-
PDF
- Split View
-
Views
-
Cite
Cite
Joseph P Merola, Joanita Ocen, Satish Kumar, James Powell, Caroline Hayhurst, Survival in melanoma brain metastases in the era of novel systemic therapies, Neuro-Oncology Advances, Volume 2, Issue 1, January-December 2020, vdaa144, https://doi.org/10.1093/noajnl/vdaa144
Close - Share Icon Share
Abstract
Melanoma brain metastases (MBMs) have historically poor overall survival (OS). Recently introduced systemic anticancer therapies (SACTs), namely targeted therapies such as BRAF inhibitors and immunotherapy, to control advanced disease have shown improved survival. Today, increasingly aggressive strategies are sought for MBM. We review outcomes in MBM after surgery or stereotactic radiosurgery (SRS) and the survival impact in advanced systemic disease when combined with novel anticancer therapies.
A retrospective cohort study of patients referred to a regional neuro-oncology multidisciplinary team (MDT) meeting with MBM. Demographic data, extent of systemic disease, and data on surgical and oncological management were collected, plus the use of SACT. The primary outcomes were median OS, 12- and 24-month survival, and progression-free survival.
Between 2010 and 2018, 142 patients with MBM were referred. Following the introduction of SACT, the rate of referrals to MDT more than doubled from 11.6 to 25.7 patients per year. Focal brain metastasis was treated surgically in 23 (16.2%) patients and by SRS in 29 (20.4%). Fifty-six (39.4%) patients underwent palliative whole-brain radiotherapy and 34 (23.9%) did not receive treatment. Median OS was 11 months for the surgical cohort, 9 months for the SRS cohort, and increased when treatment with or without SACT was considered to 23 and 12 months, respectively.
In the setting of SACTs, survival in MBM is significantly improved after surgery or SRS even in patients with advanced and uncontrolled systemic disease at the time of presentation, supporting an aggressive approach to MBM management.
Survival benefit conferred with surgery plus systemic anticancer therapies.
Survival benefit conferred with SRS plus systemic anticancer therapy.
Systemic anticancer therapies prolong survival in advanced melanoma.
Melanoma brain metastases (MBMs) have historically conferred poor overall survival. As a result, decisions to treat a focal brain lesion in the presence of systemic disease, with either surgery or radiosurgery, were finite. Since the introduction of novel systemic anticancer therapies (SACTs) overall survival, even in the presence of systemic disease, has improved. There is a paucity of information in the worldwide literature reporting outcomes following treatment of a focal brain lesion, particularly after surgery. In this study, our experience of managing advanced MBM patients in the era of SACTs has shown that survival is prolonged. It is important that an aggressive approach in the management of advanced MBM is employed and vital that the neuro-oncology multidisciplinary team is involved in an appropriate patient selection.
Advanced malignant melanoma commonly metastasizes to the brain and historically carries poor overall survival (OS) despite radical intervention with a median OS of 8 months.1 However, recent advances in novel systemic therapies have shown prolonged survival as a result of improved systemic disease control. In 2011, Chapman et al.2 demonstrated an OS advantage with vemurafenib, a potent inhibitor of mutated BRAF, over the traditional chemotherapeutic agent dacarbazine. Subsequently, combination therapy with BRAF and MEK inhibitors (eg, dabrafenib and trametinib) demonstrated an additional survival benefit.3,4 Improved outcomes have also been shown with immune checkpoint inhibitors such as the anti-programmed death-1 (PD-1) agent pembrolizumab and the CTLA-4 inhibitor ipilimumab.5
Following the National Institute of Health and Care Excellence’s (NICE) approval of these systemic anticancer therapies (SACTs) into the routine care of melanoma patients,6 we noted an increasing number of referrals to our regional neuro-oncology multidisciplinary team (MDT) meeting. In view of these additional SACT options and improved clinical outcomes, patients with advanced melanoma are now often considered for focal treatment for melanoma brain metastases (MBMs) with either surgery or stereotactic radiosurgery (SRS). In light of the recent improvements with SACT, we reviewed the outcomes of patients in our MDT with MBM following surgery or SRS and systemic therapies.
Methods
Population
All patients with MBM who were discussed at our regional neuro-oncology MDT between 2010 and 2018 were included in this study. Prior to referral to the MDT, all patients had confirmed metastases on MRI brain and up-to-date assessment of systemic disease with CT. Data were collected from clinical records and from the regional oncology database. Demographic information, baseline characteristics, and pre- and post-procedural outcomes including OS and progression-free survival (PFS) were collected.
Strategy and Procedures
In line with national guidelines,7,8 focal brain treatment was considered for patients with absent or controllable systemic disease, a WHO performance status of 0–2, and a prognosis greater than 6 months. Importantly, patients with a systemic disease deemed amenable to control with novel systemic therapies by their treating oncologist were treated accordingly. Those considered for surgical intervention underwent craniotomy and resection by the neurosurgical team at the University Hospital of Wales. In addition, SRS was considered suitable for lesions less than 20cc with no mass effect and no significant pressure symptoms.8 Patients where surgery or SRS was not suitable were treated with either whole-brain radiotherapy (WBRT) or offered best supportive care, determined by the treating oncologist.
Follow-up
All patients had regular review under the care of a medical oncologist with a specialist interest in melanoma. Surveillance MRI was undertaken every 3 months or sooner if there was a clinical indication. SACTs were employed in line with NICE recommendations to control the systemic disease.7
Performance status at discharge, 30 days, 6 months, and 12 months was recorded. OS was defined as the time in months between first treatment and time of death or last follow-up (if not treated then the time of diagnosing brain metastasis was used). PFS was defined as the time from first treatment to the first documented progression or recurrence of disease either distant or local to the treated metastasis or time to death/the last follow-up.
Statistics
Statistical analysis was performed using IBM SPSS statistics, version 25. Continuous variables are expressed as means or median and categorical variables as count or percentages. Survival was estimated using the Kaplan–Meier method and compared by the log-rank test. Statistical significance was defined as P < .05.
Ethics Statement
This project was approved by appropriate reviewers within our institution.
Results
Cohort
Between 2010 and 2018, 142 patients with MBM were discussed at our regional neuro-oncology MDT meeting. Following the introduction of novel SACT for melanoma, the rate of referrals to our MDT more than doubled, from a rate of 11.6 patients per year to 25.7 patients per year. A total of 52 (36.6%) patients had a focal brain lesion treated—23 (16.2%) patients were referred for surgery and 29 (20.4%) referred for SRS. Fifty-six (39.4%) patients were treated with palliative WBRT and 34 (23.9%) did not receive either SRS, WBRT, or surgery (Figure 1).
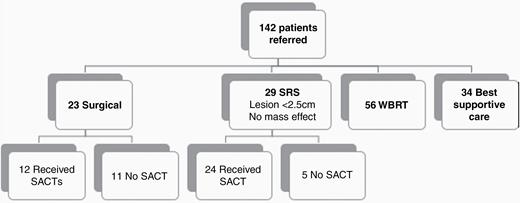
Hierarchal flow chart of the patient population. SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy; SACT, systemic anticancer therapy.
Comparability of the Groups
Table 1 summarizes the baseline characteristics of the patients who underwent surgery or SRS.
Summary of the Baseline Characteristics of Patients Undergoing Surgery or SRS for Melanoma Brain Metastasis
| . | Surgery (n = 23) . | Stereotactic Radiosurgery (n = 29) . |
|---|---|---|
| Age (mean) | 58 | 58 |
| Sex | ||
| Male | 11 | 16 |
| Female | 12 | 13 |
| Melanoma history | ||
| Synchronous presentation | 4 | 1 |
| Breslow thickness (mean) | 2.2 mm | 3.8 mm |
| Time to cerebral metastasis (median) | 38 months | 36 months |
| Presentation | ||
| Performance status (mode) | 1 | 0 |
| Symptoms/signs | ||
| Asymptomatic | 0 | 19 |
| Headache | 14 | 3 |
| Speech disturbance | 8 | 0 |
| Visual disturbance | 3 | 0 |
| Focal neurology | 14 | 4 |
| Seizure | 1 | 0 |
| Cognition | 4 | 1 |
| Location of metastasis | ||
| Frontal | 8 | 10 |
| Parietal | 8 | 5 |
| Occipital | 1 | 2 |
| Temporal | 3 | 4 |
| Insular | 0 | 1 |
| Posterior fossa | 3 | 2 |
| Not recorded | 0 | 5 |
| Number of metastases | ||
| 1 | 17 | 16 |
| 2 | 3 | 8 |
| 3 | 3 | 4 |
| 4 | 0 | 1 |
| Lateralization | ||
| Right | 10 | 12 |
| Left | 11 | 10 |
| Bilateral | 2 | 3 |
| Not recorded | 0 | 4 |
| Neuroimaging | ||
| Avg maximal diameter (mm) | 38.9 | 14.3 |
| Hemorrhage | 12 | 6 |
| Hydrocephalus | 3 | 0 |
| edema | 19 | 12 |
| Extracerebral metastases | 12 | 19 |
| SACT | ||
| Before | 4 | 18 |
| After | 8 | 6 |
| . | Surgery (n = 23) . | Stereotactic Radiosurgery (n = 29) . |
|---|---|---|
| Age (mean) | 58 | 58 |
| Sex | ||
| Male | 11 | 16 |
| Female | 12 | 13 |
| Melanoma history | ||
| Synchronous presentation | 4 | 1 |
| Breslow thickness (mean) | 2.2 mm | 3.8 mm |
| Time to cerebral metastasis (median) | 38 months | 36 months |
| Presentation | ||
| Performance status (mode) | 1 | 0 |
| Symptoms/signs | ||
| Asymptomatic | 0 | 19 |
| Headache | 14 | 3 |
| Speech disturbance | 8 | 0 |
| Visual disturbance | 3 | 0 |
| Focal neurology | 14 | 4 |
| Seizure | 1 | 0 |
| Cognition | 4 | 1 |
| Location of metastasis | ||
| Frontal | 8 | 10 |
| Parietal | 8 | 5 |
| Occipital | 1 | 2 |
| Temporal | 3 | 4 |
| Insular | 0 | 1 |
| Posterior fossa | 3 | 2 |
| Not recorded | 0 | 5 |
| Number of metastases | ||
| 1 | 17 | 16 |
| 2 | 3 | 8 |
| 3 | 3 | 4 |
| 4 | 0 | 1 |
| Lateralization | ||
| Right | 10 | 12 |
| Left | 11 | 10 |
| Bilateral | 2 | 3 |
| Not recorded | 0 | 4 |
| Neuroimaging | ||
| Avg maximal diameter (mm) | 38.9 | 14.3 |
| Hemorrhage | 12 | 6 |
| Hydrocephalus | 3 | 0 |
| edema | 19 | 12 |
| Extracerebral metastases | 12 | 19 |
| SACT | ||
| Before | 4 | 18 |
| After | 8 | 6 |
Summary of the Baseline Characteristics of Patients Undergoing Surgery or SRS for Melanoma Brain Metastasis
| . | Surgery (n = 23) . | Stereotactic Radiosurgery (n = 29) . |
|---|---|---|
| Age (mean) | 58 | 58 |
| Sex | ||
| Male | 11 | 16 |
| Female | 12 | 13 |
| Melanoma history | ||
| Synchronous presentation | 4 | 1 |
| Breslow thickness (mean) | 2.2 mm | 3.8 mm |
| Time to cerebral metastasis (median) | 38 months | 36 months |
| Presentation | ||
| Performance status (mode) | 1 | 0 |
| Symptoms/signs | ||
| Asymptomatic | 0 | 19 |
| Headache | 14 | 3 |
| Speech disturbance | 8 | 0 |
| Visual disturbance | 3 | 0 |
| Focal neurology | 14 | 4 |
| Seizure | 1 | 0 |
| Cognition | 4 | 1 |
| Location of metastasis | ||
| Frontal | 8 | 10 |
| Parietal | 8 | 5 |
| Occipital | 1 | 2 |
| Temporal | 3 | 4 |
| Insular | 0 | 1 |
| Posterior fossa | 3 | 2 |
| Not recorded | 0 | 5 |
| Number of metastases | ||
| 1 | 17 | 16 |
| 2 | 3 | 8 |
| 3 | 3 | 4 |
| 4 | 0 | 1 |
| Lateralization | ||
| Right | 10 | 12 |
| Left | 11 | 10 |
| Bilateral | 2 | 3 |
| Not recorded | 0 | 4 |
| Neuroimaging | ||
| Avg maximal diameter (mm) | 38.9 | 14.3 |
| Hemorrhage | 12 | 6 |
| Hydrocephalus | 3 | 0 |
| edema | 19 | 12 |
| Extracerebral metastases | 12 | 19 |
| SACT | ||
| Before | 4 | 18 |
| After | 8 | 6 |
| . | Surgery (n = 23) . | Stereotactic Radiosurgery (n = 29) . |
|---|---|---|
| Age (mean) | 58 | 58 |
| Sex | ||
| Male | 11 | 16 |
| Female | 12 | 13 |
| Melanoma history | ||
| Synchronous presentation | 4 | 1 |
| Breslow thickness (mean) | 2.2 mm | 3.8 mm |
| Time to cerebral metastasis (median) | 38 months | 36 months |
| Presentation | ||
| Performance status (mode) | 1 | 0 |
| Symptoms/signs | ||
| Asymptomatic | 0 | 19 |
| Headache | 14 | 3 |
| Speech disturbance | 8 | 0 |
| Visual disturbance | 3 | 0 |
| Focal neurology | 14 | 4 |
| Seizure | 1 | 0 |
| Cognition | 4 | 1 |
| Location of metastasis | ||
| Frontal | 8 | 10 |
| Parietal | 8 | 5 |
| Occipital | 1 | 2 |
| Temporal | 3 | 4 |
| Insular | 0 | 1 |
| Posterior fossa | 3 | 2 |
| Not recorded | 0 | 5 |
| Number of metastases | ||
| 1 | 17 | 16 |
| 2 | 3 | 8 |
| 3 | 3 | 4 |
| 4 | 0 | 1 |
| Lateralization | ||
| Right | 10 | 12 |
| Left | 11 | 10 |
| Bilateral | 2 | 3 |
| Not recorded | 0 | 4 |
| Neuroimaging | ||
| Avg maximal diameter (mm) | 38.9 | 14.3 |
| Hemorrhage | 12 | 6 |
| Hydrocephalus | 3 | 0 |
| edema | 19 | 12 |
| Extracerebral metastases | 12 | 19 |
| SACT | ||
| Before | 4 | 18 |
| After | 8 | 6 |
Overall Survival
The median OS for the whole cohort was 5 months (95% confidence interval [CI] 3.89–6.11). When radical treatment options are considered (surgery and SRS combined) median OS is 9 months (95% CI 4.29–13.71) versus 3 months for those treated with palliative intent (WBRT or best supportive care combined; 95% CI 1.83–4.16, P = .000).
The median OS was 11 months (95% CI 0.00–29.78) for the surgical cohort alone, 9 months (95% CI 3.72–14.27) for the SRS cohort, 6 months (95% CI 4.57–7.43) for those who underwent WBRT, and 1 month (95% CI 0.64–1.36) for those conservatively managed (Figure 2A). The above overall comparisons were statistically significant on log-rank P = .000. The median survival rates are summarized in Table 2 along with 12-month and 24-month survival.
Summary of the Median OS and 12- and 24-Month Survival Rates per Treatment Group
| . | Median OS (months) . | 12-Month Survival (%) . | 24-Month Survival (%) . |
|---|---|---|---|
| Surgery | |||
| Overall | 11 | 47.8 | 30.4 |
| SACT | 23 | 70 | 50 |
| No SACT | 6 | 30.7 | 15.4 |
| SRS | |||
| Overall | 9 | 44.8 | 30.2 |
| SACT | 12 | 52.2 | 39.1 |
| No SACT | 4 | 16.7 | 16.7 |
| WBRT | 6 | 19.6 | 6.4 |
| Conservative | 1 | 2.9 | 2.9 |
| . | Median OS (months) . | 12-Month Survival (%) . | 24-Month Survival (%) . |
|---|---|---|---|
| Surgery | |||
| Overall | 11 | 47.8 | 30.4 |
| SACT | 23 | 70 | 50 |
| No SACT | 6 | 30.7 | 15.4 |
| SRS | |||
| Overall | 9 | 44.8 | 30.2 |
| SACT | 12 | 52.2 | 39.1 |
| No SACT | 4 | 16.7 | 16.7 |
| WBRT | 6 | 19.6 | 6.4 |
| Conservative | 1 | 2.9 | 2.9 |
Summary of the Median OS and 12- and 24-Month Survival Rates per Treatment Group
| . | Median OS (months) . | 12-Month Survival (%) . | 24-Month Survival (%) . |
|---|---|---|---|
| Surgery | |||
| Overall | 11 | 47.8 | 30.4 |
| SACT | 23 | 70 | 50 |
| No SACT | 6 | 30.7 | 15.4 |
| SRS | |||
| Overall | 9 | 44.8 | 30.2 |
| SACT | 12 | 52.2 | 39.1 |
| No SACT | 4 | 16.7 | 16.7 |
| WBRT | 6 | 19.6 | 6.4 |
| Conservative | 1 | 2.9 | 2.9 |
| . | Median OS (months) . | 12-Month Survival (%) . | 24-Month Survival (%) . |
|---|---|---|---|
| Surgery | |||
| Overall | 11 | 47.8 | 30.4 |
| SACT | 23 | 70 | 50 |
| No SACT | 6 | 30.7 | 15.4 |
| SRS | |||
| Overall | 9 | 44.8 | 30.2 |
| SACT | 12 | 52.2 | 39.1 |
| No SACT | 4 | 16.7 | 16.7 |
| WBRT | 6 | 19.6 | 6.4 |
| Conservative | 1 | 2.9 | 2.9 |
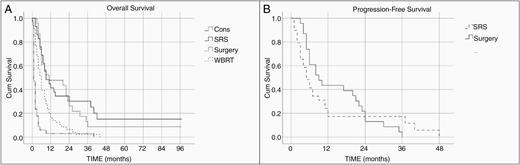
Kaplan–Meier analysis of overall survival (A) and progression-free survival (B) in the entire cohort. CONS, conservative; WBRT, whole-brain radiotherapy; SRS, stereotactic radiosurgery; Cum Survival, cumulative survival.
Progression-Free Survival
The median PFS for all patients in whom a focal brain lesion was treated was 6 months (95% CI 4.2–7.8, P = .656). PFS for both groups is shown in Figure 2B. The median PFS was 8 months in the surgical cohort (95% CI 3.3–12.7). In the SRS cohort, the median PFS was 5 months (95% CI 2.89–7.11).
In the surgical cohort, 7 (30%) patients were noted to have either recurrence or progression of the disease. Four were local (ie, at the original intracranial site of treatment) and 3 were intracranial but distant to the treated lesion. In the SRS cohort, 19 (65.5%) were noted to have progression or recurrence. Twelve were local (ie, at the site of the treated lesion) with 2 of these patients noted to have distant progression simultaneously (1 intracranially and 1 extracranially). Seven were noted to have distant progression: 4 intracranially, 1 extracranially, and 2 both simultaneously.
Secondary Analysis
Outcomes with/without SACT (targeted therapy or immunotherapy)
Overall, patients in whom a focal brain lesion was treated, the median OS survival increased to 15 months when patients were eligible for SACT (95% CI 2.05–27.95). Those not eligible for SACT had 6 months median OS (95% CI 4.31–7.69, P = .004; Figure 3A).
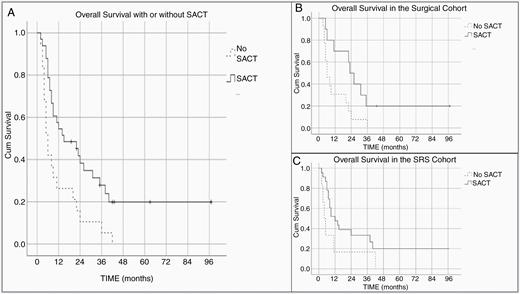
Kaplan–Meier survival curve demonstrates overall survival (A) and survival following surgical resection (B) and SRS (C) with or without SACT. SACT, systemic anticancer therapy; Cum Survival, cumulative survival.
In the surgical cohort, the median OS was 23 months in patients who were eligible for SACT therapy before or after surgery (95% CI 16.8–29.2). In those not deemed eligible, the median OS was 6 months (95% CI 2.48–9.5). This association was statistically significant on log-rank P = .022 (Figure 3B).
In the SRS cohort, the median OS was 12 months for those treated with SACT (95% CI 4.96–19.04) and 4 months for those who were not (95% CI 1.6–6.4). This association did not reach statistical significance (log-rank P = .082; Figure 3C).
Analysis according to BRAF mutation
Thirty-nine (75%) patients (surgical/SRS cohort) had a result available for BRAF mutation analysis. Fourteen (36%) were found to have the V600E BRAF mutation: 9 SRS patients and 5 surgically treated patients. The median OS was 12 months (95% CI 1.00–23.00, P = .821).
Surgical outcomes
The average length of stay in hospital was 13 days. Twenty-two (96%) patients underwent a scheduled total macroscopic resection while 1 (4%) patient having presented acutely required evacuation of an intracerebral hematoma resulting in the diagnosis of MBM.
Four (17%) patients suffered surgery-related comorbidity: 1 pseudomeningocele, 1 seizure, 1 pulmonary embolism, and 1 patient had a postoperative infection.
Eight (34%) had postsurgical SRS—3 (13%) required SRS at the site of the previously surgically resected lesion. These were all due to recurrence; however, 1 patient was found to only have reactive tissue following repeat craniotomy and resection. The remainder had SRS to distant intracranial metastases. Six (26%) patients underwent WBRT following surgery.
SRS outcomes
The symptoms described in Table 3 were noted in 9 (31%) patients. Three (10%) required re-treatment with SRS to the same lesion while a further 3 (10%) required SRS to a distant intracranial lesion. Five (17%) went on to require surgery and 4 (14%) ultimately required WBRT.
| Toxicity/Adverse Event (n = 9) . | Rate (%) . |
|---|---|
| Headache | 2 (22) |
| Hemorrhage into lesion | 2 (22) |
| Radionecrosis | 1 (11) |
| Cerebellar dysfunction | 1 (11) |
| Seizure | 1 (11) |
| Paraesthesia | 1 (11) |
| Nausea and vomiting | 1 (11) |
| Toxicity/Adverse Event (n = 9) . | Rate (%) . |
|---|---|
| Headache | 2 (22) |
| Hemorrhage into lesion | 2 (22) |
| Radionecrosis | 1 (11) |
| Cerebellar dysfunction | 1 (11) |
| Seizure | 1 (11) |
| Paraesthesia | 1 (11) |
| Nausea and vomiting | 1 (11) |
| Toxicity/Adverse Event (n = 9) . | Rate (%) . |
|---|---|
| Headache | 2 (22) |
| Hemorrhage into lesion | 2 (22) |
| Radionecrosis | 1 (11) |
| Cerebellar dysfunction | 1 (11) |
| Seizure | 1 (11) |
| Paraesthesia | 1 (11) |
| Nausea and vomiting | 1 (11) |
| Toxicity/Adverse Event (n = 9) . | Rate (%) . |
|---|---|
| Headache | 2 (22) |
| Hemorrhage into lesion | 2 (22) |
| Radionecrosis | 1 (11) |
| Cerebellar dysfunction | 1 (11) |
| Seizure | 1 (11) |
| Paraesthesia | 1 (11) |
| Nausea and vomiting | 1 (11) |
Discussion
This study has shown a survival benefit in patients following surgical resection or SRS to melanoma brain metastases treated with SACT. Early scoring algorithms that pre-date the availability of modern SACTs highlighted risk factors that impact survival. Favorable prognosis was more likely in those aged younger than 65 years, Karnofsky performance score greater than 70, and isolated brain metastases. Even with a favorable prognosis median OS was 7.1 months compared with 2.3 months in the unfavorable.9
Since the introduction of SACTs, namely targeted therapies and immunotherapy, MBM referrals to our regional neuro-oncology MDT more than doubled from 11.6 to 25.7 per year on the premise of controlled or controllable systemic disease. Overall, treatment of a focal brain lesion by either surgery or SRS in our cohort conferred a survival benefit in MBM patients. Median OS was 9 months (P = .000) that increased to 15 months if patients were eligible for SACT before or after focal brain treatment (P = .004). One-year and 2-year survival rates were 57.6% and 41.8%, respectively (Table 2 and Figure 2A). Similar outcomes are seen in previously published retrospective studies—Pessina et al.10 report median OS, 1-year and 2-year survival rates in 53 patients at 11.8 months, 47.2% and 28%, respectively.
While an MDT should continue to individualize treatment plans, the criteria for surgical resection of MBM must now take account of the improved outcome with SACT seen in patients with metastatic melanoma. In our surgical cohort, the addition of SACT was associated with a significant improvement in 1-year and 2-year OS rates of 70% and 50%, respectively, with a median OS of 23 months (Table 2 and Figure 3A). This is compared with 30% at 1-year, 15% at 2-year, and median OS 6 months if not treated with a SACT (P = .022). Despite the paucity of surgical only outcomes in the literature, similar results to ours are seen in other studies. Lonser et al.11 report a similar median OS of 12.4 months in 41 patients in whom 53 MBM were surgically resected. The authors go on to report a 70.6% 12-month survival rate in immunotherapy responders following surgery and 37.5% in those non-immunotherapy responders. A more recent article by Alvarez-Breckenridge et al.12 reported median OS in 79 patients who had undergone craniotomy for MBM. The authors found that in those with immunotherapy-naïve MBM, surgery followed by immunotherapy conferred the longest median OS at 22.7 months, while those treated with immunotherapy alone survived 10.8 months. Patients who were treated first with immunotherapy and then surgery survived a median OS of 9.4 months. This association was not statistically significant (P = .12). In patients who developed MBM following immunotherapy, median OS did not differ between further upfront immunotherapy and surgery (9.1 vs 9.0 months, respectively, P = .95). Interestingly, while we attempted to present the groups as pragmatically as possible (ie, as seen in the real clinical setting and not theoretical groups), all but one of those who were treated with SACT in our cohort were treated following surgery. The median OS survival was 23 months (P = .02) but increases to 26 months if only those treated following surgery are considered.
Twenty-nine patients in our study received SRS and the median OS was 9 months. This increased to 12 months when only those treated with SACTs are considered—23 (79%) were treated with SACT in this cohort. The 1-year and 2-year survival rates in those treated with SACT either before or after SRS were 52% and 39%, respectively. In those not treated with SACT, 1-year and 2-year survival rates were 33% and 17%, respectively (P = .082; Figure 3). Gaudy-Marqueste et al.13 reported a median OS of 6.79 months in 179 MBM patients following radiosurgery. For those who received SACT, they reported 10.95-month median OS and 2.29 months in those did not. One-year and 2-year survival rates were reported to be 50% and 24%, respectively. Other reports in the literature addressing SRS in combination with SACT therapies also show a benefit in terms of survival or local control.14–20
Melanoma is strongly associated with somatic mutations, the most frequent is the V600E BRAF mutation occurring in 35–45%. Both dabrafenib and vemurafenib have substantial activity in BRAF-mutated MBM21 and showed significant improvement in PFS and OS.4,22–24 In our study, patients with BRAF mutation conferred a survival benefit as seen in other studies both overall and when treatment methods were considered individually. However, this association was not statistically significant (P = .821). Only 75% of the surgical and SRS cohort had a test result available. It is possible that more were tested and the results were not recorded or available. In Wales, a funding establishment was set up in 2012 when BRAF testing became clinically relevant, and early demands for testing were met.25
Immune checkpoint inhibitors—selective blockers of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and PD-1—have proven to significantly prolong the survival of patients with MBM. Ugurel et al.26 reported an exploratory analysis of patients treated for MBM with novel therapeutic agents. They demonstrated superiority for BRAF and MEK inhibition within the first 6 months after treatment. However, in a follow-up review with 24 months follow-up data, a clear advantage is seen with immune checkpoint inhibitors at that point. The authors attribute this to acquired resistance and highlight the importance of this finding in clinical management. In our experience, there are several well-recognized mechanisms of acquired resistance for BRAF inhibitors leading to a PFS of approximately 7–8 months. In contrast, immunotherapy does result in longer-term survivors but there is a paucity of data in the literature regarding patients with brain metastases (since immunotherapy is typically limited to patients with smaller size/number of lesions and not on corticosteroids and hence generally excluded in randomized studies). In our study, we saw a significantly increased survival advantage when patients underwent surgical resection and were on SACT, from 11 months to 23 months (P = .022). Prolonged survival was also seen in those on SACTs who were treated with SRS (P = .08). This finding supports the efficacy of targeted or immunotherapy agents in controlling systemic disease and reinforces the need for an aggressive approach in MBM management.
A few limitations are noted in this study. Firstly, it is retrospective and in no way a comparison study of the treatment types discussed. There is also a selection bias in determining who was suitable for surgery or SRS and the subsequent numbers are small when subgroups are analyzed. The patient cohort, although representative of a real-life cohort of MBM patients, has very heterogeneous characteristics including the number of lesions, extent of extracranial disease, and treatment with SACT. The study period is long and therefore different methods of managing MBM would have been employed since treatment has been rapidly evolving. It is clear that the conclusions drawn from this study, and most comparison studies, are that multicenter studies are needed.
Conclusions
This single-center retrospective study shows a significant survival benefit of radical MBM management even in the setting of advanced systemic disease with the use of SACT. Patient selection is a crucial component of the management, and the regional MDT therefore plays a significant role in determining who would be eligible for surgery/SRS and SACT. Multicenter trials are required to confirm these drawn conclusions.
Funding
No funding supported this work.
Conflict of interest statement. There are no conflicts of interest.
Authorship Statement. Conception and design: J.P.M. and C.H.; acquisition of data: J.PM. and J.O.; analysis and interpretation: J.P.M., J.O., S.K., J.P., and C.H.; drafting manuscript: J.P.M.; revision of the manuscript: J.P.M., J.O., S.K., J.P., and C.H.; final approval: J.P.M., J.O., S.K., J.P., and C.H.
References
- sinoatrial conduction time
- patient referral
- metastatic malignant neoplasm to brain
- immunotherapy
- melanoma
- patient care team
- radiosurgery
- surgical procedures, operative
- systemic therapy
- systemic disease
- interdisciplinary treatment approach
- molecular targeted therapy
- whole brain irradiation
- neurologic oncology
- progression-free survival
- braf inhibitors
- systemic anti-cancer therapy dataset




