%) −ve: 18 (75%) FP: 6 (25%) | CL: 102 (100%) CL: 23 (95.8%) PL: 1 (4.2%) | All cases All Cases | 21.23 months (range: 1.2–103.4 months) |
| Astrocytoma Validating Cohort | 44 22 | +ve (IHC): 40 (90.9%) +ve (SS): 4 (9.1%) +ve (IHC): 24 (100%) | Loss: 34 (77.3%) Retained: 10 (22.7%) Loss: 19 (86.4%) Retained: 3 (13.6%) | +ve: 33 (75%) −ve: 11 (25%) +ve: 21 (95.5%) −ve: 1 (4.5%) | M: 36 (81.8%) Sparse: 5 (11.4%) −ve: 3 (6.8%) M: 17 (77.2%) Sparse: 1 (4.5%) −ve: 4 (18.3%) | DP: 36 (81.8%) FP: 8 (18.2%) DP: 19 (86.4%) FP: 3 (13.6%) | CL: 33 (75%) PL: 9 (20.5%) Retained: 2 (4.5%) CL: 11 (50%) PL: 11 (50%) | None None | 18.25 months (range: 2–81.4 months) |
| GBM Validating Cohort | 36 27 | −ve: 36(100%) −ve: 27 (100%) | Retained: 36 (100%) Retained: 27 (100%) | +ve: 10 (27.7%)−ve: 26 (72.3%) +ve: 6 (22.2%) −ve: 21 (77.8%) | M: 28 (77.8%) H + M: 2 (5.6%) Sparse: 3 (8.3%) −ve: 3 (8.3%) M: 19 (70.4%) H + M: 1 (3.7%) Sparse: 3 (11.1%) −ve: 4 (14.8%) | DP: 25 (69.4%) FP: 11 (30.6%) DP: 17 (62.9%) FP: 9 (33.3%) −ve: 1 (3.7%) | PL: 19 (52.7%) CL: 10 (27.7%) Retained: 7 (19.6%) PL: 12 (44.4%) CL: 9 (33.3%) Retained: 6 (22.3%) | None None | 10.93 months (range: 0.2–82.6 months) |
| P value ODG vs Astro ODG vs GBM | – | 0.19 <0.001 | <0.001 0.17 | <0.001 0.20 | <0.001 0.02 | <0.05 0.045 | 0.15 0.007 | – | <0.001 |
| Tumors . | n . | IDH R 132 H . | ATRX . | P53 . | Hip1R . | Vimentin . | H3K27Me3 . | 1p/19q co-deletion (FISH assay) . | Mean survival . |
|---|---|---|---|---|---|---|---|---|---|
| Oligodendroglioma (ODG) Validating Cohort | 102 24 | +ve (IHC): 97 (98.5%) +ve (SS): 5 (1.5%) +ve (IHC): 23 (95.8%) +ve (SS): 1 (4.2%) | Retained: 102 (100%) Retained: 24 (100%) | −ve: 102 (100%) −ve: 24 (100%) | H: 86 (84.3%) H + M: 16 (15.7%) H: 21 (87.5%) H + M: 3 (12.5%) | −ve: 93 (91.2%) FP: 9 (8.8%) −ve: 18 (75%) FP: 6 (25%) | CL: 102 (100%) CL: 23 (95.8%) PL: 1 (4.2%) | All cases All Cases | 21.23 months (range: 1.2–103.4 months) |
| Astrocytoma Validating Cohort | 44 22 | +ve (IHC): 40 (90.9%) +ve (SS): 4 (9.1%) +ve (IHC): 24 (100%) | Loss: 34 (77.3%) Retained: 10 (22.7%) Loss: 19 (86.4%) Retained: 3 (13.6%) | +ve: 33 (75%) −ve: 11 (25%) +ve: 21 (95.5%) −ve: 1 (4.5%) | M: 36 (81.8%) Sparse: 5 (11.4%) −ve: 3 (6.8%) M: 17 (77.2%) Sparse: 1 (4.5%) −ve: 4 (18.3%) | DP: 36 (81.8%) FP: 8 (18.2%) DP: 19 (86.4%) FP: 3 (13.6%) | CL: 33 (75%) PL: 9 (20.5%) Retained: 2 (4.5%) CL: 11 (50%) PL: 11 (50%) | None None | 18.25 months (range: 2–81.4 months) |
| GBM Validating Cohort | 36 27 | −ve: 36(100%) −ve: 27 (100%) | Retained: 36 (100%) Retained: 27 (100%) | +ve: 10 (27.7%)−ve: 26 (72.3%) +ve: 6 (22.2%) −ve: 21 (77.8%) | M: 28 (77.8%) H + M: 2 (5.6%) Sparse: 3 (8.3%) −ve: 3 (8.3%) M: 19 (70.4%) H + M: 1 (3.7%) Sparse: 3 (11.1%) −ve: 4 (14.8%) | DP: 25 (69.4%) FP: 11 (30.6%) DP: 17 (62.9%) FP: 9 (33.3%) −ve: 1 (3.7%) | PL: 19 (52.7%) CL: 10 (27.7%) Retained: 7 (19.6%) PL: 12 (44.4%) CL: 9 (33.3%) Retained: 6 (22.3%) | None None | 10.93 months (range: 0.2–82.6 months) |
| P value ODG vs Astro ODG vs GBM | – | 0.19 <0.001 | <0.001 0.17 | <0.001 0.20 | <0.001 0.02 | <0.05 0.045 | 0.15 0.007 | – | <0.001 |
The table shows the number of cases, their expression patterns, percentages, and statistical correlation. In addition, Sanger sequencing (SS) was performed in IDH-negative cases.
Abbreviations: CL = complete loss, DP = diffuse positive, H = homogenous, H + M = homogenous + mosaic, FP = focal positive, PL = partial loss, +ve = positive, −ve = negative.
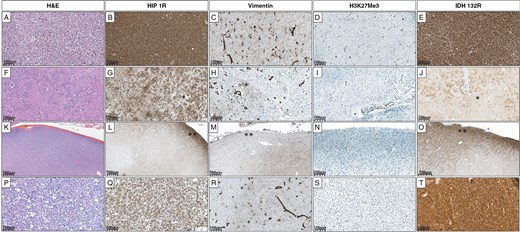
Grade 2 oligodendroglioma (A) shows diffuse HIP1R positivity (B), is negative for Vimentin (C), and exhibits complete loss of H3K27me3 (D). IDH1 R132H is diffusely positive (E). Oligodendroglioma (F) showing heterogeneous HIP1R positivity attributed to white matter* fragments (G). Vimentin is focally positive in white matter* and negative in tumor cells (H). H3K27me3 is completely lost (I). IDHR132 H positivity is variable due to white matter* (J). Cortical infiltrating oligodendroglioma (K) with diffuse HIP1R positivity in the subpial region** and patchy positivity in the underlying cortex (L). Vimentin is negative in the subpial region** and shows focal positivity in the underlying cortex (M). H3K27me3 is completely lost (N). IDH1 R132 H is diffusely positive in the subpial region** highlighting a few tumor cells in the underlying cortex (O). A case of Oligodendroglioma (P), HIP1R is diffusely positive (Q), Vimentin is negative but highlights the reactive astrocytes (R), H3K27me3 is completely lost (S), and IDHR132 H is diffusely positive (T).
HIP1R immunoreactivity demonstrated distinct patterns. Among the 102 cases, 86 (84.3%) cases showed homogeneous staining (H), while 16 (15.7%) cases exhibited a combination of homogeneous staining with focal areas of mosaic staining (H+M), which could be attributed to regions with white matter or cortical infiltration. Immunoreactivity for vimentin was absent, in predominantly 93/102 (91.2%) cases. However, 9 (8.8%) cases displayed focal immunopositivity, attributed to the presence of reactive astrocytes, mini-gemistocytes, white matter fragments, and areas of cortical infiltration. H3K27me3 expression was lost in all cases (Table 1).
Astrocytoma, IDH-mutant
The analysis of 44 astrocytomas, comprising 29 cases of grade 2, 11 of grade 3, and 4 of grade 4 tumors, revealed distinct patterns for IDH, ATRX, and P53 expression. IDH1 R132H was positive in 40 (90.9%) cases while 4 (9.1%) cases showed non-canonical mutations by Sanger sequencing, including 2 cases with IDH1 R132C, 1 case with IDH1 R132S, and 1 case with IDH1 R132G mutation. For ATRX, 10 (22.7%) cases with intact IHC expression and 34 (77.3%) cases with lost ATRX were taken up for analysis. P53 was positive in 33 cases and negative in 11 cases. Importantly, none of the astrocytoma cases showed 1p/19q co-deletion. The mean MIB-1 LI was 2.36% (range: 1%–8%) for grade 2, 14.5% (range: 3%–40%) for grade 3, and 15.25% (range: 10%–70%) for grade 4 astrocytomas (Figure 2). The mean overall survival for patients was 18.25 months (range: 2–81.4 months) (Table 1). 36 (81.8%) patients were alive, while 8 (18.2%) patients had either died from the disease or were lost to follow-up (Figure 3).
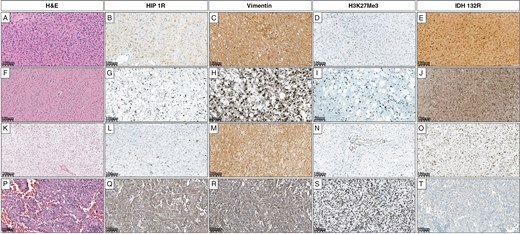
Grade 3 astrocytoma (A) shows focal HIP1R positivity (B), diffuse Vimentin positivity (C), complete loss of H3K27me3 (D), and positive IDH1 R132H expression (E). Grade 2 astrocytoma (F) shows sparse HIP1R positivity (G), focal Vimentin positivity (H), partial loss of H3K27me3 (I), and positive IDH1 R132H expression (J). Another Grade 2 astrocytoma (K) is negative for HIP1R (L), shows diffuse Vimentin positivity (M), complete loss of H3K27me3 (N), and positive IDH1 R132H expression (O). Glioblastoma (P) shows diffuse HIP1R positivity (Q), diffuse Vimentin positivity (R), retained H3K27me3 expression (S), and is negative for IDH1 R132H (T).
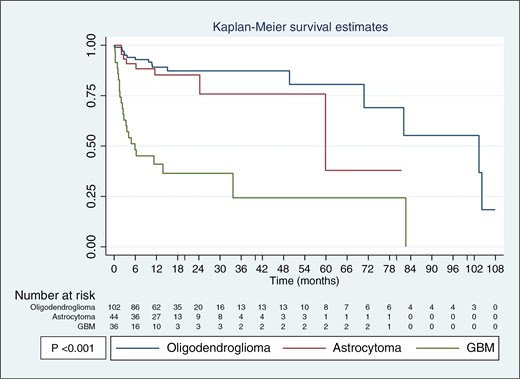
Kaplan–Meier survival estimates for oligodendroglioma, astrocytoma, and GBM. Oligodendroglioma shows the best survival outcomes, followed by astrocytoma, while GBM has the worst prognosis.
The patterns of HIP1R expression demonstrated notable heterogeneity. The majority of the astrocytomas, 36 (81.8%) cases, exhibited a mosaic pattern of HIP1R expression. A smaller subset, comprising 5 (11.4%) cases, showed a sparse pattern. Additionally, 3 (6.8%) cases were characterized by a negative HIP1R expression pattern. The majority of cases, 36 out of 44 (81.8%), exhibited a diffuse positive staining pattern for Vimentin, and 8 (18.2%) cases showed a focal staining pattern. The patterns of H3K27me3 staining revealed significant variability. 33 out of 44 (75.0%) cases, exhibited a CL of H3K27me3 expression. Additionally, 9 (20.5%) cases demonstrated a partial loss of H3K27me3 expression. Only 2 (4.5%) cases retained H3K27me3 expression (Table 1).
Glioblastoma, IDH-wild-type
All 36 glioblastomas were immune-negative for IDH1 R132H. All Patients younger than 55 years were assessed for IDH mutations with none showing these mutations. ATRX was retained in all. The p53 expressions were negative in 26 (72.3%) cases and positive in 10 (27.7%) cases. None of the cases showed 1p/19q co-deletion. The mean MIB-1 LI was 20.5% (range: 12-80%) (Figure 2). The mean overall survival for patients was 10.93 months (range: 0.2–82.6 months) (Table 1). Of the patients, 13 (36.2%) were alive, while 23 (63.8%) had either died from the disease or were lost to follow-up (Figure 3).
Among the 36 GBM cases analyzed, the majority, 28 (77.8%) cases, exhibited a mosaic pattern of HIP1R expression. Additionally, 3 (8.3%) cases were negative for HIP1R expression, and another 3 (8.3%) cases showed a sparse pattern. Two (5.6%) cases demonstrated a mixed pattern described as H + M, indicating both homogeneous and mosaic expression akin to that observed in oligodendrogliomas. 25 (69.4%) cases exhibited a diffuse positive staining pattern for Vimentin and 11 (30.6%) cases showed a focal pattern of Vimentin expression The expression patterns of H3K27me3 staining demonstrated significant variability. 19 (52.7%) cases, exhibited a partial loss of H3K27me3 expression. Additionally, 10 (27.7%) cases showed a CL of H3K27me3 expression while 7 (19.6%) cases showed retained H3K27me3 expression (Table 1).
Performance of Individual Markers
The performance of individual IHC markers and their combined use demonstrates significant diagnostic value in differentiating glioma subtypes. HIP1R, with a pattern of either homogeneous or mixed homogeneous and mosaic staining (H or H+M), showed a sensitivity of 100%, specificity of 94.1%, positive predictive value (PPV) of 95.1%, and negative predictive value (NPV) of 100%. Vimentin had a sensitivity of 92.8%, specificity of 96.5%, PPV of 96.8%, and NPV of 92.1%. H3K27Me3 had a sensitivity of 100% but a lower specificity of 45.9%, with a PPV of 67.6% and NPV of 100% (Table 2 and 3).
Demonstrating the Staining Patterns of HIP1R, Vimentin, and H3K27me3 To Aid in the Differential Diagnosis Between Oligodendrogliomas and Astrocytomas.
| Pattern . | HIP1R . | Vimentin . | H3K27me3 . | Diagnosis . |
|---|---|---|---|---|
| Pattern 1 | Homogenous | Negative/focal positive | Complete loss | Oligodendroglioma |
| Pattern 2 | Homogenous + Mosaic | Negative/focal positive | Complete loss | Oligodendroglioma with cortical infiltration along with fragments of normal white matter or mini-gemistocytes, or reactive astrocytes |
| Pattern 3 | Mosaic | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 4 | Sparse | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 5 | Negative | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 6 | Homogenous + Mosaic | Diffuse/focal positive | Complete losss or partially loss or intact | Astrocytoma or GBM |
| Pattern . | HIP1R . | Vimentin . | H3K27me3 . | Diagnosis . |
|---|---|---|---|---|
| Pattern 1 | Homogenous | Negative/focal positive | Complete loss | Oligodendroglioma |
| Pattern 2 | Homogenous + Mosaic | Negative/focal positive | Complete loss | Oligodendroglioma with cortical infiltration along with fragments of normal white matter or mini-gemistocytes, or reactive astrocytes |
| Pattern 3 | Mosaic | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 4 | Sparse | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 5 | Negative | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 6 | Homogenous + Mosaic | Diffuse/focal positive | Complete losss or partially loss or intact | Astrocytoma or GBM |
Demonstrating the Staining Patterns of HIP1R, Vimentin, and H3K27me3 To Aid in the Differential Diagnosis Between Oligodendrogliomas and Astrocytomas.
| Pattern . | HIP1R . | Vimentin . | H3K27me3 . | Diagnosis . |
|---|---|---|---|---|
| Pattern 1 | Homogenous | Negative/focal positive | Complete loss | Oligodendroglioma |
| Pattern 2 | Homogenous + Mosaic | Negative/focal positive | Complete loss | Oligodendroglioma with cortical infiltration along with fragments of normal white matter or mini-gemistocytes, or reactive astrocytes |
| Pattern 3 | Mosaic | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 4 | Sparse | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 5 | Negative | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 6 | Homogenous + Mosaic | Diffuse/focal positive | Complete losss or partially loss or intact | Astrocytoma or GBM |
| Pattern . | HIP1R . | Vimentin . | H3K27me3 . | Diagnosis . |
|---|---|---|---|---|
| Pattern 1 | Homogenous | Negative/focal positive | Complete loss | Oligodendroglioma |
| Pattern 2 | Homogenous + Mosaic | Negative/focal positive | Complete loss | Oligodendroglioma with cortical infiltration along with fragments of normal white matter or mini-gemistocytes, or reactive astrocytes |
| Pattern 3 | Mosaic | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 4 | Sparse | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 5 | Negative | Diffuse/focal positive | Complete loss or partially loss or intact | Astrocytoma or GBM |
| Pattern 6 | Homogenous + Mosaic | Diffuse/focal positive | Complete losss or partially loss or intact | Astrocytoma or GBM |
Summarization of the Sensitivity, Specificity, PPV, and NPV for Various Combinations of Immunohistochemical Markers in Differentiating Oligodendrogliomas From Astrocytomas and Glioblastomas.
| Markers . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|
| HIP1R | 100% | 94.1% | 95.1% | 100% |
| Vimentin | 92.8% | 96.5% | 96.8% | 92.1% |
| H3K27Me3 | 100% | 45.9% | 67.6% | 100% |
| HIP1R (H or H + M)+ Vimentin (N)+ H3K27me3 (CL) OR Vimentin (N)+ H3K27me3 (CL) OR HIP1R (H) + H3K27me3 (CL) OR HIP1R (H or H + M)+ Vimentin (N) | 100% | 100% | 100% | 100% |
| Markers . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|
| HIP1R | 100% | 94.1% | 95.1% | 100% |
| Vimentin | 92.8% | 96.5% | 96.8% | 92.1% |
| H3K27Me3 | 100% | 45.9% | 67.6% | 100% |
| HIP1R (H or H + M)+ Vimentin (N)+ H3K27me3 (CL) OR Vimentin (N)+ H3K27me3 (CL) OR HIP1R (H) + H3K27me3 (CL) OR HIP1R (H or H + M)+ Vimentin (N) | 100% | 100% | 100% | 100% |
The markers and their respective staining patterns are defined as follows: HIP1R (H or H + M), indicating homogenous or predominantly homogenous staining with a mosaic pattern for HIP1R; Vimentin (N), indicating complete immunonegativity for Vimentin; and H3K27Me3 (CL), indicating complete loss of H3K27Me3. H3K27Me3 (CL): Complete loss of H3K27Me3.
Summarization of the Sensitivity, Specificity, PPV, and NPV for Various Combinations of Immunohistochemical Markers in Differentiating Oligodendrogliomas From Astrocytomas and Glioblastomas.
| Markers . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|
| HIP1R | 100% | 94.1% | 95.1% | 100% |
| Vimentin | 92.8% | 96.5% | 96.8% | 92.1% |
| H3K27Me3 | 100% | 45.9% | 67.6% | 100% |
| HIP1R (H or H + M)+ Vimentin (N)+ H3K27me3 (CL) OR Vimentin (N)+ H3K27me3 (CL) OR HIP1R (H) + H3K27me3 (CL) OR HIP1R (H or H + M)+ Vimentin (N) | 100% | 100% | 100% | 100% |
| Markers . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|
| HIP1R | 100% | 94.1% | 95.1% | 100% |
| Vimentin | 92.8% | 96.5% | 96.8% | 92.1% |
| H3K27Me3 | 100% | 45.9% | 67.6% | 100% |
| HIP1R (H or H + M)+ Vimentin (N)+ H3K27me3 (CL) OR Vimentin (N)+ H3K27me3 (CL) OR HIP1R (H) + H3K27me3 (CL) OR HIP1R (H or H + M)+ Vimentin (N) | 100% | 100% | 100% | 100% |
The markers and their respective staining patterns are defined as follows: HIP1R (H or H + M), indicating homogenous or predominantly homogenous staining with a mosaic pattern for HIP1R; Vimentin (N), indicating complete immunonegativity for Vimentin; and H3K27Me3 (CL), indicating complete loss of H3K27Me3. H3K27Me3 (CL): Complete loss of H3K27Me3.
Performance of Combined Markers
HIP1R (H or H + M) + Vimentin (N) + H3K27Me3 (CL):
The combination of all three markers showing homogenous or predominantly homogenous staining with a mosaic pattern for HIP1R (H or H+M), complete immunonegative for Vimentin (N), and CL of H3K27Me3 (CL) achieved perfect diagnostic accuracy. The presence of these specific staining patterns in tumor samples resulted in sensitivity, specificity, PPV, and NPV all at 100%. This high level of accuracy indicates that this combination of markers is highly reliable for the clinical diagnosis of oligodendroglioma, effectively differentiating them from astrocytomas and glioblastomas.
Other combinations:
Other combinations, such as Vimentin (N) + H3K27Me3 (CL), HIP1R (H or H+M) + H3K27Me3 (CL), or HIP1R (H or H+M) + Vimentin (N), also resulted in perfect diagnostic metrics, with sensitivity, specificity, PPV, and NPV all at 100% with area under ROC curve (AUC) 1.00 (Supplementary Figure S1).
This data demonstrates the high diagnostic value of using HIP1R, Vimentin, and H3K27Me3, in different combinations, for differentiating oligodendrogliomas from astrocytomas and glioblastomas. The combined use of these markers provides exceptional diagnostic accuracy, making them highly reliable for clinical application in distinguishing these tumor types (Table 2).
Validation Cohort Results
The study evaluated 73 adult patients with diffuse infiltrating gliomas, serving as a validation cohort for the findings. This cohort included 24 oligodendrogliomas (IDH-mutant, 1p/19q co-deleted), 22 astrocytomas (IDH-mutant), and 27 glioblastomas (IDH-wild-type), with a mean age of 42.7 years (range: 21–72). Male predominance was observed with a male-to-female ratio of 1.8:1 (47 males, 26 females).
Oligodendrogliomas (IDH-mutant, 1p/19q co-deleted)
Among 24 oligodendrogliomas, 13 were grade 2 and 11 were grade 3, with a mean age of 39.8 years (range: 28–58). IDH1 R132H positivity was confirmed in 23 cases, while one case had a non-canonical IDH2 R172K mutation identified via Sanger sequencing. ATRX expression was retained, P53 was negative, and all cases showed 1p/19q co-deletion. HIP1R showed homogeneous (H) staining in 87.5% and mixed homogeneous/mosaic (H + M) staining in 12.5%. Vimentin was absent in 75% but showed focal positivity in 25%, linked to reactive changes. H3K27me3 expression was lost in 23/24 cases, with one case showing partial loss in 60% of tumor cells.
Astrocytomas (IDH-mutant)
The cohort included 12 grade 2, 3 grade 3, and 7 grade 4 astrocytomas. All cases were IDH1 R132H positive, with ATRX loss in 86.4% and P53 positivity in 21/22 cases. None of the astrocytomas showed 1p/19q co-deletion. HIP1R expression was predominantly mosaic (77.2%), with one sparse and four negative patterns. Vimentin was diffusely positive in 86.4% and focal in 13.6%. H3K27me3 showed CL in 50% of cases, while the remaining 50% demonstrated partial loss.
Glioblastomas (IDH-wild-type)
All 27 glioblastomas were IDH1 R132H-negative, with ATRX retained in all cases. P53 was negative in 77.8% and positive in 22.2%. None of the cases exhibited 1p/19q codeletion. HIP1R was mosaic in 70.4%, negative in 14.8%, sparse in 11.1%, and H + M in 3.7%. Vimentin staining was diffuse in 62.9%, focal in 33.3%, and negative in one case. H3K27me3 showed partial loss in 44.4%, CL in 33.3%, and retention in 22.3%.
Performance of Markers
The combined use of HIP1R (H or H+M), Vimentin (negative), and H3K27me3 (CL) continued to demonstrate 100% diagnostic accuracy in distinguishing oligodendrogliomas from astrocytomas and glioblastomas. These results validate the robustness and reproducibility of the marker combination across both the test and validation cohorts.
Comprehensive cost-benefit analysis:
Traditional molecular diagnostics for assessing 1p/19q co-deletion typically costs around 8000 rupees (approximately 97 USD) per case. In contrast, using IHC for markers such as HIP1R, ATRX, and Vimentin significantly reduces the cost, with each antibody priced at approximately 1000 rupees (around 12 USD). Assessing one case involves the use of multiple antibodies, bringing the total cost per case using IHC to about 3000 rupees (approximately 36 USD). This represents a cost reduction of over 60% compared to traditional molecular methods. This substantial decrease highlights the feasibility and economic advantage of IHC as a reliable alternative for diagnosing oligodendrogliomas, especially in low and middle-income countries (LMICs).
Discussion
Current standard approaches for determining 1p/19q status include FISH, MLPA, NGS sequencing, and array-based analyses.10 While NGS and array methods are effective, their availability for routine diagnostics is limited. FISH is the most commonly used method but has several limitations, including its inability to differentiate between segmental and whole-arm deletions, an estimated false-positive rate of 3.6%, and potential variability in interpretation due to operator experience.4,11 Additionally, FISH may not detect certain types of deletions and can be affected by sample quality11 According to the WHO 2021 guidelines, p53 expression supports the diagnosis of astrocytoma, while loss of ATRX is the only essential criterion for substituting 1p/19q exclusion in IDH-mutant gliomas. Although several surrogate markers for oligodendroglioma have been proposed, none have been officially accepted by the WHO central nervous system (CNS) 5.1 A recent study has demonstrated that immunohistochemistry for HIP1R, vimentin, and ATRX can accurately predict the 1p/19q status in IDH-mutant gliomas.7
H3K27me3, a tri-methylation of histone H3 at lysine 27, plays a crucial role in the epigenetic regulation of gene expression and has significant implications in the classification and prognosis of CNS tumors.12,13 Studies on the use of H3K27me3 immunohistochemistry to differentiate astrocytic from oligodendroglial diffuse gliomas have yielded notable findings. Loss of H3K27me3 is strongly associated with oligodendroglial tumors, as evidenced by its loss in 96% (25/26) of IDH-mutant and 1p/19q co-deleted oligodendrogliomas in a study of 161 diffuse gliomas.14 Habiba et al. (2021) screened 145 adult gliomas, and observed H3K27me3 loss in 90% of IDH1 R132H mutant oligodendrogliomas, while it was not observed in non-canonical (non-IDH1 R132H) mutated 1p/19q co-deleted oligodendrogliomas. Nuclear staining was highly preserved in Astrocytoma, IDH-mutant (87%), IDH-wild-type astrocytomas (94%), and glioblastomas (91%).13 Peckmezci et al. (2020) reported limitations in using H3K27me3 immunohistochemistry to differentiate astrocytic from oligodendroglial diffuse gliomas, noting retained H3K27me3 expression in 25% of oligodendrogliomas suggesting that H3K27me3 loss is not a completely sensitive or specific marker for oligodendroglial tumors, emphasizing the need to integrate H3K27me3 with other markers, like IDH mutation status and ATRX immunostaining, for more accurate classification of diffuse gliomas.15 Additionally, a study in 2020 by Kitahama et.al used histone epiproteomic profiling to reveal less H3K27 tri-methylation in oligodendrogliomas compared to IDH-mutant astrocytomas.16 This epigenetic difference serves as a new marker for glioma classification, aiding in more accurate diagnosis and treatment planning. The variability in the proportions of cases with H3 K27me3 loss compared to other studies can be attributed to several factors. Our study utilized a standardized Ventana automated platform for IHC, ensuring consistent and reproducible staining by precisely controlling parameters such as incubation times, reagent volumes, and temperature. In contrast, manual methods or other platforms may exhibit variability due to differences in sensitivity, antibody performance, and protocol standardization.
Additional factors include differences in case selection, sample preparation, and pre-analytical variables such as fixation time, and tissue handling, all of which can impact results. Variations in antibody specificity and scoring criteria, including thresholds for determining “loss” versus “retention,” further contribute to discrepancies.
HIP1R is a crucial protein involved in cellular processes such as endocytosis and vesicle trafficking. It facilitates the uptake of proteins from the extracellular environment and aids in the intracellular transport of these proteins to their designated locations within the cell. HIP1R interacts with the actin cytoskeleton, which is essential for the movement of vesicles and proteins.17,18 Mutations in the HIP1R gene have been linked to Parkinson’s disease, notably the R934C mutation, which impairs HIP1R’s ability to bind to actin and delays. Additionally, its expression is associated with patient outcomes in diffuse large B-cell lymphoma (DLBCL), where lower levels of HIP1R correlate with poorer survival rates in patients undergoing R-CHOP chemotherapy. The transcription factor FOXP1 has been shown to directly repress HIP1R expression in DLBCL, creating an inverse relationship between FOXP1 and HIP1R levels.18 HIP1R was found to be overexpressed in brain tumors, correlating significantly with the expression of EGFR and PDGF receptors. The researchers analyzed tissue samples and discovered that 63% of brain tumor tissues exhibited HIP1R expression compared to only 28% in normal cortical tissues. Notably, they identified that serum samples from brain cancer patients contained anti-HIP1R antibodies at a significantly higher frequency than controls, with 93% positivity in glioma patients. Furthermore, HIP1R was shown to physically associate with EGFR, independent of its lipid, clathrin, and actin-binding domains, indicating a direct interaction that may enhance EGFR overexpression in tumors, thereby contributing to tumorigenesis.19 The study by Marius Felix et al. in 2022 demonstrated that immunohistochemistry for HIP1R and vimentin can effectively predict 1p/19q co-deletion status in IDH-mutant gliomas with high accuracy. Using mass spectrometry-based proteomic analysis on a discovery series of 35 fresh-frozen and 72 formalin-fixed tumors, the authors identified an inverse abundance pattern for HIP1R and vimentin in astrocytomas and oligodendrogliomas. Subsequent validation through immunohistochemistry in two independent cohorts revealed distinct protein patterns: oligodendrogliomas exhibited high levels of HIP1R and low levels of vimentin, while astrocytomas showed the reverse expressions. In blinded evaluations, the prediction of 1p/19q status achieved 100% positive and NPVs in one cohort, and in the second cohort, it reached an 83% PPV, 100% NPV, and 92% accuracy. The loss of nuclear ATRX expression further enhanced sensitivity to 96% and specificity to 100%. These findings suggest that immunohistochemistry for HIP1R, vimentin, and ATRX offers a reliable and cost-effective alternative to genetic testing for determining 1p/19q co-deletion status, which is crucial for the classification and management of IDH-mutant gliomas. Overall, this research highlighted the importance of utilizing these immunohistochemical markers to enhance diagnostic accuracy and improve treatment strategies for patients.7
In our study we have observed that the use of HIP1R, Vimentin, and H3K27me3 IHC staining is crucial in differentiating oligodendrogliomas from astrocytoma and glioblastomas. HIP1R expression patterns provide significant diagnostic insights, with oligodendrogliomas typically exhibiting homogeneous (H) or mixed homogeneous and mosaic staining (H+M), while astrocytomas and GBM show a more heterogeneous expression with mosaic, sparse, or negative patterns. Vimentin staining is predominantly negative in oligodendrogliomas, with occasional focal positivity due to reactive astrocytes, mini-gemistocytes, white matter or cortical infiltration fragments. In contrast, astrocytomas and GBMs demonstrate diffuse or focal positive staining, highlighting the utility of Vimentin as a distinguishing marker. H3K27me3 expression is consistently lost in oligodendrogliomas, contrasting with the variable loss and retention seen in astrocytomas and GBM, thereby serving as a reliable marker to differentiate these tumor types. These markers collectively enhance the accuracy of histopathological diagnosis, aiding in the effective classification and subsequent management of diffuse gliomas. In the present study, the 2 or 3 markers combination of HIP1R, vimentin, and H3K27me3 exhibited 100% accuracy in correlating with 1p/19q co-deletion and identifying oligodendrogliomas, making it an excellent choice for use in resource-constrained areas. Pattern 1 (Diffusely positive HIP1R, negative vimentin, and H3K27me3 completely lost) is diagnostic of oligodendroglioma, and even a combination of any two of these markers ensures accurate classification in resource-limited settings. However, for other staining patterns, we recommend using all three antibodies to achieve 100% diagnostic accuracy. To further reduce the cost of assessment, these markers can be restricted for cases with retained ATRX expression as ATRX loss is exclusively seen in astrocytomas.
The cost of FISH per case is high as compared to the IHC and necessitates specialized equipment, including a FISH microscope, along with trained personnel for accurate assessment. The procedure takes about two days, signals may fade, potentially impacting the reliability of challenges for analysis as standard cutoff values cannot be applied in these scenarios. In contrast, IHC is much more cost-effective.9 IHC can be performed on small sample sizes and provides robust results within a single day, making it particularly advantageous in peripheral centers where resources may be limited. Given these advantages, we strongly recommend this three-marker panel as a practical alternative to FISH testing for the diagnosis of oligodendrogliomas, especially in settings with restricted access to advanced molecular diagnostic techniques.
One key limitation of our study is the use of FISH as the reference standard for assessing 1p/19q codeletion. More advanced techniques, such as SNP array or methylation profiling, could provide a more comprehensive assessment of chromosome alterations but were not utilized in this study due to resource constraints. Despite these limitations, FISH remains the standard diagnostic tool in many settings, including ours. Although we included a separate validation cohort in this study, also we have incorporated IHC for HIP1R, Vimentin, and H3K27Me3 into our routine diagnostic workflow for all relevant cases. Our ongoing application of these markers has continued to yield the same findings, as reported in this study. This further reinforces the reliability and robustness of these markers for assessing 1p/19q codeletion status in routine diagnostics.
Conclusion
In conclusion, our study demonstrates that the assessment for immunohistochemical markers HIP1R, vimentin, and H3K27me3 provide a reliable and cost-effective method for determining 1p/19q co-deletion in oligodendrogliomas, achieving 100% accuracy. This three-marker panel serves as a practical alternative to traditional genetic testing, particularly in resource-constrained settings, as it offers rapid results and reduced costs. Our findings highlight the potential for improved diagnostic accuracy and patient management, emphasizing the need for further validation in larger cohorts to solidify its clinical applicability.
Supplementary Material
Supplementary material is available online at Neuro-Oncology Advances (https://dbpia.nl.go.kr/noa).
Funding
None.
Acknowledgments
We extend our sincere gratitude to our technical staff for their meticulous work in performing the IHC staining and molecular testing. CS thanks the Indian Council of Medical Research for ICMR Emeritus Scientist Position.
Conflict of interest statement. None declared.
Authorship statement
C.S. and V.S. conceptualized the study theme and design. V.S., M.C.S., C.S., A.W., and H.J. performed the histological, immunohistochemical, and molecular analysis. A.W., H.J., S.S., C.R., S.D., and A.S. were responsible for material preparation and data collection. A.W. and H.J. wrote the first draft of the manuscript. All authors have read and approved the final manuscript.
Data availability
The data will be made available upon reasonable request and available with the corresponding author.
References
Author notes
Ashish Wadekar and Hemlata Jangir contributed equally to this work.
Ashish Wadekar and Hemlata Jangir considered first co-authors.




