-
PDF
- Split View
-
Views
-
Cite
Cite
Katherine G Holste, Daniel A Orringer, Laser interstitial thermal therapy, Neuro-Oncology Advances, Volume 2, Issue 1, January-December 2020, vdz035, https://doi.org/10.1093/noajnl/vdz035
Close - Share Icon Share
Abstract
Laser interstitial thermal therapy (LITT) is becoming an increasingly popular technique for the treatment of brain lesions. More minimally invasive that open craniotomy for lesion resection, LITT may be more appropriate for lesions that are harder to access through an open approach, deeper lesions, and for patients who may not tolerate open surgery.
A search of the current primary literature on LITT for brain lesions on PubMed was performed. These studies were reviewed and updates on the radiological, pathological, and long-term outcomes after LITT for brain metastases, primary brain tumors, and radiation necrosis as well as common complications are included.
Larger extent of ablation and LITT as frontline treatment were potential predictors of favorable progression-free and overall survival for primary brain tumors. In brain metastases, larger extent of ablation was more significantly associated with survival benefit, whereas tumor size was a possible predictor. The most common complications after LITT are transient and permanent weakness, cerebral edema, hemorrhage, seizures, and hyponatremia.
Although the current literature is limited by small sample sizes and primarily retrospective studies, LITT is a safe and effective treatment for brain lesions in the correct patient population.
Extent of ablation, tumor size, and LITT as frontline treatment are potential predictors of progression-free and overall survival in brain metastases and primary brain tumors.
LITT may be a good option for patients with brain lesions that are deep seated or harder to access through craniotomy or who may not be good open surgery candidates.
Laser interstitial thermal therapy (LITT), first described for brain tumors by Sugiyama et al.1 in 1990, has been increasingly utilized in the treatment of primary brain tumors, metastases, and even radiation necrosis.2 Given its minimally invasive nature, compared with an open craniotomy, it has begun to gain ground as a treatment option, especially for patients who cannot safely undergo a major operation or who have deep-seated lesions that are challenging to safely remove. As LITT has matured as a technology for treating intracranial tumors, there has been a dramatic increase in the number of studies on LITT for brain lesions. Of the 245 studies on the topic indexed in PubMed, 117 have been published since 2016. Table 1 summarizes recently published important papers on LITT. This review will cover the principles of LITT as well as advances in understanding outcomes of this treatment for primary brain tumors, brain metastases (BM), and radiation necrosis (RN).
Summary of important studies of laser interstitial thermal therapy (LITT) included within this review paper
| Study . | (n) . | Study Type . | Finding . |
|---|---|---|---|
| Kamath et al. 20193 | 54 | Retrospective | There was no significant difference in steroid use at 1 month, progression-free survival or overall survival in patients treated with LITT for newly discovered or recurrent glioblastoma multiforme tumors |
| Hong et al. 20194 | 75 | Retrospective | Progression-free survival, overall survival and number of patients remaining on steroids were not significantly different between patients who underwent LITT or craniotomy for symptomatic lesions |
| Smith et al. 20165 | 25 | Retrospective | Mental health and vitality scores were higher at 1 year after LITT for biopsy-proven radiation necrosis |
| Hernandez et al. 20186 | 59 | Retrospective | Local control of radiation necrosis lesions occurred in 83.1% of patients, with 10 patients requiring further intervention for their lesion |
| Ahluwalia et al. 20197 | 42 | Prospective multi-center study | Progression was seen in 25% of completely ablated brain metastases, whereas 62.5% of incompletely ablated brain metastases progressed |
| Salehi et al. 20188 | 25 | Retrospective | Progression-free survival was significantly longer in patients with a brain metastasis > 5.62 cm3 (median volume in the study) and >97% lesion ablated |
| Ali et al. 20189 | 3 | Retrospective | Patients who received bevacizumab within 30 days after LITT did not experience any complications, although all died due to disease progression |
| Study . | (n) . | Study Type . | Finding . |
|---|---|---|---|
| Kamath et al. 20193 | 54 | Retrospective | There was no significant difference in steroid use at 1 month, progression-free survival or overall survival in patients treated with LITT for newly discovered or recurrent glioblastoma multiforme tumors |
| Hong et al. 20194 | 75 | Retrospective | Progression-free survival, overall survival and number of patients remaining on steroids were not significantly different between patients who underwent LITT or craniotomy for symptomatic lesions |
| Smith et al. 20165 | 25 | Retrospective | Mental health and vitality scores were higher at 1 year after LITT for biopsy-proven radiation necrosis |
| Hernandez et al. 20186 | 59 | Retrospective | Local control of radiation necrosis lesions occurred in 83.1% of patients, with 10 patients requiring further intervention for their lesion |
| Ahluwalia et al. 20197 | 42 | Prospective multi-center study | Progression was seen in 25% of completely ablated brain metastases, whereas 62.5% of incompletely ablated brain metastases progressed |
| Salehi et al. 20188 | 25 | Retrospective | Progression-free survival was significantly longer in patients with a brain metastasis > 5.62 cm3 (median volume in the study) and >97% lesion ablated |
| Ali et al. 20189 | 3 | Retrospective | Patients who received bevacizumab within 30 days after LITT did not experience any complications, although all died due to disease progression |
Summary of important studies of laser interstitial thermal therapy (LITT) included within this review paper
| Study . | (n) . | Study Type . | Finding . |
|---|---|---|---|
| Kamath et al. 20193 | 54 | Retrospective | There was no significant difference in steroid use at 1 month, progression-free survival or overall survival in patients treated with LITT for newly discovered or recurrent glioblastoma multiforme tumors |
| Hong et al. 20194 | 75 | Retrospective | Progression-free survival, overall survival and number of patients remaining on steroids were not significantly different between patients who underwent LITT or craniotomy for symptomatic lesions |
| Smith et al. 20165 | 25 | Retrospective | Mental health and vitality scores were higher at 1 year after LITT for biopsy-proven radiation necrosis |
| Hernandez et al. 20186 | 59 | Retrospective | Local control of radiation necrosis lesions occurred in 83.1% of patients, with 10 patients requiring further intervention for their lesion |
| Ahluwalia et al. 20197 | 42 | Prospective multi-center study | Progression was seen in 25% of completely ablated brain metastases, whereas 62.5% of incompletely ablated brain metastases progressed |
| Salehi et al. 20188 | 25 | Retrospective | Progression-free survival was significantly longer in patients with a brain metastasis > 5.62 cm3 (median volume in the study) and >97% lesion ablated |
| Ali et al. 20189 | 3 | Retrospective | Patients who received bevacizumab within 30 days after LITT did not experience any complications, although all died due to disease progression |
| Study . | (n) . | Study Type . | Finding . |
|---|---|---|---|
| Kamath et al. 20193 | 54 | Retrospective | There was no significant difference in steroid use at 1 month, progression-free survival or overall survival in patients treated with LITT for newly discovered or recurrent glioblastoma multiforme tumors |
| Hong et al. 20194 | 75 | Retrospective | Progression-free survival, overall survival and number of patients remaining on steroids were not significantly different between patients who underwent LITT or craniotomy for symptomatic lesions |
| Smith et al. 20165 | 25 | Retrospective | Mental health and vitality scores were higher at 1 year after LITT for biopsy-proven radiation necrosis |
| Hernandez et al. 20186 | 59 | Retrospective | Local control of radiation necrosis lesions occurred in 83.1% of patients, with 10 patients requiring further intervention for their lesion |
| Ahluwalia et al. 20197 | 42 | Prospective multi-center study | Progression was seen in 25% of completely ablated brain metastases, whereas 62.5% of incompletely ablated brain metastases progressed |
| Salehi et al. 20188 | 25 | Retrospective | Progression-free survival was significantly longer in patients with a brain metastasis > 5.62 cm3 (median volume in the study) and >97% lesion ablated |
| Ali et al. 20189 | 3 | Retrospective | Patients who received bevacizumab within 30 days after LITT did not experience any complications, although all died due to disease progression |
Principles of LITT
Operative Principles
LITT is performed stereotactically and generally in an interventional or intraoperative MRI suite while patients are under general anesthesia (Figure 1). Using preoperative imaging, the lesion is targeted, a simple burr hole is created and, when required, a needle biopsy may be performed before treatment for definitive tissue diagnosis. An optical fiber is then delivered to the desired depth, and laser light is interstitially delivered with low power over a long period of time to heat the tissues.2 Temperature within the lesion is measured throughout the procedure using MRI thermometry. There are two different systems on the market, both of which provide visual representations of temperature over a certain time to induce cell death, usually 43°C for 10 min.8
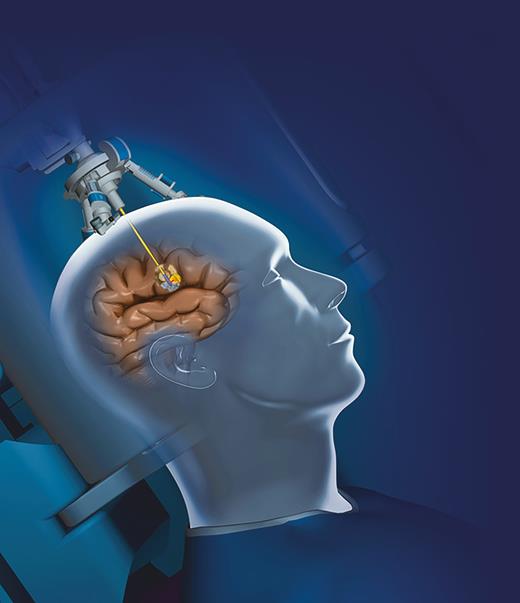
The optical fiber, attached to the laser light system, is passed through a burr hole to the lesion of interest.
Lesions that are more favorable to LITT are deep seated, spherical/oblong, and well circumscribed and have a trajectory that misses important structures like the ventricles or large arteries. Lesions that are not favorable are hypervascular, diffuse, and very large, as these would require multiple stages of treatment that could lead to more complications.3 Ideally the complete lesion would be covered by the thermal damage threshold (TDT) lines, although the presence of eloquent areas or heat sinks such as ventricles and blood vessels can limit lesion coverage.8 The size of the lesion also limits ablation. Four cases of malignant cerebral edema requiring hemicraniectomy have been described after LITT, with the volumes ranging from 29 to 70 cm3.10 For this reason, ablation in patients with large lesions should be completed in stages with adequate temporal spacing. In addition, anticoagulation should be reversed in patients before proceeding with LITT, given the known risk of hemorrhage.9
Physiology
Laser light is distributed throughout the tissue based on its optical properties. The type of fiber used and the shape of the fiber tip can affect light distribution as well. Optical properties of brain and tumor tissue vary; thus, different doses of light energy are required to achieve ablation.11 Absorption of light is affected by the tissue chromophores, generally hemoglobin and water, which can conduct heat to the rest of the tissue.2 Intralesional temperatures >100°C cause vaporization of water and carbonization of the tissues, and the steam then causes cavity formation. Conversely, an intralesional temperature between 45°C and 90°C is thought to cause irreversible coagulation of healthy and malignant tissues without vaporization. Given their different optical properties, an ablation temperature of 42.5–45.5°C is slightly more effective for tumor ablation while causing less damage to healthy tissue. This difference is thought to arise from the relatively fragile microvasculature and metabolic microenvironments of tumors.2
The histological response to LITT has been studied extensively in animal models. LITT created a zonal architecture in tissues heated to less than 100°C. The central zone around the fiber tip consists of densely coagulated tissue called the core. The middle zone was less coagulated, with some damage to cellular and subcellular membranes. Finally, the outer zone demonstrated marked edema amongst viable brain, neuronal shrinkage, and axonal swelling.12 In the first 48 h after LITT the total lesional volume increased, thought to be due to an increase in the outer edematous zone in particular. By 1 week after LITT, the central necrotic core started to retract and granulation tissue grew towards the center of the lesion as the volume of the lesion decreased.2,12
Imaging of Ablated Brain Lesions
The MR appearance of ablated tissues can be explained by the histological/pathological findings described by Tracz et al.12 MRI performed immediately after LITT demonstrates a centrally necrotic core surrounded by an outer area of edematous tissue corresponding to a hypo- or isointense core of the lesion surrounded by a hyperintense zone. Specifically, a pattern of concentric zones surrounding the light tract and necrotic core consisted of a thin enhancing rim and perilesional edema. Over time the zonal architecture disappeared and the thin enhancing rim subjectively reduced in size.12 In the initial studies of all lesions treated with LITT, lesions increased in size, described as the contrast-enhancing volume (CEv), 1 month after treatment by 0–45%. In one volumetric study of 18 patients,13 this initial period of lesion volume increase was followed by lesion shrinkage to 50% of the initial volume within 93 days after LITT. Peri-lesional edema tended to peak 1–3 days after the procedure and lasted 15–45 days in this same group.13
These patterns hold true in patients with BMs and RN as well. In a study of brain metastases after LITT, there was a characteristic pattern of expansion and shrinkage in about 2/3 of tumors. The lesion would expand as early as 1 h, reaching up to 300% of the pre-LITT CEv, and then shrinkage of the lesion would begin about 15–30 days after treatment. Fluid-attenuated inversion recovery volume would expand 1–3 days after treatment, peak at about 1 month, and then regress to pre-LITT volume generally within 6 months.10 Similarly on imaging after LITT for radiation necrosis, there was an increase in volume 1 day and 1–2 weeks postoperatively (220% and 430%, respectively), with shrinkage to 69% of the preoperative size by 6 months.10 Patterns in imaging after LITT are important to understand as clinicians follow their patients months after treatment.
Treatment Outcomes
Primary Brain Tumors
LITT has been described in a variety of primary brain tumors, predominantly glioblastoma multiforme (GBM), and can be used in both newly diagnosed and recurrent tumors. The progression-free survival (PFS) and overall survival (OS) are mixed in primary compared with recurrent GBMs after LITT. In one study of 58 LITT treatments in 54 patients with GBMs, those who underwent LITT as the frontline therapy had reduced PFS and OS compared to recurrent tumors (PFS: 3.6 vs. 7.3 months, respectively; OS: 9.1 vs. 11.8 months, respectively). The lesions treated with LITT as frontline therapy were chosen based on tumor location, age, and functional status of the patient. There was no difference in OS and PFS in patients with O[6]-methylguanine-DNA methyltransferase methylation in the 45 patients with known mutation status, and there were not enough patients with isocitrate dehydrogenase-1 R132 (IDH-1) mutations for survival analysis.3 In a smaller cohort of patients, 8 patients treated with up front LITT for GBM showed no reduction in CEv, whereas 5 of 13 recurrent GBMs demonstrated reduction in tumor volume. The cohort of patients treated with up front LITT were older and had larger tumors and more IDH wild-type mutations than the recurrent GBM group.14 A recent multi-institutional retrospective study at 4 centers comparing biopsy-proven GBM treated with LITT compared with biopsy only before chemotherapy and radiation found multiple predictors of PFS and OS.15 Tumor volume less than 11 cm3 and age less than 70 were associated with significantly improved OS (Hazard ratio 4.89 and 4.32, respectively). Extent of coverage, in this study divided into three groups based on the percentage of tumor covered, was significantly associated with improved PFS on multivariate analysis. PFS was not significantly different between patients treated with LITT vs. biopsy only before chemoradiation.15 Currently it seems that patients treated with LITT frontline for GBM have worse PFS and OS compared with recurrent GBM, but this could be confounded by other important variables such as tumor mutations, size, location, and the functional status of the patient preoperatively.
The extent of ablation would appear to be another significant predictor of outcomes after LITT. A review of 6 studies,16 including 63 patients with recurrent high-grade gliomas, found that more complete ablation of the tumor (less than 0.05 cm3 of contrast-enhancing tumor not ablated) was associated with increased OS of 9.7 compared with 4.6 months. Mean coverage was 78% of the enhancing lesion.16 On the other hand, a meta-analysis of 4 papers on LITT for newly diagnosed malignant gliomas, including primarily GBMs, anaplastic oligodendrogliomas, and anaplastic astrocytomas, found in 25 patients that there was no correlation between the extent of ablation or tumor volume and outcome. Mean extent of ablation was 82.9%.17 Given the small number of patients in these studies and the heterogeneity of high-grade gliomas included, additional work is required to study the potential benefits of LITT in GBM.
One theoretical benefit of LITT is that this minimally invasive technique may be a better fit with rapid initiation of radiation and chemotherapy than a large craniotomy. Some investigators suggest that LITT can be safely integrated into cycles of bevacizumab, immunotherapy, or chemo without interruptions of scheduled cycles.10 Generally bevacizumab is not started until 4–6 weeks after craniotomy due to increased risk of hemorrhage. Three patients with GBM were started on bevacizumab 13–20 days after LITT without hemorrhagic complications. Unfortunately, all three died due to local disease progression.9
Metastatic Brain Tumors
LITT is also used to treat BMs up front and in the context of recurrence. Overall PFS for BMs after LITT is promising. One prospective multicenter study of 42 patients, an open-label phase 2 study of one of the two commercially available LITT systems, reported PFS of 54% at 12 weeks and 62% at 26 weeks; however, only 16 patients completed the follow-up and one patient was started on chemotherapy between assessments.7 Median overall survival ranged from 5.8 to 19.6 months. One-year survival was between 0% and 65% in a systematic review of 13 papers.10 When comparing LITT with open craniotomy for tumor resection for BMs, there was no significant difference in the PFS, OS, or the number of patients on steroids by 1 month. Hong et al.4 compared a series of 41 patients who underwent craniotomy with 34 who underwent LITT for comparable lesions and found that more patients had resolution of their preoperative symptoms with craniotomy compared with LITT (26 of 29 compared with 20 of 23, respectively). The numbers of patients who could be weaned off steroids at 1 month (47.4% compared with 34.8%), PFS at 2 years (61.1% compared with 60%), and OS (49.5% compared with 56.6%) did not differ significantly between the two groups. Even when accounting for size of lesion, there was no difference in PFS and OS in lesions that were smaller than 3 cm.4
The degree of ablation seems to be more strongly associated with outcome in the BM data. In the prospective multicenter study noted above, local disease progression was seen in 25% of completely ablated BMs compared with 62.5% of incompletely ablated BMs.7 In a systematic review of 13 publications using LITT after radiation therapy for BMs, local control and PFS occurred more often in completely ablated lesions. Local control, defined as a smaller postoperative CEv when compared with the preoperative CEv, occurred in 60% of partially ablated lesions and 85% of completely ablated lesions at 6 months. There were no cases of progression when the lesion was completely ablated.10 Data in these studies combine BMs treated with LITT first and lesions that underwent prior craniotomy and/or radiosurgery. In a retrospective study of 25 LITT procedures on 24 patients with BM, PFS was significantly longer when greater than 97% of the tumor was ablated. Furthermore, tumor size was found to be a significant predictor of PFS; patients with tumors larger than 5.62 cm3, the median volume of the lesions in this cohort, had a shorter PFS.8 For metastatic brain tumors, it appears that degree of ablation is associated with significantly longer PFS. The significance of tumor size on PFS still remains in question given the limited data.
LITT is not restricted to supratentorial lesions. Although the data for cerebellar lesions are less robust, in 2 small cohorts of posterior fossa lesions treated with LITT, outcomes appeared to be favorable. A case series of 4 cerebellar metastases found that all 4 patients had resolution of their preoperative symptoms without need for prolonged steroid use. The lesions increased on average 486.9% of the preoperative CEv and required 294.5 days to return back to the initial size calculated by extrapolated time. There was also a decrease in the average edema volume from 17.8 cm3 to 3.4 cm3 at last follow up.18 Another study of 8 posterior fossa lesions, including 2 RNs, 3 BMs, and 3 primary brain tumors (2 pilocytic astrocytomas and 1 GBM) found that 1 needed open resection 7.7 months after LITT and 2 had disease progression (GBM and one BM); all other lesions remained stable or diminished at median follow-up of 14.8 months.19 It appears that posterior fossa lesions can be treated with LITT, although their location should be taken into account as significant lesion volume expansion can be expected after treatment.
Radiation Necrosis
RN is a known complication after stereotactic radiosurgery and occurs in about 6.7–25.8% of patients. RN lesions can progress irreversibly or may stabilize without need for treatment, but predicting which patients will fall into either category is extremely difficult.6 In addition, distinguishing between tumor progression and RN on imaging is very challenging, even with the increasing utilization of advanced imaging such as perfusion MRI. In patients who could not tolerate open surgical resection, LITT has also been used successfully for symptomatic radiation necrosis.
Patients with RN respond very well to LITT in regard to symptom control and PFS. In a prospective multicenter study, 19 patients found to have RN on biopsy before LITT had 100% PFS at 12 weeks and 91% PFS at last follow-up.7 In one study of biopsy-proved RN, PFS was significantly longer for patients who previously had grade 2 tumors compared with BMs, grade 3 or grade 4 primary CNS tumors. At 12 months postoperative, patients had improved mental status and vitality when surveyed and only 5 of the 25 needed treatment with bevacizumab after LITT.5 In a study of 10 patients with progressive neurologic deficits or steroid dependence caused by RN, 7 patients were able to discontinue steroids completely following LITT treatment.20 Unfortunately, less patient data have been published on LITT for RN than for other lesions, and much of this is heterogenous retrospective observational data. LITT may be a good option for patients with RN who require long-term steroids or have progressive neurologic symptoms, as they tend to respond well to LITT and are often weaned off steroids.
Complications
Adverse effects have been cited to occur in as many as 83% of patients undergoing LITT.7 The most common complications of LITT include temporary or permanent neurologic deficits (8.82–35.5% and 2.17–7.14%, respectively),,7,10,17,20 cerebral edema,3,7,8,21 seizures,8,20,21 and intracranial hemorrhage (0.98–14.2%).7,10 Hemorrhage is thought to result from passage of the fiber or stereotactic biopsy. Other less common complications include hyponatremia,17 hydrocephalus, headache,7 malignant cerebral edema,17 and meningitis.21 Catheter misplacement has been quoted as high as 14.2%, but this problem has likely been reduced as stereotaxis and surgical planning have improved.10 In a series of 120 patients, mortality occurred in 3 cases.21
Post-LITT motor weakness has been of great interest. A study of 80 patients who underwent preoperative diffusion tensor imaging before LITT examined the overlap between the treatment lines and the motor fibers. Postoperative motor deficits were seen in 14 patients: 11 permanent and 3 temporary cases of weakness. There was a significant difference between those who developed motor deficits and those without deficits in the volume and surface area of the corticospinal tract covered by the TDT lines.22 These data suggest that coverage of the corticospinal tract during surgical planning of lesion ablation is associated with increased risk of temporary or permanent motor weakness.
Given the amount of cerebral edema after LITT in the acute period, corticosteroid use is common. Postoperative steroid use varies by surgeon and can range from 1 week to chronic maintenance therapy. In a small study of 8 patients who underwent LITT and RT in close succession, the median time until steroids could be weaned was 60.5 days. Three patients required prolonged use of steroids (>65 days).23 Other studies have reported patients weaning within 2–4 weeks after LITT.4
Conclusions
An increasingly robust body of literature has been published on the safety and efficacy of LITT for brain lesions. Like many clinical studies in brain tumor patients, those on LITT are generally retrospective observational studies with small numbers of patients. Nonetheless, published data suggest that LITT may be as effective as craniotomy in select lesions, with respect to PFS, OS, and steroid use. Factors that may increase PFS and OS include the extent of ablation for BMs, tumor size and whether the lesion is a recurrent or primary brain tumor. Adverse events are common in LITT. The most frequent serious complications include motor deficits, hemorrhage, cerebral edema, and seizures. Overall, LITT appears to be well-tolerated and presents a minimally invasive treatment option for increasing local control and PFS in patients whose lesions are not accessible by craniotomy or who are otherwise poor surgical candidates.
Funding
None
Conflict of interest statement. None declared.




