-
PDF
- Split View
-
Views
-
Cite
Cite
Scott Raskin, Peter de Blank, Catherine A Billups, Yimei Li, James M Olson, Sarah E S Leary, Isotretinoin has no effect on event-free survival across high-risk medulloblastoma molecular groups when added to maintenance: A secondary analysis of the Children's Oncology Group ACNS0332 data, Neuro-Oncology Advances, Volume 7, Issue 1, January-December 2025, vdaf054, https://doi.org/10.1093/noajnl/vdaf054
Close - Share Icon Share
Abstract
The Children’s Oncology Group (COG) study ACNS0332 examined the effect of adding carboplatin and isotretinoin to high-risk medulloblastoma therapy. Isotretinoin arms were closed early due to futility, but the effect of carboplatin was shown to vary by individual medulloblastoma subgroups. Because isotretinoin arms were closed before subgroup classification was available, a differential effect of isotretinoin among various subgroups was not examined. Here, we conduct a secondary analysis of ACNS0332 data examining the effect of isotretinoin on event-free survival (EFS) among individual medulloblastoma subgroups.
Among 261 patients enrolled in ACNS0332, a subgroup was evaluable in 231 patients. Fisher’s exact tests and chi-square tests were used to compare distributions of categorical variables among patients with and without exposure to isotretinoin. EFS for subgroups was estimated, and the log-rank test was used to examine differences in outcome distributions among patient groups.
Among 231 evaluable patients, 85 were randomized to isotretinoin, 85 were randomized to no isotretinoin, and 61 received no isotretinoin without randomization. All 4 medulloblastoma groups were identified: Randomization to isotretinoin was not associated with any difference in EFS in patients with group 3 (n = 79, P = .87), group 4 (n = 101, P = .53), SHH (n = 37, P = .69) or WNT (n = 14, P = 1) medulloblastoma.
This study confirms that isotretinoin in addition to radiation and chemotherapy did not improve EFS in pediatric high-risk medulloblastoma regardless of molecular subgroup.
• ACNS0332 closed the isotretinoin arm prior to study completion due to futility.
• Secondary analysis was done to investigate if isotretinoin has an effect by subgroup.
• Isotretinoin had no effect on event-free survival in high-risk medulloblastoma, regardless of molecular group.
Medulloblastoma is the most common malignant brain tumor in children. A randomized phase 3 clinical trial (ACNS0332) was conducted by the Children’s Oncology Group between March 2007 and September 2018 for patients aged 3 to 21 years with newly diagnosed high-risk medulloblastoma. ACNS0332 examined the effect of adding carboplatin during radiation and isotretinoin during maintenance cycles. Therapy intensification with carboplatin improved event-free survival (EFS) for children with high-risk group 3 medulloblastoma only, highlighting the importance of molecular subgroup on patient outcomes as previously published. However, isotretinoin randomization was discontinued in January 2015 following a futility analysis and was not assessed by the molecular subgroup. This secondary analysis of ACNS0332 data demonstrates that isotretinoin did not have an effect on EFS in high-risk medulloblastoma regardless of molecular subgroup. This study continues to reinforce the value of an integrated clinical and molecular risk stratification for medulloblastoma.
Medulloblastoma is the most common malignant brain tumor in children.1,2 With current multimodal therapy (including neurosurgical resection, craniospinal radiation, and combination chemotherapy), the majority of patients will be cured of their disease.3–5 However, patients who present with high-risk disease, including metastases, diffuse anaplasia, or incomplete surgical resection have worse outcomes.6,7 Some studies have shown an exception in that the traditional high-risk features did not significantly impact outcomes in the WNT-MB cohorts of patients further highlighting the significance of the molecular group and its effect on outcomes.8–10 Although medulloblastoma had long been considered a single disease, new biological insights have redefined medulloblastoma as belonging to 4 distinct molecular subgroups (Wingless [WNT], Sonic Hedgehog [SHH], groups 3 and 4) with different prognoses and responses to treatment.11
Recently, The Children’s Oncology Group examined the effect of carboplatin and isotretinoin in children with high-risk medulloblastoma (ACNS0332, NCT00392327).1 Randomization to isotretinoin was discontinued prior to complete accrual due to an interim futility analysis that showed that isotretinoin during the maintenance phase of therapy was unlikely to substantially improve event-free survival (EFS). The effect of carboplatin during craniospinal radiation also did not show a significant effect on EFS or overall survival (OS) within the whole cohort. However, a significant benefit in EFS and OS was seen exclusively in group 3 medulloblastoma with nearly a 20% improvement in EFS (73.2% [95% CI: 56.9%–89.5%] with carboplatin vs. 53.7% [95% CI: 35.3%–72.1%] without carboplatin, P = .047).1 There was also a nearly 20% improvement in OS 82.8% (95% CI: 68.7%–96.9%) with carboplatin versus 63.7% (95% CI: 46.1%–81.3%) without carboplatin, P = .06.1 At the time of creating ACNS0332, the 4 distinct medulloblastoma groups were not yet recognized. It is therefore worth noting that the difference in EFS and OS for group 3 patients receiving carboplatin concurrently with radiation was also completed as part of a post hoc analysis.
The results from ACNS0332 highlight that therapies may have differential effects based on the medulloblastoma group. Although no effect of isotretinoin on EFS was observed in the analysis performed prior to the closure of the isotretinoin randomization, subset analyses by molecular group have not been presented. Therefore, we conducted a secondary analysis of the primary data from ACNS0332 reviewing isotretinoin effects specifically by the patient’s molecular group to further elucidate potential tumor characteristics where isotretinoin could prove effective.
Methods
Patients
Trial design and patient eligibility for ACNS0332 have been previously described in prior publications.1,12,13 Briefly, ACNS0332 was a randomized, phase 3, factorial-designed study with 4 arms. Although supratentorial primitive neuroectodermal tumors were initially included, their enrollment was later discontinued due to emerging data on biological differences with medulloblastoma.13 This analysis includes only patients with medulloblastoma.
Patients 3–21 years old with newly diagnosed high-risk medulloblastoma were enrolled. Metastatic disease, diffuse anaplasia, or residual disease greater than 1.5 cm2 were considered criteria for high-risk disease. Race and ethnicity were self-described according to National Institute of Health-defined categories. The authors confirm that written consent for submission and publication of this report, including the images and associated text, have been obtained from the patients in line with Committee on Publication Ethics (COPE) guidance. Institutional review board approval was obtained at each institution and written informed consent and assent when appropriate were obtained for all participants. Diagnosis and anaplastic features were confirmed by retrospective central pathology review. Initial staging, extent of resection, radiologic progression, relapse, or second malignant neoplasm were confirmed by retrospective central radiologic review. The molecular group was determined by array (Illumina Infinium Methylation EPIC BeadChip, Illumina Inc.) as previously described14–16 in consenting patients from tumors with sufficient genomic DNA for analysis.
Patients with medulloblastoma who were evaluable for ACNS0332 study aims were included in this analysis if they were also evaluable for molecular group analysis.
Study Design
At the time of enrollment, all patients were stratified by high-risk criteria, including (1) M0 medulloblastoma with greater than 1.5 cm2 residual, (2) M + medulloblastoma, and (3) M0 diffusely anaplastic medulloblastoma. Treatment has been described in previous publications.1,13 This included craniospinal radiation (36 Gy) with a boost to the posterior fossa (55.8 Gy) concurrent with weekly vincristine. Patients randomized to receive carboplatin were given 30 doses daily during radiation. Maintenance therapy included 6 cycles of cisplatin, cyclophosphamide, and vincristine. Patients randomized to isotretinoin received 80 mg/m2 twice daily on days 15 to 28 for 12 cycles during and following maintenance therapy. Randomization to Isotretinoin study arms was discontinued on January 27, 2015, and patients receiving isotretinoin were instructed to discontinue isotretinoin at that time.
Statistical Analysis
Study data as of September 30, 2019, were used for this analysis, similar to prior manuscripts.1,12,13 Analyses were based on intent-to-treat, and no adjustments were made for multiple comparisons. Patient numbers for some subgroups were small and no adjustments were made for multiple comparisons in this exploratory study. Results should be considered hypothesis-generating only, not confirmatory. Fisher’s exact tests and chi-square tests were used to compare distributions of categorical variables among patient groups. Wilcoxon rank sum and Kruskal–Wallis tests were used to compare distributions of continuous variables among patient groups. EFS was defined as the time interval from the date of study enrollment to the date of the first event (disease progression or recurrence, second malignant neoplasm, or death from any cause) or to the date of the last follow-up for patients without events. EFS estimates were obtained using the method of Kaplan and Meier. The reverse Kaplan–Meier method was used to calculate the median follow-up time and its range. The log-rank test was used to examine differences in outcome distributions among patient groups. P-values were two-sided except for the log-rank test P-value used for the futility analysis performed in 2014 per the statistical design.
Results
A total of 261 patients with medulloblastoma were evaluable for ACNS0332 primary study aims. Molecular group analysis was evaluable in 231 patients: 15 patients did not consent to molecular analysis, 14 had insufficient tissue or poor data quality, and 1 patient had molecular grouping not consistent with medulloblastoma. Table 1 shows patient characteristics by medulloblastoma group and isotretinoin randomization. WNT and SHH were the least frequently seen groups, with 14 (6.1%) and 37 (16%) patients, respectively; groups 3 and 4 were the most common groups with 79 (34.2%) and 101 (43.7%) patients. Between molecular groups, there was no evidence of differences in isotretinoin randomization (P = .76) or in carboplatin randomization (P = .85). There were statistically significant differences in sex and age by group (P = .016 for sex; P = .017 for age on study and P = .016 for age at diagnosis). Of the 231 patients included, 166 were male and 65 were female. The median age at diagnosis differed by subgroup (group 3 = 7.2 years, group 4 = 8.7 years, SHH = 8.8 years, and WNT = 9.2 years). There was also evidence of differences in the distribution of stratum by group (P < .001) and M-stage (P < .001).
Patient Characteristics by Molecular Group and Isotretinoin Randomization Group
| Randomized to isotretinoin (N = 85) | Randomized to no isotretinoin (N = 85) | Post closure of iso: no isotretinoin (N = 61) | P1 Analysis of all 3 patient groups | P 2 Analysis of patients with no isotretinoin exposure (randomized vs. assigned) | All patients (N = 231) | |
| N (%) | N (%) | N (%) | N (%) | |||
| Sex | ||||||
| Female | 25 (29.4) | 29 (34.1) | 11 (18) | .098 | .032 | 65 (28.1) |
| Male | 60 (70.6) | 56 (65.9) | 50 (82) | 166 (71.9) | ||
| Stratum | ||||||
| M + medulloblastoma | 63 (74.1) | 58 (68.2) | 46 (75.4) | .62 | .38 | 167 (72.3) |
| M0 diffusely anaplastic medullo with <1.5 cm2 residual | 17 (20) | 22 (25.9) | 14 (23) | 53 (22.9) | ||
| M0 medullo with >1.5 cm2 residual | 5 (5.9) | 5 (5.9) | 1 (1.6) | 11 (4.8) | ||
| M stage | ||||||
| M0 | 22 (25.9) | 27 (31.8) | 15 (24.6) | .39 | .18 | 64 (27.7) |
| M1 | 12 (14.0 | 12 (49.4) | 6 (9.8) | 30 (13) | ||
| M2 | 14 (16.5) | 13 (12.9) | 10 (16.4) | 37 (16) | ||
| M3 | 37 (43.5) | 33 (5.9) | 30 (49.2) | 100 (43.3) | ||
| Molecular group | ||||||
| Group 3 | 32 (37.6) | 27 (31.8) | 20 (32.8) | .64 | .4 | 79 (34.2) |
| Group 4 | 35 (41.2) | 42 (49.4) | 24 (39.3) | 101 (43.7) | ||
| SHH | 12 (14.1) | 11 (12.9) | 14 (23) | 37 (16) | ||
| WNT | 6 (7.1) | 5 (5.9) | 3 (4.9) | 14 (6.1) | ||
| Treatment group | ||||||
| Carboplatin/isotretinoin | 46 (54.1) | 0 (0) | 0 (0) | - | - | 46 (19.9) |
| Carboplatin/no isotretinoin | 0 (0) | 47 (55.3) | 31 (50.8) | 78 (33.8) | ||
| No Carboplatin/isotretinoin | 39 (45.9) | 0 (0) | 0 (0) | 39 (16.9) | ||
| No carboplatin/no isotretinoin | 0 (0) | 38 (44.7) | 30 (49.2) | 68 (29.4) | ||
| Total | 85 (100) | 85 (100) | 61 (100) | 231 (100) | ||
| Randomized to isotretinoin (N = 85) | Randomized to no isotretinoin (N = 85) | Post closure of iso: no isotretinoin (N = 61) | P1 Analysis of all 3 patient groups | P 2 Analysis of patients with no isotretinoin exposure (randomized vs. assigned) | All patients (N = 231) | |
| N (%) | N (%) | N (%) | N (%) | |||
| Sex | ||||||
| Female | 25 (29.4) | 29 (34.1) | 11 (18) | .098 | .032 | 65 (28.1) |
| Male | 60 (70.6) | 56 (65.9) | 50 (82) | 166 (71.9) | ||
| Stratum | ||||||
| M + medulloblastoma | 63 (74.1) | 58 (68.2) | 46 (75.4) | .62 | .38 | 167 (72.3) |
| M0 diffusely anaplastic medullo with <1.5 cm2 residual | 17 (20) | 22 (25.9) | 14 (23) | 53 (22.9) | ||
| M0 medullo with >1.5 cm2 residual | 5 (5.9) | 5 (5.9) | 1 (1.6) | 11 (4.8) | ||
| M stage | ||||||
| M0 | 22 (25.9) | 27 (31.8) | 15 (24.6) | .39 | .18 | 64 (27.7) |
| M1 | 12 (14.0 | 12 (49.4) | 6 (9.8) | 30 (13) | ||
| M2 | 14 (16.5) | 13 (12.9) | 10 (16.4) | 37 (16) | ||
| M3 | 37 (43.5) | 33 (5.9) | 30 (49.2) | 100 (43.3) | ||
| Molecular group | ||||||
| Group 3 | 32 (37.6) | 27 (31.8) | 20 (32.8) | .64 | .4 | 79 (34.2) |
| Group 4 | 35 (41.2) | 42 (49.4) | 24 (39.3) | 101 (43.7) | ||
| SHH | 12 (14.1) | 11 (12.9) | 14 (23) | 37 (16) | ||
| WNT | 6 (7.1) | 5 (5.9) | 3 (4.9) | 14 (6.1) | ||
| Treatment group | ||||||
| Carboplatin/isotretinoin | 46 (54.1) | 0 (0) | 0 (0) | - | - | 46 (19.9) |
| Carboplatin/no isotretinoin | 0 (0) | 47 (55.3) | 31 (50.8) | 78 (33.8) | ||
| No Carboplatin/isotretinoin | 39 (45.9) | 0 (0) | 0 (0) | 39 (16.9) | ||
| No carboplatin/no isotretinoin | 0 (0) | 38 (44.7) | 30 (49.2) | 68 (29.4) | ||
| Total | 85 (100) | 85 (100) | 61 (100) | 231 (100) | ||
Patient Characteristics by Molecular Group and Isotretinoin Randomization Group
| Randomized to isotretinoin (N = 85) | Randomized to no isotretinoin (N = 85) | Post closure of iso: no isotretinoin (N = 61) | P1 Analysis of all 3 patient groups | P 2 Analysis of patients with no isotretinoin exposure (randomized vs. assigned) | All patients (N = 231) | |
| N (%) | N (%) | N (%) | N (%) | |||
| Sex | ||||||
| Female | 25 (29.4) | 29 (34.1) | 11 (18) | .098 | .032 | 65 (28.1) |
| Male | 60 (70.6) | 56 (65.9) | 50 (82) | 166 (71.9) | ||
| Stratum | ||||||
| M + medulloblastoma | 63 (74.1) | 58 (68.2) | 46 (75.4) | .62 | .38 | 167 (72.3) |
| M0 diffusely anaplastic medullo with <1.5 cm2 residual | 17 (20) | 22 (25.9) | 14 (23) | 53 (22.9) | ||
| M0 medullo with >1.5 cm2 residual | 5 (5.9) | 5 (5.9) | 1 (1.6) | 11 (4.8) | ||
| M stage | ||||||
| M0 | 22 (25.9) | 27 (31.8) | 15 (24.6) | .39 | .18 | 64 (27.7) |
| M1 | 12 (14.0 | 12 (49.4) | 6 (9.8) | 30 (13) | ||
| M2 | 14 (16.5) | 13 (12.9) | 10 (16.4) | 37 (16) | ||
| M3 | 37 (43.5) | 33 (5.9) | 30 (49.2) | 100 (43.3) | ||
| Molecular group | ||||||
| Group 3 | 32 (37.6) | 27 (31.8) | 20 (32.8) | .64 | .4 | 79 (34.2) |
| Group 4 | 35 (41.2) | 42 (49.4) | 24 (39.3) | 101 (43.7) | ||
| SHH | 12 (14.1) | 11 (12.9) | 14 (23) | 37 (16) | ||
| WNT | 6 (7.1) | 5 (5.9) | 3 (4.9) | 14 (6.1) | ||
| Treatment group | ||||||
| Carboplatin/isotretinoin | 46 (54.1) | 0 (0) | 0 (0) | - | - | 46 (19.9) |
| Carboplatin/no isotretinoin | 0 (0) | 47 (55.3) | 31 (50.8) | 78 (33.8) | ||
| No Carboplatin/isotretinoin | 39 (45.9) | 0 (0) | 0 (0) | 39 (16.9) | ||
| No carboplatin/no isotretinoin | 0 (0) | 38 (44.7) | 30 (49.2) | 68 (29.4) | ||
| Total | 85 (100) | 85 (100) | 61 (100) | 231 (100) | ||
| Randomized to isotretinoin (N = 85) | Randomized to no isotretinoin (N = 85) | Post closure of iso: no isotretinoin (N = 61) | P1 Analysis of all 3 patient groups | P 2 Analysis of patients with no isotretinoin exposure (randomized vs. assigned) | All patients (N = 231) | |
| N (%) | N (%) | N (%) | N (%) | |||
| Sex | ||||||
| Female | 25 (29.4) | 29 (34.1) | 11 (18) | .098 | .032 | 65 (28.1) |
| Male | 60 (70.6) | 56 (65.9) | 50 (82) | 166 (71.9) | ||
| Stratum | ||||||
| M + medulloblastoma | 63 (74.1) | 58 (68.2) | 46 (75.4) | .62 | .38 | 167 (72.3) |
| M0 diffusely anaplastic medullo with <1.5 cm2 residual | 17 (20) | 22 (25.9) | 14 (23) | 53 (22.9) | ||
| M0 medullo with >1.5 cm2 residual | 5 (5.9) | 5 (5.9) | 1 (1.6) | 11 (4.8) | ||
| M stage | ||||||
| M0 | 22 (25.9) | 27 (31.8) | 15 (24.6) | .39 | .18 | 64 (27.7) |
| M1 | 12 (14.0 | 12 (49.4) | 6 (9.8) | 30 (13) | ||
| M2 | 14 (16.5) | 13 (12.9) | 10 (16.4) | 37 (16) | ||
| M3 | 37 (43.5) | 33 (5.9) | 30 (49.2) | 100 (43.3) | ||
| Molecular group | ||||||
| Group 3 | 32 (37.6) | 27 (31.8) | 20 (32.8) | .64 | .4 | 79 (34.2) |
| Group 4 | 35 (41.2) | 42 (49.4) | 24 (39.3) | 101 (43.7) | ||
| SHH | 12 (14.1) | 11 (12.9) | 14 (23) | 37 (16) | ||
| WNT | 6 (7.1) | 5 (5.9) | 3 (4.9) | 14 (6.1) | ||
| Treatment group | ||||||
| Carboplatin/isotretinoin | 46 (54.1) | 0 (0) | 0 (0) | - | - | 46 (19.9) |
| Carboplatin/no isotretinoin | 0 (0) | 47 (55.3) | 31 (50.8) | 78 (33.8) | ||
| No Carboplatin/isotretinoin | 39 (45.9) | 0 (0) | 0 (0) | 39 (16.9) | ||
| No carboplatin/no isotretinoin | 0 (0) | 38 (44.7) | 30 (49.2) | 68 (29.4) | ||
| Total | 85 (100) | 85 (100) | 61 (100) | 231 (100) | ||
Patient, disease, and treatment characteristics were also evaluated based on randomization to isotretinoin. Three groups were defined: patients randomized to isotretinoin, patients randomized not to receive isotretinoin, and patients treated after isotretinoin arms were closed. A comparison of characteristics between all 3 groups is shown in Table 1; the P value for this comparison is reported as P1. To compare patient characteristics before and after isotretinoin randomization was ended, patients randomized to no isotretinoin and those assigned to no isotretinoin (after isotretinoin arms were closed) were also compared in Table 1 and reported as P2. There were no significant differences in stratum, M stage, molecular group, or randomization to carboplatin among groups in either comparison. There was a difference in sex distribution among patients randomized and assigned to no isotretinoin (P = .032). The difference in sex distribution is similar to what has been seen in other medulloblastoma trials including the Children’s Oncology Group average-risk medulloblastoma study, ACNS0331 (NCT00085735),17 and aligns with the overall male predominance of medulloblastoma around a 1.8:1 male:female ratio.11
Among the whole cohort, there was no difference in EFS between patients who were assigned to receive isotretinoin or not. This was seen at the time of data freeze in 2014 (Figure 1, P = .34) that was used to determine isotretinoin futility. It was also confirmed using the data up to September 30, 2019 (Figure 1, P = .30), which was the data cut-off used for the primary analysis of ACNS0332.1 Median follow-up time was 7.0 years (95% CI: 6.5–7.5 years).1 The effect of isotretinoin on EFS was also evaluated by molecular group (Figure 2). When divided by molecular groups and stratified by carboplatin randomization, there was also no evidence of differences in EFS by isotretinoin arms among group 3 (P = .87), group 4 (P = .53), SHH (P = .69), or WNT patients (P = 1; Figure 2). Finally, as seen in Figure 3, EFS was also evaluated in each medulloblastoma group by 3 categories of isotretinoin randomization: randomized to isotretinoin, randomized to no isotretinoin, and received no isotretinoin following closure of this arm. In this analysis, there was no significant difference in EFS by isotretinoin category among group 3 (P = .47), group 4 (P = .82), SHH (P = .41), or WNT patients (P = .54).
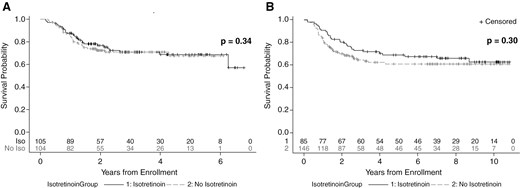
EFS by isotretinoin exposure based on data up to (A) June 30, 2014 (data used for isotretinoin futility analysis) using One-sided log-rank used to calculate P-values, stratified by carboplatin randomization and (B) September 30, 2019 (data used for study primary analysis) using Two-sided log-rank used to calculate P-values, stratified by carboplatin randomization. Number of subjects at risk for each group is listed at the bottom of each plot.
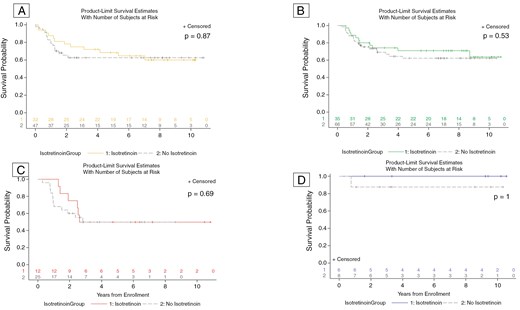
EFS for patients by isotretinoin exposure using data as of September 30, 2019, for individual medulloblastoma groups: (A) group 3, (B) group 4, (C) SHH, (D) WNT. The number at risk for each category is shown at the bottom of the plot. There was no significant difference in EFS by molecular subgroup in patients who did or did not use isotretinoin.
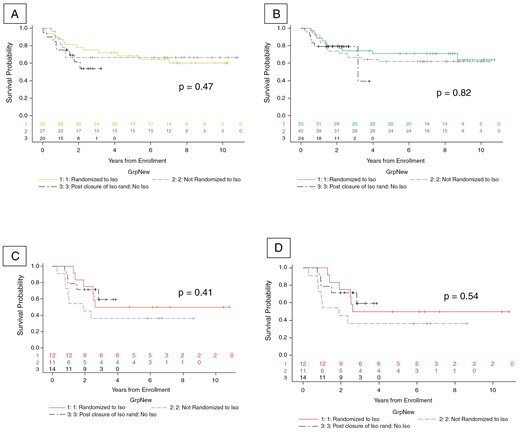
EFS for patients by isotretinoin randomization groups using data as of September 30, 2019, for each medulloblastoma group: (A) group 3, (B) group 4, (C) SHH, (D) WNT. There was no significant difference in EFS by molecular group in patients who did or did not use isotretinoin.
Discussion
Our secondary analysis of data from ACNS0332 shows no difference in EFS between groups that received isotretinoin and those that did not. Although isotretinoin futility was shown in an earlier analysis based on data up until isotretinoin arms were closed,1 earlier analyses were unable to examine the effect of the medulloblastoma group. Our analysis also extends follow-up for nearly 5 additional years and expands the number of patients analyzed by including those assigned to treatment arms without isotretinoin after the closure of those study arms. Unlike the effect of carboplatin which was only beneficial in group 3 medulloblastoma, our analysis showed no differences in the effect of isotretinoin based on molecular group.
Isotretinoin was added to the ACNS0332 treatment regimen based on preclinical evidence that Isotretinoin and all-trans retinoic acid (ATRA) induced programmed cell death in medulloblastoma cells. Isotretinoin also acted synergistically with cisplatin in cultured medulloblastoma cell lines.18 Previous studies using retinoids in combination with cisplatin demonstrated durable responses in patients with recurrent or advanced squamous cell carcinoma of the head and neck, advanced squamous cell carcinoma of the skin, and advanced non-small-cell lung cancer.19–23 Isotretinoin and ATRA were evaluated in 2 medulloblastoma cell lines, D283 and D341.24 Casciati et al demonstrated that the D283 cell line is in fact a group 3/4 medulloblastoma and D341 is a group 3 medulloblastoma.25 This preclinical data would suggest that isotretinoin may be more effective in group 3 or group 4 medulloblastoma. However, in our analysis, no difference was seen in these molecular groups, although they accounted for more than 75% of all patients analyzed.
Although the overall sample size of 231 evaluable patients makes ACNS0332 one of the largest medulloblastoma studies reported, our analysis examines smaller subpopulations. These smaller groups, especially WNT (n = 14) and SHH (n = 37) may have had less power to find a significant association between EFS and isotretinoin exposure.
This study is unusual in that randomization to isotretinoin was discontinued before all data was collected. In our analysis, patients randomized to receive no isotretinoin were statistically similar to those non-randomly assigned to no isotretinoin in terms of their stratum at study entry, M stage, or molecular group. However, sex did differ significantly between these 2 groups (P = .032) which is consistent with the male predominance of medulloblastoma. Previous studies such as those done by See et al. using 13-cis-retinoinc acid for recurrent adult GBM and Leary et al. testing the use of Vorinostat and Isotretinoin for recurrent embryonal tumors have utilized isotretinoin or other retinoids as differentiating agents in brain tumors. However, the data is limited with a small number of patients and it is still unclear if sex plays a role in response to retinoids.26,27 Because of the overall similarity between patients randomly and non-randomly assigned to no isotretinoin, these groups were combined in the primary analysis. We additionally conducted a sub-analysis using all 3 isotretinoin exposure groups to determine if this would have an effect on our results. Since our secondary analysis applied the differences in molecular group both retrospectively and prospectively there is a low likelihood for selection bias between any of the groups based on non-randomization following the closure of the isotretinoin group. The results confirm isotretinoin has no added benefit in every molecular group.
This analysis is subject to important limitations. Data were analyzed with the intention of treating analysis. Some patients randomized to isotretinoin may have had their exposure to isotretinoin abbreviated if they were still receiving therapy at the time of isotretinoin discontinuation. In addition, the assignment of the trial cohort into 4 molecular groups left few patients in some groups. This is particularly true for the WNT and SHH groups which were less frequently seen in this high-risk medulloblastoma study. However, groups with the strongest preclinical data (groups 3 and 4) were best represented in the cohort and still showed no significant effect due to isotretinoin. Although our results show no significant effect of isotretinoin among any medulloblastoma group, reporting these findings remains important. Negative results are important to the field as they help interpret the significance of positive findings. The main goals of stopping for futility are to preserve resources such as time and money and to prevent patients from being exposed to ineffective treatments with potential side effects unnecessarily.28 It is important to note that isotretinoin was discontinued due to statistical futility as the interim analysis made it clear that results were likely to not be statistically significant as opposed to safety concerns or operational aspects such as slow accrual. Reporting these results also enables a flawed concept to not be continuously studied or helps scientific researchers alter studies and plans to better understand why a concept was not effective.29 The omission of negative publications is often referred to as publication bias. Butler et al. investigated the discontinuation and nonpublication of neurooncological randomized clinical trials (RCT) and found that almost one-half of neurooncological RCTs were discontinued and nearly one-half of the clinical trials were unpublished.30 Jitlal et al noted that studies with modest treatment effects could be inappropriately stopped early following a futility analysis and provided examples of one study in epidermoid anal cancer (ACT I)31 plus one in locally advanced head and neck cancer (UKHAN_2)32 where interim hazard ratios (HR) are close to or exceed 1.0, with low conditional power, but the final HR indicated a clinically important effect.33 There are previous examples of clinical trials in pediatric neuro-oncology that were closed due to futility, not safety, and still published including a Children’s Oncology Group phase 2 trial in high-grade glioma by Karajannis et al.34 In pediatric neuro-oncology, where therapeutic advances are critical, publication bias can waste resources and delay the identification of more promising interventions. Reporting both successful and unsuccessful outcomes is essential for achieving meaningful progress in treating challenging diseases. It will continue to be important to publish data and note differences between closures due to safety versus futility when deliberating if therapies should still be considered for future clinical application.
The results of this study confirm that isotretinoin is not effective in pediatric high-risk medulloblastoma regardless of molecular group in the context of the high-risk backbone of high-dose radiation and intensive cytotoxic chemotherapy. Although integrated molecular diagnosis of medulloblastoma remains essential for proper treatment, the molecular group did not alter the effect of isotretinoin in this population. Retinoids may still be worthy of exploration for Central Nervous System (CNS) tumors, and preclinical testing has shown that they may be effective in ependymoma and glioma models.35,36 While Isotretinoin did not add benefit in medulloblastoma patients, future therapies may differentially target the varied biologies of different molecular groups. This secondary analysis continues to solidify the importance of analyzing medulloblastoma data by specific molecular groups in future studies.
Funding
ACNS0332 was funded by the National Cancer Institute through the National Clinical Trials Network (NCTN). The study was supported by NCTN Operations Center grant U10CA180886, NCTN Statistics & Data Center grant U10CA180899, St Baldrick’s Foundation. (Dr Northcott), Sontag Foundation (Dr Northcott), NIH 5R01CA114567 (Dr Olson), and The Brain Tumor Charity Clinical Biomarkers Award (Dr Northcott).
Authorship statement
Authorship: S.E.S.L. and J.M.O. are co-senior authors. Concept and design: S.R. and P.deB.. Statistical Analysis and interpretation of data: Y.L., C.A.B., and S.R.. Drafting of the manuscript and Critical revision: S.R., P.deB., S.E.S.L., and J.M.O..
Conflict of interest statement
Dr Olson reported receiving grants from the NIH during the conduct of the study. No other disclosures were reported.
Data availability
Raw data were generated/analyzed by Children’s Oncology Group Statisticians Y.L. and C.A.B. Deidentified participant data and derived data supporting the findings of this study are available with publication using childrensoncologygroup.org/data request. Data will be shared according to COG data sharing policy. Individual-level de-identified datasets that would be sufficient to reproduce results will be provided to the NCTN/NCORP Data Archive within 6 months after a PMID is assigned.




