-
PDF
- Split View
-
Views
-
Cite
Cite
Masayuki Kanamori, Hirokazu Takami, Tomonari Suzuki, Teiji Tominaga, Jun Kurihara, Shota Tanaka, Seiji Hatazaki, Motoo Nagane, Masahide Matsuda, Atsuo Yoshino, Manabu Natsumeda, Masayoshi Yamaoka, Naoki Kagawa, Yukinori Akiyama, Junya Fukai, Tetsuya Negoto, Ichiyo Shibahara, Kazuhiro Tanaka, Akihiro Inoue, Mitsuhiro Mase, Takahiro Tomita, Daisuke Kuga, Noriyuki Kijima, Tadateru Fukami, Yukiko Nakahara, Atsushi Natsume, Koji Yoshimoto, Dai Keino, Tsutomu Tokuyama, Kenichiro Asano, Kenta Ujifuku, Hiroshi Abe, Mitsutoshi Nakada, Ken-ichiro Matsuda, Yoshiki Arakawa, Naokado Ikeda, Yoshitaka Narita, Naoki Shinojima, Atsushi Kambe, Masahiko Nonaka, Shuichi Izumoto, Yu Kawanishi, Kohei Kanaya, Sadahiro Nomura, Kohei Nakajima, Shohei Yamamoto, Keita Terashima, Koichi Ichimura, Ryo Nishikawa, Necessity for craniospinal irradiation of germinoma with positive cytology without spinal lesion on MR imaging—A controversy, Neuro-Oncology Advances, Volume 3, Issue 1, January-December 2021, vdab086, https://doi.org/10.1093/noajnl/vdab086
Close - Share Icon Share
Abstract
Cerebrospinal fluid (CSF) cytology and spinal MR imaging are routinely performed for staging before treatment of intracranial germinoma. However, the interpretation of the results of CSF cytology poses 2 unresolved clinical questions: (1) Does positive CSF cytology correlate with the presence of spinal lesion before treatment? and (2) Is craniospinal irradiation (CSI) necessary for patients with positive CSF cytology in the absence of spinal lesion?
Multicenter retrospective analyses were performed based on a questionnaire on clinical features, spinal MR imaging finding, results of CSF cytology, treatments, and outcomes which was sent to 86 neurosurgical and 35 pediatrics departments in Japan. Pretreatment frequencies of spinal lesion on MR imaging were compared between the patients with positive and negative cytology. Progression-free survival (PFS) rates were compared between patients with positive CSF cytology without spinal lesion on MR imaging treated with CSI and with whole brain or whole ventricular irradiation (non-CSI).
A total of 92 germinoma patients from 45 institutes were evaluated by both CSF cytology and spinal MR images, but 26 patients were excluded because of tumor markers, the timing of CSF sampling or incomplete estimation of spinal lesion. Of the remaining 66 germinoma patients, spinal lesions were equally identified in patients with negative CSF cytology and positive cytology (4.9% and 8.0%, respectively). Eleven patients treated with non-CSI had excellent PFS comparable to 11 patients treated with CSI.
CSI is unnecessary for germinoma patients with positive CSF cytology without spinal lesions on MR imaging.
Positive CSF cytology was not correlated with spinal lesions on MR imaging.
PFS rate of patients with non-CSI was comparable to that of cases receiving CSI.
CSI is unnecessary for cases with positive CSF cytology without spinal lesions.
Interpretation of the results of CSF cytology poses 2 unresolved clinical questions: (1) Does positive CSF cytology correlate with the presence of spinal lesion? and (2) Is craniospinal irradiation (CSI) necessary for patients with positive CSF cytology in the absence of spinal lesion? This multicenter retrospective study demonstrated the following results: (1) Spinal lesions on MR imaging were equally identified in patients with negative CSF cytology and positive cytology and (2) No difference in PFS rates was found between patients with positive CSF cytology without spinal lesion on MR imaging treated by CSI and by radiation therapy not covering the craniospinal axis. We concluded that positive CSF cytology did not carry high risk for the presence of spinal disease and that CSI is not necessary for germinoma patients with positive CSF cytology without spinal lesions on MR imaging.
The Delphi consensus statements on the management of intracranial germ cell tumors (GCTs) reported in 2015 failed to reach agreement on several areas.1 For example, the statement suggesting that only craniospinal irradiation (CSI) was sufficient treatment for metastatic germinoma documented on craniospinal imaging was rejected, because acceptance would preclude the use of chemotherapy for reduction of radiation dose and long-term morbidity. Therefore, the statement was revised to suggest only CSI was sufficient to secure excellent overall survival, but pre-irradiation chemotherapy might allow reduction of the radiation dose. This statement was supported by 68% of participants but rejected because of the lack of evidence. Consequently, standard treatment strategies for metastatic germinoma documented on craniospinal imaging have not been established.
In addition, the definition of metastatic disease is controversial. According to the modified Chang staging system,2 which is widely used in the field of pediatric neuro-oncology, cerebrospinal fluid (CSF) cytology and spinal MR imaging are routinely examined for metastatic disease before treatment for various types of pediatric CNS tumors.3–7 In this system, patients with positive cytology are diagnosed with metastatic disease, and receive more intense treatments even in the absence of macroscopic spinal lesion.8 However, conflicting interpretations of positive cytology in germinoma patients have been reported in various clinical trials and countries. The SIOP CNS GCT 96 and GCT II trial in Europe regarded positive cytology as metastases and the patients were treated with a regimen including CSI.3 Similarly, the ACNS1123 trial in North America focused on patients with localized germinoma and excluded patients with positive cytology. In contrast, the current phase II study for newly diagnosed intracranial GCTs (UMIN000004528) in Japan does not vary the treatment strategy according to the cytology findings because of the rarity of spinal disease at initial treatment.3,9–11
The present multicenter retrospective analysis was designed to elucidate 2 clinical questions about the interpretation of CSF cytology in germinoma patients: (1) Does positive CSF cytology correlate with the presence of spinal lesion on MR imaging before treatment? and (2) Is CSI necessary to prevent recurrence in patients with positive CSF cytology without spinal lesion on MR imaging.
Methods
This multicenter retrospective study was conducted after obtaining the necessary ethical clearance from the institutional ethics board for study on human subjects in each institution. For this retrospective study, all institutional ethics boards waived the requirement for written informed consent. Patients were provided the opportunity to opt out of participation in this study by signaling their opposition. Data were collected for newly diagnosed, histologically verified or clinically diagnosed, germinoma patients from January 1990 to December 2015, in whom both CSF cytology and spinal MR imaging were evaluated before treatment and tissue sampling. Criteria for inclusion were histologically diagnosed pure germinoma, germinoma with syncytiotrophoblastic giant cells, or germinoma/mature teratoma, and clinically diagnosed germinoma based on clinical findings of age, sex, radiological appearance, and negative or modestly elevated tumor markers that fulfilled α-fetoprotein (AFP) <10 ng/mL, human chorionic gonadotropin (HCG) <50 mIU/mL, and human chorionic gonadotropin-β subunit (HCG-β) <5 ng/mL in serum and CSF.2,12,13
A questionnaire on clinical features including age, sex, levels of pretreatment serum and CSF tumor markers, histological diagnosis, details of pretreatment brain and spinal MR imaging, methods of CSF sampling (lumbar tap or ventricular drainage), diagnosis of cytology, details of radiation and chemotherapy, and recurrence and survival at last follow-up examination were sent to 121 institutes in Japan, including 86 neurosurgical and 35 pediatrics departments.14 These institutes belong to the intracranial Germ Cell Tumor Consortium or Japan Children’s Cancer Group or are training hospitals of the Japan Neurosurgical Society.
The findings of CSF cytology were classified according to the Papanicolaou classification15 which is commonly used in Japan as follows: Class I, absence of atypical or abnormal cells; class II, atypical, but no evidence of malignancy; class III, suggestive, but not conclusive for malignancy; class IV, strongly suggestive for malignancy; and class V, conclusive for malignancy. In this study, classes IV and V were regarded as positive CSF cytology, whereas classes I, II, and III as negative CSF cytology.
Presence of spinal lesions on MR imaging was compared between patients with positive and negative cytology. We analyzed the timing, sequence, and magnetic field intensity, and included patients in whom spinal disease was detected by gadolinium-enhanced T1-weighted images or T1-, T2-, and diffusion-weighted MR images if the administration of gadolinium was impossible.
Progression-free survival (PFS) rate of patients treated with CSI was compared to that of patients treated with whole ventricle or whole brain irradiation (non-CSI) in patients with positive CSF cytology without spinal lesion on MR imaging. Patients with spinal lesions on MR imaging or who underwent radiation therapy to the local field not covering the whole ventricle or only chemotherapy were excluded from this analysis because the former should be treated with CSI and the latter had high rate of recurrence.16
Categorical and continuous variables were compared using Fisher’s exact test and Student’s t test, respectively. The PFS rate of patients treated with or without CSI was analyzed with Kaplan–Meier analysis. P-values below .05 were regarded as statistically significant.
Results
Patient Demographics
A total of 45 institutes (37.2%) answered our inquiry and reported 92 patients evaluated by spinal MR imaging and CSF cytology. Our criteria excluded 26 patients: 8 with positive tumor markers or no estimation of tumor markers, 10 with CSF analysis after tissue sampling or no information on the timing of CSF analysis, and 8 with incomplete estimation of spinal lesion on MR imaging (Figure 1).

Inclusion and exclusion criteria of germinoma patients in this study.
Therefore, the analysis of the correlation between positive CSF cytology and presence of spinal disease included 66 patients (Figure 1), 52 males and 14 females aged 7–40 years (median 16 years) at diagnosis. CSF samples were obtained from lumbar puncture in 25 (38%) patients and the ventricles in 41 (62%). The findings of cytology were negative in 41 patients (class I in 11, class II in 9, and class III in 21), and positive in 25 patients (class IV in 7 and class V in 18) (Table 1). No differences in distribution of the age at diagnosis, locations of the primary site, method of CSF sampling, proportion of patients with HCG or HCG-β elevation, and histological diagnosis were found between the 2 groups, but females and patients without histological diagnosis were more frequent in patients with positive CSF cytology (Table 1).
Demographics of Germinoma Patients Undergoing Both Cytology and Spinal MR Imaging
| . | Classes I, II, and III (n = 41) . | Classes IV and V (n = 25) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–34 (16) | 7–40 (16) | .59b |
| Sex, male:female | 36:5 | 16:9 | .039a |
| Location | .40a | ||
| Pineal | 14 | 6 | |
| Neurohypophysis | 6 | 3 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 18 | 15 | |
| Pineal/basal ganglia | 2 | 0 | |
| Unknown | 1 | 0 | |
| CSF sampling | .80a | ||
| Lumbar tap | 15 | 10 | |
| Ventricle | 26 | 15 | |
| HCG/HCG-β elevation | .12a | ||
| Yes | 19 | 17 | |
| No | 22 | 8 | |
| Verification of histological diagnosis | .0036a | ||
| Unverified | 1 | 7 | |
| Verified | 40 | 18 | |
| Histological diagnosis | .71a | ||
| Pure germinoma | 37 | 16 | |
| Germinoma with STGC | 2 | 2 | |
| Germinoma/mature teratoma | 1 | 0 |
| . | Classes I, II, and III (n = 41) . | Classes IV and V (n = 25) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–34 (16) | 7–40 (16) | .59b |
| Sex, male:female | 36:5 | 16:9 | .039a |
| Location | .40a | ||
| Pineal | 14 | 6 | |
| Neurohypophysis | 6 | 3 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 18 | 15 | |
| Pineal/basal ganglia | 2 | 0 | |
| Unknown | 1 | 0 | |
| CSF sampling | .80a | ||
| Lumbar tap | 15 | 10 | |
| Ventricle | 26 | 15 | |
| HCG/HCG-β elevation | .12a | ||
| Yes | 19 | 17 | |
| No | 22 | 8 | |
| Verification of histological diagnosis | .0036a | ||
| Unverified | 1 | 7 | |
| Verified | 40 | 18 | |
| Histological diagnosis | .71a | ||
| Pure germinoma | 37 | 16 | |
| Germinoma with STGC | 2 | 2 | |
| Germinoma/mature teratoma | 1 | 0 |
STGC, syncytiotrophoblastic giant cell.
aFisher’s exact test;
bStudent’s t-test.
Demographics of Germinoma Patients Undergoing Both Cytology and Spinal MR Imaging
| . | Classes I, II, and III (n = 41) . | Classes IV and V (n = 25) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–34 (16) | 7–40 (16) | .59b |
| Sex, male:female | 36:5 | 16:9 | .039a |
| Location | .40a | ||
| Pineal | 14 | 6 | |
| Neurohypophysis | 6 | 3 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 18 | 15 | |
| Pineal/basal ganglia | 2 | 0 | |
| Unknown | 1 | 0 | |
| CSF sampling | .80a | ||
| Lumbar tap | 15 | 10 | |
| Ventricle | 26 | 15 | |
| HCG/HCG-β elevation | .12a | ||
| Yes | 19 | 17 | |
| No | 22 | 8 | |
| Verification of histological diagnosis | .0036a | ||
| Unverified | 1 | 7 | |
| Verified | 40 | 18 | |
| Histological diagnosis | .71a | ||
| Pure germinoma | 37 | 16 | |
| Germinoma with STGC | 2 | 2 | |
| Germinoma/mature teratoma | 1 | 0 |
| . | Classes I, II, and III (n = 41) . | Classes IV and V (n = 25) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–34 (16) | 7–40 (16) | .59b |
| Sex, male:female | 36:5 | 16:9 | .039a |
| Location | .40a | ||
| Pineal | 14 | 6 | |
| Neurohypophysis | 6 | 3 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 18 | 15 | |
| Pineal/basal ganglia | 2 | 0 | |
| Unknown | 1 | 0 | |
| CSF sampling | .80a | ||
| Lumbar tap | 15 | 10 | |
| Ventricle | 26 | 15 | |
| HCG/HCG-β elevation | .12a | ||
| Yes | 19 | 17 | |
| No | 22 | 8 | |
| Verification of histological diagnosis | .0036a | ||
| Unverified | 1 | 7 | |
| Verified | 40 | 18 | |
| Histological diagnosis | .71a | ||
| Pure germinoma | 37 | 16 | |
| Germinoma with STGC | 2 | 2 | |
| Germinoma/mature teratoma | 1 | 0 |
STGC, syncytiotrophoblastic giant cell.
aFisher’s exact test;
bStudent’s t-test.
The effect of CSI on the prevention of recurrence in patients with positive CSF cytology without spinal lesions on MR imaging was analyzed in 22 of the 25 patients with positive cytology, excluding 2 patients who had spinal disease on MR images and 1 patient who received radiation therapy to the local site (Figure 1), of whom 11 patients were treated with CSI, whereas 11 were treated with non-CSI (Table 2). Chemotherapies of platinum-based regimens were administered in similar numbers in both groups. Age at diagnosis, sex, location of the primary site, method of CSF sampling, proportion of patients with HCG or HCG-β elevation, histological diagnosis, and radiation dosage to the primary site, whole ventricle, and whole brain were also similar in both groups. Follow-up periods were not significantly different between the 2 groups (Table 2). Patients treated with non-CSI received radiation therapy to the whole ventricle in 5 cases and the whole brain in 6.
Demographics of Germinoma Patients With Positive Cytology Treated with CSI and Non-CSI
| . | CSI (n = 11) . | non-CSI (n = 11) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–40 (12) | 9–28 (16) | .94b |
| Sex, male:female | 7:3 | 7:4 | 1.00a |
| Location | .15a | ||
| Pineal | 1 | 4 | |
| Neurohypophysis | 1 | 2 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 9 | 4 | |
| CSF sampling | .66 | ||
| Lumbar tap | 5 | 3 | |
| Ventricle | 6 | 8 | |
| HCG/HCG-β elevation | .18a | ||
| Yes | 9 | 5 | |
| No | 2 | 6 | |
| Verification of histological diagnosis | 1.00a | ||
| Unverified | 3 | 4 | |
| Verified | 8 | 7 | |
| Histological diagnosis | .47a | ||
| Pure germinoma | 8 | 6 | |
| Germinoma with STGC | 0 | 1 | |
| Radiation field | |||
| Whole ventricle | 0 | 5 | |
| Whole brain | 0 | 6 | |
| CSI | 11 | 0 | |
| Radiation dose, range (median), Gy | |||
| Primary site | 24–52 (44) | 24–51 (40) | .39b |
| Whole ventricle | 23–44 (24) | 24–45 (30) | .57b |
| Whole brain | 23–32 (24) | 10–45 (30) | .31b |
| Craniospinal | 18–35 (24) | 0 | |
| Chemotherapy | 1.00a | ||
| Yes | 8 | 7 | |
| No | 3 | 4 | |
| Follow-up periods, range (median), months | 12–265 (101) | 2–228 (75) | .25b |
| . | CSI (n = 11) . | non-CSI (n = 11) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–40 (12) | 9–28 (16) | .94b |
| Sex, male:female | 7:3 | 7:4 | 1.00a |
| Location | .15a | ||
| Pineal | 1 | 4 | |
| Neurohypophysis | 1 | 2 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 9 | 4 | |
| CSF sampling | .66 | ||
| Lumbar tap | 5 | 3 | |
| Ventricle | 6 | 8 | |
| HCG/HCG-β elevation | .18a | ||
| Yes | 9 | 5 | |
| No | 2 | 6 | |
| Verification of histological diagnosis | 1.00a | ||
| Unverified | 3 | 4 | |
| Verified | 8 | 7 | |
| Histological diagnosis | .47a | ||
| Pure germinoma | 8 | 6 | |
| Germinoma with STGC | 0 | 1 | |
| Radiation field | |||
| Whole ventricle | 0 | 5 | |
| Whole brain | 0 | 6 | |
| CSI | 11 | 0 | |
| Radiation dose, range (median), Gy | |||
| Primary site | 24–52 (44) | 24–51 (40) | .39b |
| Whole ventricle | 23–44 (24) | 24–45 (30) | .57b |
| Whole brain | 23–32 (24) | 10–45 (30) | .31b |
| Craniospinal | 18–35 (24) | 0 | |
| Chemotherapy | 1.00a | ||
| Yes | 8 | 7 | |
| No | 3 | 4 | |
| Follow-up periods, range (median), months | 12–265 (101) | 2–228 (75) | .25b |
CSI, craniospinal irradiation; STGC, syncytiotrophoblastic giant cell.
aFisher’s exact test;
bStudent’s t-test.
Demographics of Germinoma Patients With Positive Cytology Treated with CSI and Non-CSI
| . | CSI (n = 11) . | non-CSI (n = 11) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–40 (12) | 9–28 (16) | .94b |
| Sex, male:female | 7:3 | 7:4 | 1.00a |
| Location | .15a | ||
| Pineal | 1 | 4 | |
| Neurohypophysis | 1 | 2 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 9 | 4 | |
| CSF sampling | .66 | ||
| Lumbar tap | 5 | 3 | |
| Ventricle | 6 | 8 | |
| HCG/HCG-β elevation | .18a | ||
| Yes | 9 | 5 | |
| No | 2 | 6 | |
| Verification of histological diagnosis | 1.00a | ||
| Unverified | 3 | 4 | |
| Verified | 8 | 7 | |
| Histological diagnosis | .47a | ||
| Pure germinoma | 8 | 6 | |
| Germinoma with STGC | 0 | 1 | |
| Radiation field | |||
| Whole ventricle | 0 | 5 | |
| Whole brain | 0 | 6 | |
| CSI | 11 | 0 | |
| Radiation dose, range (median), Gy | |||
| Primary site | 24–52 (44) | 24–51 (40) | .39b |
| Whole ventricle | 23–44 (24) | 24–45 (30) | .57b |
| Whole brain | 23–32 (24) | 10–45 (30) | .31b |
| Craniospinal | 18–35 (24) | 0 | |
| Chemotherapy | 1.00a | ||
| Yes | 8 | 7 | |
| No | 3 | 4 | |
| Follow-up periods, range (median), months | 12–265 (101) | 2–228 (75) | .25b |
| . | CSI (n = 11) . | non-CSI (n = 11) . | P-value . |
|---|---|---|---|
| Age at diagnosis, range (median), years | 7–40 (12) | 9–28 (16) | .94b |
| Sex, male:female | 7:3 | 7:4 | 1.00a |
| Location | .15a | ||
| Pineal | 1 | 4 | |
| Neurohypophysis | 1 | 2 | |
| Basal ganglia | 0 | 1 | |
| Bifocal | 9 | 4 | |
| CSF sampling | .66 | ||
| Lumbar tap | 5 | 3 | |
| Ventricle | 6 | 8 | |
| HCG/HCG-β elevation | .18a | ||
| Yes | 9 | 5 | |
| No | 2 | 6 | |
| Verification of histological diagnosis | 1.00a | ||
| Unverified | 3 | 4 | |
| Verified | 8 | 7 | |
| Histological diagnosis | .47a | ||
| Pure germinoma | 8 | 6 | |
| Germinoma with STGC | 0 | 1 | |
| Radiation field | |||
| Whole ventricle | 0 | 5 | |
| Whole brain | 0 | 6 | |
| CSI | 11 | 0 | |
| Radiation dose, range (median), Gy | |||
| Primary site | 24–52 (44) | 24–51 (40) | .39b |
| Whole ventricle | 23–44 (24) | 24–45 (30) | .57b |
| Whole brain | 23–32 (24) | 10–45 (30) | .31b |
| Craniospinal | 18–35 (24) | 0 | |
| Chemotherapy | 1.00a | ||
| Yes | 8 | 7 | |
| No | 3 | 4 | |
| Follow-up periods, range (median), months | 12–265 (101) | 2–228 (75) | .25b |
CSI, craniospinal irradiation; STGC, syncytiotrophoblastic giant cell.
aFisher’s exact test;
bStudent’s t-test.
Correlation Between Positive CSF Cytology and Spinal Disease on MR Images
Pretreatment spinal lesion was estimated by gadolinium-enhanced T1-weighted MR imaging in all patients except one. One patient was evaluated by T1-, T2-, and diffusion-weighted MR imaging because gadolinium could not be administered due to asthma. Magnetic field intensity was 0.5 T in 1 patient, 1.5 T in 59, 3.0 T in 4, and unknown in 2. Spinal lesions were identified in 2 (4.9%) of 41 patients with negative CSF cytology and in 2 (8.0%) of 25 patients with positive cytology. The CSF cytology of these 4 patients was class II in 2 and class V in 2. The proportion of spinal disease was not significantly different between the 2 groups (Fisher’s exact test, P = 0.63)
Treatment Outcomes of the Patients Treated With or Without CSI
To clarify the indication for CSI in patients with positive cytology without spinal disease on MR images, PFS rates were compared between the patients treated with and without CSI. The follow-up periods were 12–265 months (median, 101 months) in the patients treated with CSI and 2–228 months (median, 75 months) in the patients treated with non-CSI (Table 2).
Recurrence was found in 1 of 22 patients with positive cytology without spinal lesion on MR images during the follow-up period. This patient was a 7-year-old female with pineal and neurohypophyseal tumor with modestly elevated CSF HCG/HCG-β (CSF HCG, 29.0 mIU/mL; HCG-β, 0.6 ng/mL). She had positive CSF cytology obtained by lumbar tap before treatment and received CSI and boost irradiation to the local site and chemotherapy. She had recurrence at the primary site 8 months after initiation of treatment and was treated by chemotherapy. Thereafter, she has survived without recurrence for 195 months after diagnosis. The PFS rate did not reach statistical significance between the CSI and non-CSI groups (log-rank test, P = 0.37; Figure 2).
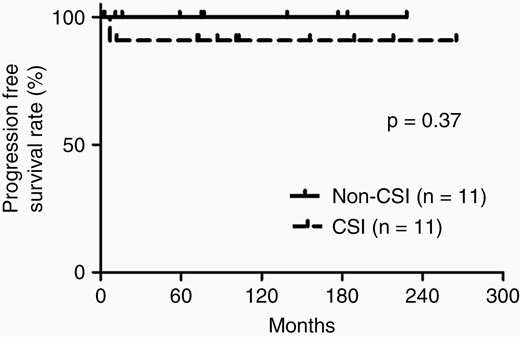
Kaplan–Meier analysis demonstrating the PFS rates of patients treated with craniospinal irradiation (CSI) and non-CSI.
Discussion
The present study demonstrated that positive CSF cytology was not correlated with the presence of spinal lesion on MR images, and that non-CSI achieved excellent PFS, comparable to that of CSI in germinoma patients with positive CSF cytology without spinal lesion on MR imaging.
CSF cytology and spinal MR imaging are frequently used in clinical practice and clinical trials for the detection of distant lesions. However, correlations between the findings of CSF cytology and spinal lesions, and the clinical significance of CSF cytology remain unclear. A study of medulloblastoma and primitive neuroectodermal tumors demonstrated that spinal lesions on MR images were more frequently found in patients with positive cytology than those with negative cytology (52% vs 11%; P < .001).17 Another study of various pediatric CNS tumors demonstrated that spinal lesions developed equally in patients with positive and negative cytology (25% vs 15%; not significant).17 These differences might be attributed to the type of tumor, as the former study included only medulloblastoma or primitive neuroectodermal tumors,18 whereas the latter had various types of pediatric CNS tumors including ependymomas, choroid plexus tumors, atypical teratoid rhabdoid tumors, astrocytic or oligodendroglial tumors, and GCTs, in addition to medulloblastoma or primitive neuroectodermal tumors.17 Positive CSF cytology and presence of spinal lesions are rare events with incidences of 1.1%–8.9% and 0%–6.3% in germinoma, respectively,2,9–11,19–21 so few reports have discussed the incidence of spinal disease in patients with positive cytology in GCTs. A previous case series analyzed the correlations between CSF cytology and spinal lesion.20 In that study, spinal lesions were mainly assessed by neurological examination, but MR imaging and myelography were also performed in only 6 and 2 cases, respectively, among the 42 patients. Spinal lesions were found only in patients with positive cytology (2 of 22 patients), and not in 20 patients with negative cytology.20 Our larger series based on the findings of spinal MR images found no difference in the frequency of spinal lesions between patients with positive and negative cytology. The previous study may have underestimated the occurrence of spinal disease, but the frequency of spinal disease in germinoma patients with positive cytology was not significantly higher than that of patients with negative cytology.
Spinal lesions are rare in newly diagnosed germinoma9,21 and the recurrence rate of isolated spinal lesions showed no difference between CSI and non-CSI,22 so CSI is considered to be unnecessary for patients with negative cytology without spinal lesions on MR imaging. In contrast, the risk and benefit of CSI for patients with positive cytology without spinal lesion remains unclear. One retrospective study demonstrated that excellent tumor control was achieved with whole brain irradiation in 2 germinoma patients with positive cytology without spinal lesions.20 Consistent with that finding, this study demonstrated that the PFS rate of patients with non-CSI was comparable to that of patients receiving CSI and that no patients suffered from spinal recurrence during the median follow-up period of 75 months. Therefore, excellent PFS can be expected by treatment with non-CSI in patients with positive cytology without spinal lesions, although our and previous studies had limited numbers of cases. CSI carries the risks of the following complications: infertility for adolescent/young adult women,23 chest wall deformity,24 low sitting and standing height,25 primary hypothyroidism,26 and secondary neoplasm including thyroid carcinoma, and bone and soft tissue tumors.27 These complications have mainly occurred in patients with medulloblastoma24,26,27 or hematological neoplasms26 with younger age of onset than germinoma. Studies of the late adverse effects of CSI have been limited in germinoma patients, and these issues should be resolved in the future.
This study has some limitations. First, we analyzed selected patients evaluated by both spinal MR imaging and CSF cytology, leading to selection bias because these screenings tended to be performed in patients with suspected metastatic disease or those with positive findings in either test. The high frequency of patients with bifocal pineal and neurohypophyseal tumors could reflect such a selection bias. Second, this study included a significant number of patients without histological verification, and with negative AFP and negative to modestly elevated HCG in the serum and/or CSF. This study could have included patients with tumors other than germinomatous GCT because the histological diagnosis was germinoma only in 69%, 51%, 89%, and 75% of patients with serum HCG <50 IU/l, CSF HCG <50 IU/l, serum AFP <10 ng/mL, and CSF AFP <10 ng/mL, respectively.13 However, the diagnosis of germinoma was probably more accurate because this study included germinoma patients who were diagnosed by local physicians in charge based on the clinical findings as well as the level of tumor markers. Third, as the number of patients analyzed was limited and recurrence did not occur frequently, this retrospective analysis did not lead to a definitive conclusion about the necessity of CSI for germinoma patient with positive cytology. In addition, the clinical course of patients with recurrence after CSI, which developed as early as 7 months at the primary site, was not typical of recurrent germinoma. However, few reports have demonstrated tumor control after the non-CSI approach.20 Therefore, we believe that the present study provides important evidence to propose the concept of the non-CSI approach. A prospective analysis is necessary to confirm this idea. Fourth, the CSF for cytology in this study was obtained by either lumbar tap or ventricular sampling. Direct comparison of lumbar and ventricular CSF in 52 patients with pediatric patients with primary CNS tumors including 3 germinomas showed that lumbar CSF cytology is clearly superior to ventricular CSF cytology for the detection of tumor cells.28 This finding could influence the interpretation of this result. For example, negative ventricular CSF cytology in 2 patients with spinal lesion might have been false negative in these patients. Similarly, the distribution of the patients diagnosed by ventricular CSF cytology was different between the patients treated with CSI and non-CSI (50% and 27%, respectively). The difference in the clinical significance of positive lumber and ventricular CSF cytology remains unclear due to the lack of information for germinoma patients, and this topic should also be resolved in the future.
In conclusion, based on the findings of this series, CSI can be omitted in the patients with positive cytology without spinal lesion on MR imaging.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement. The authors declare that they have no conflicts of interest pertaining to this work.
Authorship Statement. Conceptualization and design: M.K, H.T., K.I., and R.N. Acquisition of data: M.K., T.S., T.Tominaga, J.K., S.T., S.H., M.Nagane, M.Matsuda, A.Y., M.Natsumeda, M.Y., N.Kagawa, Y.Akiyama, J.F., T.N., I.S., K.Tanaka, A.I., M.Mase, T.Tomita, D.Kuga, N.Kijima, T.F., Y.Nakahara, A.N., K.Y., D.Keino, T.Tokuyama, K.A., K.U., H.A., M.Nakada, K.M., Y.Arakawa, N.I., Y.Narita, N.S., A.K., M.Nonaka, S.I., Y.K., K.K., S.N., K.N., S.Y., and K.Terashima. Analysis of data: M.K. and H.T. Manuscript writing: M.K, H.T., and R.N. Final editing and approval of the manuscript: M.K, H.T., T.S., T.Tominaga, J.K., S.T., S.H., M.Nagane, M.Matsuda, A.Y., M.Natsumeda, M.Y., N.Kagawa, Y.Akiyama, J.F., T.N., I.S., K.Tanaka, A.I., M.Mase, T.Tomita, D.Kuga, N.Kijima, T.F., Y.Nakahara, A.N., K.Y., D.Keino, T.Tokuyama, K.A., K.U., H.A., M.Nakada, K.M., Y.Arakawa, N.I., Y.Narita, N.S., A.K., M.Nonaka, S.I., Y.K., K.K., S.N., K.N., S.Y., K.Terashima, K.I., and R.N.




