-
PDF
- Split View
-
Views
-
Cite
Cite
Sirui Ma, Soumon Rudra, Jian L Campian, Milan G Chheda, Tanner M Johanns, George Ansstas, Christopher D Abraham, Michael R Chicoine, Eric C Leuthardt, Joshua L Dowling, Gavin P Dunn, Albert H Kim, Jiayi Huang, Salvage therapies for radiation-relapsed isocitrate dehydrogenase-mutant astrocytoma and 1p/19q codeleted oligodendroglioma, Neuro-Oncology Advances, Volume 3, Issue 1, January-December 2021, vdab081, https://doi.org/10.1093/noajnl/vdab081
Close - Share Icon Share
Abstract
Optimal management for recurrent IDH-mutant glioma after radiation therapy (RT) is not well-defined. This study assesses practice patterns for managing recurrent IDH-mutant astrocytoma (Astro) and 1p/19q codeleted oligodendroglioma (Oligo) after RT and surveys their clinical outcomes after different salvage approaches.
Ninety-four recurrent Astro or Oligo patients after RT who received salvage systemic therapy (SST) between 2001 and 2019 at a tertiary cancer center were retrospectively analyzed. SST was defined as either alkylating chemotherapy (AC) or nonalkylating therapy (non-AC). Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method from the start of SST. Multivariable analysis (MVA) was conducted using Cox regression analysis.
Recurrent Oligo (n = 35) had significantly higher PFS (median: 3.1 vs 0.8 years, respectively, P = .002) and OS (median: 6.3 vs 1.5 years, respectively, P < .001) than Astro (n = 59). Overall, 90% of recurrences were local. Eight-three percent received AC as the first-line SST; 50% received salvage surgery before SST; approximately 50% with local failure >2 years after prior RT received reirradiation. On MVA, non-AC was associated with worse OS for both Oligo and Astro; salvage surgery was associated with improved PFS and OS for Astro; early reirradiation was associated with improved PFS for Astro.
Recurrent radiation-relapsed IDH-mutant gliomas represent a heterogeneous group with variable treatment approaches. Surgery, AC, and reirradiation remain the mainstay of salvage options for retreatment.
Recurrent IDH-mutant oligodendroglioma has significantly better clinical outcomes than recurrent IDH-mutant astrocytoma.
Alkylating chemotherapy should be considered first-line salvage systemic therapy for recurrent IDH-mutant glioma.
Reirradiation with concurrent temozolomide is feasible and safe in recurrent IDH-mutant glioma.
Management of IDH-mutant glioma in the setting of recurrence is not well-defined. This study details our institution’s experience with treating patients with these tumors who developed the recurrent disease after initial radiation therapy. We report treatment patterns and clinical outcomes of a retrospective cohort with IDH-mutant astrocytoma (Astro) and 1p/1q codeleted oligodendroglioma (Oligo). Alkylating chemotherapy (AC) demonstrated improved overall survival (OS) compared with non-AC, suggesting that these agents should be employed as first-line salvage systemic therapy whenever possible. Consistent with previously reported outcomes, our results indicate that salvage surgery may prolong survival for recurrent glioma, a benefit possibly further enhanced in Astro. Reirradiation was associated with nonsignificant trends towards improved survival and could safely be combined with AC, but its observed benefit may be confounded by selection bias. Our data support multimodality management of recurrent IDH-mutant glioma and highlights the need to identify other active systemic agents for AC-refractory disease.
Diffuse gliomas are a heterogeneous set of central nervous system tumors, and the 2016 World Health Organization classification has incorporated molecular markers in their diagnoses, including the isocitrate dehydrogenase (IDH) mutation as a key marker.1 IDH-mutant gliomas have a significantly better prognosis than IDH-wildtype gliomas.2 IDH-mutant astrocytoma is typically characterized by ATRX loss or absence of 1p/19q codeletion, and IDH-mutant oligodendroglioma is typically characterized by 1p/19q codeletion or mutation of the TERT promoter.3–5 Currently, the standard of care for IDH-mutant astrocytoma and oligodendroglioma consists of maximum safe resection followed by radiation therapy (RT) with chemotherapy (either temozolomide [TMZ] or procarbazine, lomustine, and vincristine [PCV]).6–8 However, there is limited available data to guide optimal salvage therapy at recurrence after chemoradiotherapy.9 As there is increasing interest to develop novel therapies for these patients at recurrence, a thorough understanding of the pattern of care for recurrent IDH-mutant gliomas and their natural history after existing salvage therapies will be crucial to guide clinical practice and future clinical trial development.
To address this unmet need, we retrospectively analyzed our tertiary care center’s experience of treating recurrent IDH-mutant astrocytomas (Astro) and 1p/19q codeleted oligodendrogliomas (Oligo) to establish an overview of the pattern of care, evaluate for prognostic factors to guide risk stratification, and survey the impact of different salvage therapies.
Methods
Patient Cohort
We conducted a single-institution retrospective review of adult patients (≥18 years old) with either Astro or Oligo who developed recurrence after initial RT and who had received salvage systemic therapy (SST). Recurrence was considered a local failure (LF) if, within the initial RT field, focal distant failure (fDF) if a single site of recurrence outside of the RT field, or disseminated distant failure if more than one site of distant recurrence or leptomeningeal disease (dDF). Glioblastoma with 1p/19q codeletion but without IDH mutation were excluded. Oligo was defined as histological oligodendroglioma or mixed oligoastrocytoma with 1p/19q codeletion. Astro was defined as glioma with IDH mutation but was either negative for 1p/19q codeletion or positive for ATRX loss. 1p/19q codeletion was assessed by fluorescence in situ hybridization (FISH) and was routinely performed at our institution for histological oligodendroglioma and mixed oligoastrocytoma since 1999. IDH mutation and ATRX loss were evaluated by either immunohistochemistry (IHC) or next-generation sequencing (NGS) as previously described.10 Although some of our patients were before the routine testing of IDH at our institution (which started in 2012), their IDH status was determined a posteriori based on the later analysis of tumor samples. Oligo without confirmed IDH mutation was included as long as it was not shown to be IDH-wildtype on NGS. Tumor grade at recurrence was defined by pathological evaluation if surgery or biopsy was performed; if no specimen was obtained, the original pathological diagnosis was used. This study was conducted with the approval of the institutional review board.
Salvage Systemic Therapy
Only first-line SST was analyzed for this study and was categorized as either alkylating chemotherapy (AC) or nonalkylating therapy (non-AC). AC included TMZ, PCV, or lomustine. Non-AC included bevacizumab, immunotherapy (either vaccine or anti-PD-1 antibody), biological agents, or tumor-treating field (TTF).
Salvage Surgery
Surgeries before SST were analyzed and were categorized as biopsy, laser interstitial thermal therapy (LITT), subtotal resection (STR), or gross-total resection (GTR) based on operative and imaging reports by the treating physicians. Salvage surgery was defined as either LITT, STR, or GTR but excluded biopsy alone.
Reirradiation
Early reRT was defined if delivered either concurrently or sequentially with the first-line SST, and delayed reRT was defined if delivered after progression on the initial SST. ReRT techniques included photon-based intensity-modulated RT (IMRT) using the Varian Medical Systems linear accelerators (Palo Alto, CA), proton beam therapy (PBT) using the Mevion Medical Systems proton accelerator (Littleton, MA), or stereotactic radiosurgery (SRS) using Gamma Knife (Elekta, Atlanta, GA). Gross tumor volume (GTV) included the surgical cavity of the salvage surgery if performed as well as the progressive T1 and T2 abnormality. For external beam RT (EBRT) using either IMRT or PBT, a clinical target volume (CTV) margin of approximately 0–5 mm from GTV was applied by the treating physician based on the clinical assessment, and a planning target volume (PTV) margin of approximately 3 mm was applied to account for setup uncertainty. SRS typically treated only GTV without additional CTV and PTV margin expansion. RT fractionation and critical structure constraints were individualized by the treating physician based on the tumor location and the previous radiation exposure. A composite plan was made with the previous EBRT whenever possible. PBT dose was prescribed as gray relative biological equivalents (GyRBE) using a relative biological effectiveness (RBE) value of 1.1. To allow the cumulative dose to the normal brain tissue from different RT courses, biologically equivalent doses in 2 Gy fractions (EQD2) were estimated for each course using the linear-quadratic model: , where N is the number of fractions, d is the dose per fraction, and α/β of 3 is used for brain tissue.
Statistical Analysis
Baseline patient, tumor, and treatment characteristics were compared using the Mann-Whitney U test and Fisher’s Exact test for continuous and categorical variables, respectively. Freedom-from progression (FFP), progression-free survival (PFS), and overall survival (OS) rates were calculated using the Kaplan-Meier method from the start of SST and then compared using the log-rank test. Univariable (UVA) and multivariable analyses (MVA) to identify prognostic factors for PFS and OS were performed using Cox regression. MVA was performed with all variables with P < .20 on UVA using the backward-conditional method. Symptomatic radiation necrosis (sRN) was characterized as previously described and was defined as grade 2 or higher based on the Common Terminology Criteria for Adverse Events (CTCAE) v4.0.11 Briefly, it was defined as a radiologic abnormality that resulted in medical or surgical therapy and excluded tumor progression through surgical evaluation or serial MRI. Time to sRN was measured from the start of reRT. All tests were two-sided, and statistical analyses were performed with the Statistical Package for Social Sciences, version 23.0 (IBM SPSS Statistics, Chicago, IL, USA).
Results
Patient and Treatment Characteristics
Between 2001 and 2019, 108 patients with grade 2–4 gliomas with IDH mutation or 1p/19q codeletion developed recurrence after prior RT or chemoradiotherapy and were captured in our institutional database at Washington University in St Louis. Six patients had IDH-wildtype GBM and were excluded. Eight patients were excluded for lack of follow-up: 4 went to another facility for salvage treatment, 3 went to hospice, and one had no additional follow-up. The remaining 94 patients received SST, including 35 Oligo and 59 Astro. Before the SST, 20 patients had prior RT alone, 71 had RT and one line of chemotherapy (90% TMZ and 10% PCV), and 3 had RT and 2 lines of chemotherapy (TMZ and PCV). Among the 35 Oligo patients, 11 had RT alone, 22 had RT and one line of chemotherapy (82% TMZ), and 2 had RT and 2 lines of chemotherapy. Among the 59 Astro patients, 9 had RT alone, 49 had RT and one line of chemotherapy (94% TMZ), and 1 had RT and 2 lines of chemotherapy. Figure 1 depicts the pattern of salvage therapy approaches stratified by tumor type, recurrence pattern, and timing since initial RT. Of the 35 Oligo patients, 17 did not have IDH evaluation. However, based on our previous study, the FISH assay’s false positive rate of 1p/19q codeletion to detect a molecular IDH-mutant and 1p/19q codeleted oligodendroglioma is only approximately 2%.12Table 1 summarizes baseline patient and treatment characteristics of the study cohort. The median age was 42 years, and 73% were male. The median time to recurrence after initial RT was significantly longer for Oligo than Astro (7.7 years vs 3.3 years, P = .01). The most common type of recurrence was LF (90%), and rarely fDF (5%) or dDF (5%). Among 12 initially grade 2 Oligo patients who had repeat biopsy or surgery at recurrence, 50% had transformed into grade 3 after a median interval of 10.4 years (range: 6.2–26.0). Among 17 initially grade 2 Astro patients who had repeat surgery at recurrence, 65% had transformed into grade 3–4 after a median interval of 6.5 years (range: 2.6–16.7). Among 12 initial grade 3 Astro patients who had repeat surgery, 50% had transformed into grade 4 after a median interval of 3.4 years (range: 0.7–15.3). Fifty percent of the entire cohort had salvage surgeries before SST, and more than 50% of patients with LF had salvage surgeries (61% for Oligo and 53% for Astro). Of all the salvage surgeries, 13% were performed with LITT. Overall, 37% received either early or delayed reRT, including 56% of Oligo and 50% of Astro patients with LF >2 years after initial RT. Most patients with DF (80%) received reRT, and patients with LF <2 years after initial RT rarely had reRT (4%). The most common first-line SST was TMZ (70%), followed by a lomustine-based regimen (13%) and a bevacizumab-based regimen (10%). The remaining regimens included anti-PD-1 antibody or T-cell vaccine (3%), TTF (2%), anti-PD1 plus TTF (1%), and mTOR inhibitor (1%).
| Baseline Factors . | All . | Oligo . | Astro . | P-value . |
|---|---|---|---|---|
| Tumor Type | – | |||
| Oligo | 35 | 35 | – | |
| Astro | 59 | – | 59 | |
| Median Age | 42 (23–75) | 46 (23–75) | 40 (23–70) | .07 |
| Male | 69 (73%) | 30 (86%) | 39 (66%) | .053 |
| Female | 25 (27%) | 5 (14%) | 20 (34%) | |
| White race | 89 (95%) | 35 (100%) | 54 (92%) | .15 |
| Nonwhite race | 5 (5%) | 5 (9%) | ||
| Grade at relapsea | <.001 | |||
| 2 | 22 (23%) | 10 (29%) | 12 (20%) | |
| 3 | 44 (47%) | 25 (71%) | 19 (32%) | |
| 4 | 28 (30%) | 28 (48%) | ||
| Time to recurrence from initial RT to SST (years) | 3.6 (0.2–26.2) | 7.7 (0.5–26.2) | 3.3 (0.2–16.7) | .01 |
| Prior Treatment | .07 | |||
| Chemoradiotherapy | 74 (79%) | 24 (69%) | 50 (85%) | |
| RT alone | 20 (21%) | 11 (31%) | 9 (15%) | |
| Type of recurrence | .21 | |||
| LF | 84 (89%) | 33 (94%) | 51 (86%) | |
| fDF | 5 (5%) | 2 ((6%) | 3 (5%) | |
| dDF | 5 (5%) | – | 5 (9%) | |
| Surgery before SST | .39 | |||
| None/biopsy | 47 (50%) | 15 (43%) | 32 (54%) | |
| Surgery/LITT | 47 (50%) | 20 (57%) | 27 (46%) | |
| SST Type | .59 | |||
| TMZ regimen | 66 (70%) | 25 (71%) | 41 (70%) | |
| Lomustine regimen | 12 (13%) | 6 (17%) | 6 (10%) | |
| Bevacizumab regimen | 9 (10%) | 2 (6%) | 7 (12%) | |
| Other | 7 (7%) | 2 (6%) | 5 (8%) | |
| Early reRT | .32 | |||
| No | 72 (77%) | 29 (83%) | 43 (73%) | |
| Yes | 22 (23%) | 6 (17%) | 16 (27%) |
| Baseline Factors . | All . | Oligo . | Astro . | P-value . |
|---|---|---|---|---|
| Tumor Type | – | |||
| Oligo | 35 | 35 | – | |
| Astro | 59 | – | 59 | |
| Median Age | 42 (23–75) | 46 (23–75) | 40 (23–70) | .07 |
| Male | 69 (73%) | 30 (86%) | 39 (66%) | .053 |
| Female | 25 (27%) | 5 (14%) | 20 (34%) | |
| White race | 89 (95%) | 35 (100%) | 54 (92%) | .15 |
| Nonwhite race | 5 (5%) | 5 (9%) | ||
| Grade at relapsea | <.001 | |||
| 2 | 22 (23%) | 10 (29%) | 12 (20%) | |
| 3 | 44 (47%) | 25 (71%) | 19 (32%) | |
| 4 | 28 (30%) | 28 (48%) | ||
| Time to recurrence from initial RT to SST (years) | 3.6 (0.2–26.2) | 7.7 (0.5–26.2) | 3.3 (0.2–16.7) | .01 |
| Prior Treatment | .07 | |||
| Chemoradiotherapy | 74 (79%) | 24 (69%) | 50 (85%) | |
| RT alone | 20 (21%) | 11 (31%) | 9 (15%) | |
| Type of recurrence | .21 | |||
| LF | 84 (89%) | 33 (94%) | 51 (86%) | |
| fDF | 5 (5%) | 2 ((6%) | 3 (5%) | |
| dDF | 5 (5%) | – | 5 (9%) | |
| Surgery before SST | .39 | |||
| None/biopsy | 47 (50%) | 15 (43%) | 32 (54%) | |
| Surgery/LITT | 47 (50%) | 20 (57%) | 27 (46%) | |
| SST Type | .59 | |||
| TMZ regimen | 66 (70%) | 25 (71%) | 41 (70%) | |
| Lomustine regimen | 12 (13%) | 6 (17%) | 6 (10%) | |
| Bevacizumab regimen | 9 (10%) | 2 (6%) | 7 (12%) | |
| Other | 7 (7%) | 2 (6%) | 5 (8%) | |
| Early reRT | .32 | |||
| No | 72 (77%) | 29 (83%) | 43 (73%) | |
| Yes | 22 (23%) | 6 (17%) | 16 (27%) |
Oligo = 1p/19q codeleted oligodendroglioma; Astro = IDH-mutant astrocytoma; RT = radiation therapy; SST = salvage systemic therapy; LF = local failure within prior RT field; fDF = focal distant failure outside of prior RT field; dDF = disseminated distant failure; LITT = laser interstitial thermal therapy; TMZ = temozolomide; reRT = reirradiation.
aFor tumors that did not have a repeat surgery or biopsy at the time of recurrence, the initial tumor grade was assumed.
| Baseline Factors . | All . | Oligo . | Astro . | P-value . |
|---|---|---|---|---|
| Tumor Type | – | |||
| Oligo | 35 | 35 | – | |
| Astro | 59 | – | 59 | |
| Median Age | 42 (23–75) | 46 (23–75) | 40 (23–70) | .07 |
| Male | 69 (73%) | 30 (86%) | 39 (66%) | .053 |
| Female | 25 (27%) | 5 (14%) | 20 (34%) | |
| White race | 89 (95%) | 35 (100%) | 54 (92%) | .15 |
| Nonwhite race | 5 (5%) | 5 (9%) | ||
| Grade at relapsea | <.001 | |||
| 2 | 22 (23%) | 10 (29%) | 12 (20%) | |
| 3 | 44 (47%) | 25 (71%) | 19 (32%) | |
| 4 | 28 (30%) | 28 (48%) | ||
| Time to recurrence from initial RT to SST (years) | 3.6 (0.2–26.2) | 7.7 (0.5–26.2) | 3.3 (0.2–16.7) | .01 |
| Prior Treatment | .07 | |||
| Chemoradiotherapy | 74 (79%) | 24 (69%) | 50 (85%) | |
| RT alone | 20 (21%) | 11 (31%) | 9 (15%) | |
| Type of recurrence | .21 | |||
| LF | 84 (89%) | 33 (94%) | 51 (86%) | |
| fDF | 5 (5%) | 2 ((6%) | 3 (5%) | |
| dDF | 5 (5%) | – | 5 (9%) | |
| Surgery before SST | .39 | |||
| None/biopsy | 47 (50%) | 15 (43%) | 32 (54%) | |
| Surgery/LITT | 47 (50%) | 20 (57%) | 27 (46%) | |
| SST Type | .59 | |||
| TMZ regimen | 66 (70%) | 25 (71%) | 41 (70%) | |
| Lomustine regimen | 12 (13%) | 6 (17%) | 6 (10%) | |
| Bevacizumab regimen | 9 (10%) | 2 (6%) | 7 (12%) | |
| Other | 7 (7%) | 2 (6%) | 5 (8%) | |
| Early reRT | .32 | |||
| No | 72 (77%) | 29 (83%) | 43 (73%) | |
| Yes | 22 (23%) | 6 (17%) | 16 (27%) |
| Baseline Factors . | All . | Oligo . | Astro . | P-value . |
|---|---|---|---|---|
| Tumor Type | – | |||
| Oligo | 35 | 35 | – | |
| Astro | 59 | – | 59 | |
| Median Age | 42 (23–75) | 46 (23–75) | 40 (23–70) | .07 |
| Male | 69 (73%) | 30 (86%) | 39 (66%) | .053 |
| Female | 25 (27%) | 5 (14%) | 20 (34%) | |
| White race | 89 (95%) | 35 (100%) | 54 (92%) | .15 |
| Nonwhite race | 5 (5%) | 5 (9%) | ||
| Grade at relapsea | <.001 | |||
| 2 | 22 (23%) | 10 (29%) | 12 (20%) | |
| 3 | 44 (47%) | 25 (71%) | 19 (32%) | |
| 4 | 28 (30%) | 28 (48%) | ||
| Time to recurrence from initial RT to SST (years) | 3.6 (0.2–26.2) | 7.7 (0.5–26.2) | 3.3 (0.2–16.7) | .01 |
| Prior Treatment | .07 | |||
| Chemoradiotherapy | 74 (79%) | 24 (69%) | 50 (85%) | |
| RT alone | 20 (21%) | 11 (31%) | 9 (15%) | |
| Type of recurrence | .21 | |||
| LF | 84 (89%) | 33 (94%) | 51 (86%) | |
| fDF | 5 (5%) | 2 ((6%) | 3 (5%) | |
| dDF | 5 (5%) | – | 5 (9%) | |
| Surgery before SST | .39 | |||
| None/biopsy | 47 (50%) | 15 (43%) | 32 (54%) | |
| Surgery/LITT | 47 (50%) | 20 (57%) | 27 (46%) | |
| SST Type | .59 | |||
| TMZ regimen | 66 (70%) | 25 (71%) | 41 (70%) | |
| Lomustine regimen | 12 (13%) | 6 (17%) | 6 (10%) | |
| Bevacizumab regimen | 9 (10%) | 2 (6%) | 7 (12%) | |
| Other | 7 (7%) | 2 (6%) | 5 (8%) | |
| Early reRT | .32 | |||
| No | 72 (77%) | 29 (83%) | 43 (73%) | |
| Yes | 22 (23%) | 6 (17%) | 16 (27%) |
Oligo = 1p/19q codeleted oligodendroglioma; Astro = IDH-mutant astrocytoma; RT = radiation therapy; SST = salvage systemic therapy; LF = local failure within prior RT field; fDF = focal distant failure outside of prior RT field; dDF = disseminated distant failure; LITT = laser interstitial thermal therapy; TMZ = temozolomide; reRT = reirradiation.
aFor tumors that did not have a repeat surgery or biopsy at the time of recurrence, the initial tumor grade was assumed.
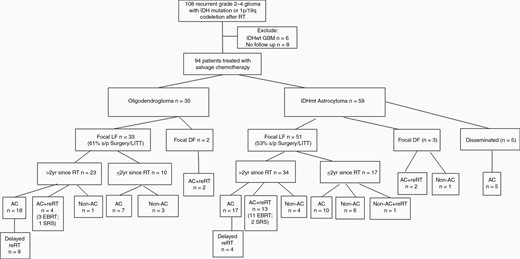
CONSORT (Consolidated Standards of Reporting Trials) diagram of the salvage treatment approaches for the study patients stratified by tumor type, recurrence pattern, and timing. IDH = isocitrate dehydrogenase, RT = radiation therapy, IDHwt = IDH-wildtype, GBM = glioblastoma, LF = local failure, DF = distant failure, LITT = laser interstitial thermal therapy, AC = alkylating chemotherapy, Non-AC = nonalkylating therapy, reRT = reirradiation.
Outcomes After Recurrence
After a median follow-up of 1.3 years, the median PFS and OS for the entire cohort after recurrence were 0.9 and 1.5 years, respectively. Oligo patients had significantly higher FFP (median: 3.1 vs 0.8 years, respectively, P = .006; Figure 2A), PFS (median: 3.1 vs 0.8 years, respectively, P = .002; Figure 2B), and OS (median: 6.3 vs 1.5 years, respectively, P < .001; Figure 2C) as compared to Astro patients. Given the significantly different natural history of recurrent Oligo vs Astro, MVA to explore prognostic factors was performed separately for each cohort. For the Oligo cohort, increased age and non-AC were associated with the significantly worse OS on MVA (Table 2). For the Astro cohort, lower tumor grade and salvage surgery were associated with significantly better PFS and OS. Non-AC use was associated with worse OS. Early reRT was associated with higher PFS (Table 3). Among a more homogeneous subset of Astro patients who experienced LF >2 years after initial RT, 17 patients received AC alone, while 11 patients received AC+EBRT. The baseline characteristics of these two subsets are shown in Supplementary Table S1. The most significant difference is that the AC+EBRT cohort had a longer disease-free interval from the initial RT than the AC alone cohort (median 10.5 vs 3.6 years, P = .004). AC+EBRT also had nonsignificantly higher proportion of grade 4 disease (46% vs 24%, P = .11) and salvage surgery (73% vs 41%, P = .14). FFP, PFS, and OS were nonsignificantly higher for AC+EBRT than AC alone (Figure 2D–F).
Univariable and Multivariable Cox Regression Analysis for Factors Associated with Worse PFS and OS for Oligodendroglioma
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P- value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P- value . |
| Median age | 1.03 (0.99–1.06) | .15 | 1.03 (0.99–1.06) | .15 | 1.04 (1.00–1.08 | .08 | 1.06 (1.02–1.11) | .008 |
| Male | 1.86 (0.54–6.39) | .33 | – | – | 5.18 (0.65–41.3) | .12 | NS | NS |
| Nonwhite race | N/A | N/A | – | – | N/A | N/A | – | – |
| Grade 3 at relapse | 0.99 (0.36–2.68) | .98 | – | – | 1.26 (0.36–4.38) | .72 | – | – |
| Time to recurrence (months) | 1.04 (0.97–1.11) | .30 | – | – | 1.04 (0.97–1.12) | .30 | – | – |
| Prior Chemoradiotherapy (vs RT alone) | 0.79 (0.33–1.88) | .59 | – | – | 0.60 (0.22–1.62) | .31 | – | – |
| fDF (vs LF) | 1.17 (0.27–5.07) | .83 | – | – | 1.79 (0.40–7.98) | .44 | – | – |
| Salvage surgery/LITT | 1.43 (0.60–3.38) | .42 | – | – | 1.03 (0.40–2.69) | .95 | – | – |
| Nonalkylating therapy | 1.88 (0.54–6.50) | .32 | – | – | 2.55 (0.73–8.95) | .15 | 8.82 (1.74–44.7) | .009 |
| Early reRT | 1.48 (0.58–3.81) | .41 | – | – | 2.59 (0.95–7.07) | .06 | NS | NS |
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P- value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P- value . |
| Median age | 1.03 (0.99–1.06) | .15 | 1.03 (0.99–1.06) | .15 | 1.04 (1.00–1.08 | .08 | 1.06 (1.02–1.11) | .008 |
| Male | 1.86 (0.54–6.39) | .33 | – | – | 5.18 (0.65–41.3) | .12 | NS | NS |
| Nonwhite race | N/A | N/A | – | – | N/A | N/A | – | – |
| Grade 3 at relapse | 0.99 (0.36–2.68) | .98 | – | – | 1.26 (0.36–4.38) | .72 | – | – |
| Time to recurrence (months) | 1.04 (0.97–1.11) | .30 | – | – | 1.04 (0.97–1.12) | .30 | – | – |
| Prior Chemoradiotherapy (vs RT alone) | 0.79 (0.33–1.88) | .59 | – | – | 0.60 (0.22–1.62) | .31 | – | – |
| fDF (vs LF) | 1.17 (0.27–5.07) | .83 | – | – | 1.79 (0.40–7.98) | .44 | – | – |
| Salvage surgery/LITT | 1.43 (0.60–3.38) | .42 | – | – | 1.03 (0.40–2.69) | .95 | – | – |
| Nonalkylating therapy | 1.88 (0.54–6.50) | .32 | – | – | 2.55 (0.73–8.95) | .15 | 8.82 (1.74–44.7) | .009 |
| Early reRT | 1.48 (0.58–3.81) | .41 | – | – | 2.59 (0.95–7.07) | .06 | NS | NS |
RT = radiation therapy; LF = local failure within prior RT field; fDF = focal distant failure outside of prior RT field; dDF = disseminated distant failure; LITT = laser interstitial thermal therapy; reRT = reirradiation.
Univariable and Multivariable Cox Regression Analysis for Factors Associated with Worse PFS and OS for Oligodendroglioma
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P- value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P- value . |
| Median age | 1.03 (0.99–1.06) | .15 | 1.03 (0.99–1.06) | .15 | 1.04 (1.00–1.08 | .08 | 1.06 (1.02–1.11) | .008 |
| Male | 1.86 (0.54–6.39) | .33 | – | – | 5.18 (0.65–41.3) | .12 | NS | NS |
| Nonwhite race | N/A | N/A | – | – | N/A | N/A | – | – |
| Grade 3 at relapse | 0.99 (0.36–2.68) | .98 | – | – | 1.26 (0.36–4.38) | .72 | – | – |
| Time to recurrence (months) | 1.04 (0.97–1.11) | .30 | – | – | 1.04 (0.97–1.12) | .30 | – | – |
| Prior Chemoradiotherapy (vs RT alone) | 0.79 (0.33–1.88) | .59 | – | – | 0.60 (0.22–1.62) | .31 | – | – |
| fDF (vs LF) | 1.17 (0.27–5.07) | .83 | – | – | 1.79 (0.40–7.98) | .44 | – | – |
| Salvage surgery/LITT | 1.43 (0.60–3.38) | .42 | – | – | 1.03 (0.40–2.69) | .95 | – | – |
| Nonalkylating therapy | 1.88 (0.54–6.50) | .32 | – | – | 2.55 (0.73–8.95) | .15 | 8.82 (1.74–44.7) | .009 |
| Early reRT | 1.48 (0.58–3.81) | .41 | – | – | 2.59 (0.95–7.07) | .06 | NS | NS |
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P- value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P- value . |
| Median age | 1.03 (0.99–1.06) | .15 | 1.03 (0.99–1.06) | .15 | 1.04 (1.00–1.08 | .08 | 1.06 (1.02–1.11) | .008 |
| Male | 1.86 (0.54–6.39) | .33 | – | – | 5.18 (0.65–41.3) | .12 | NS | NS |
| Nonwhite race | N/A | N/A | – | – | N/A | N/A | – | – |
| Grade 3 at relapse | 0.99 (0.36–2.68) | .98 | – | – | 1.26 (0.36–4.38) | .72 | – | – |
| Time to recurrence (months) | 1.04 (0.97–1.11) | .30 | – | – | 1.04 (0.97–1.12) | .30 | – | – |
| Prior Chemoradiotherapy (vs RT alone) | 0.79 (0.33–1.88) | .59 | – | – | 0.60 (0.22–1.62) | .31 | – | – |
| fDF (vs LF) | 1.17 (0.27–5.07) | .83 | – | – | 1.79 (0.40–7.98) | .44 | – | – |
| Salvage surgery/LITT | 1.43 (0.60–3.38) | .42 | – | – | 1.03 (0.40–2.69) | .95 | – | – |
| Nonalkylating therapy | 1.88 (0.54–6.50) | .32 | – | – | 2.55 (0.73–8.95) | .15 | 8.82 (1.74–44.7) | .009 |
| Early reRT | 1.48 (0.58–3.81) | .41 | – | – | 2.59 (0.95–7.07) | .06 | NS | NS |
RT = radiation therapy; LF = local failure within prior RT field; fDF = focal distant failure outside of prior RT field; dDF = disseminated distant failure; LITT = laser interstitial thermal therapy; reRT = reirradiation.
Univariable and Multivariable Cox Regression Analysis for Factors Associated with Worse PFS and OS for IDH-Mutant Astrocytoma
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P value . | HR (95% CI) . | P-value . |
| Median age | 0.97 (0.95–1.00) | .046 | NS | NS | 0.98 (0.96–1.01 | .20 | NS | NS |
| Male | 1.59 (0.83–3.06) | .16 | NS | NS | 2.05 (1.01–4.17) | .047 | 3.05 (1.35–6.87) | .007 |
| Nonwhite race | 2.56 (0.97–6.77) | .06 | NS | NS | 2.87 (1.08–7.65) | .04 | NS | NS |
| Grade | .06 | .003 | .09 | .03 | ||||
| Grade 2 | Ref | – | Ref | – | Ref | – | Ref | – |
| Grade 3 | 1.88 (0.75–4.69) | .18 | 3.73 (1.37–10.2) | .01 | 2.02 (0.79–5.18) | .15 | 3.78 (1.37–10.4) | .01 |
| Grade 4 | 2.83 (1.19–6.72) | .02 | 4.80 (1.94–11.9) | .001 | 2.66 (1.11–6.38) | .03 | 3.19 (1.15–8.81) | .03 |
| Time to recurrence (months) | 0.03 (0.00–347.4) | .46 | – | – | 0.91 (0.83–0.995) | .04 | NS | NS |
| Prior Chemoradiotherapy (vs RT alone) | 0.60 (0.25–1.43) | .25 | – | – | 0.49 (0.17–1.37) | .17 | NS | NS |
| dDF (vs LF/fDF) | 2.45 (0.93–6.43) | .07 | NS | NS | 2.19 (0.76–6.33) | .15 | NS | NS |
| Salvage surgery/LITT | 0.36 (0.19–0.69) | .002 | 0.31 (0.16–0.60) | .001 | 0.40 (0.21–0.78) | .007 | 0.36 (0.18–0.73) | .005 |
| Nonalkylating therapy | 3.24 (1.58–6.63) | .001 | NS | NS | 3.33 (1.62–6.84) | .001 | 2.78 (1.11–6.92) | .03 |
| Early reRT | 0.48 (0.23–1.01) | .054 | 0.43 (0.19–0.95) | .04 | 0.49 (0.22–1.11) | .09 | NS | NS |
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P value . | HR (95% CI) . | P-value . |
| Median age | 0.97 (0.95–1.00) | .046 | NS | NS | 0.98 (0.96–1.01 | .20 | NS | NS |
| Male | 1.59 (0.83–3.06) | .16 | NS | NS | 2.05 (1.01–4.17) | .047 | 3.05 (1.35–6.87) | .007 |
| Nonwhite race | 2.56 (0.97–6.77) | .06 | NS | NS | 2.87 (1.08–7.65) | .04 | NS | NS |
| Grade | .06 | .003 | .09 | .03 | ||||
| Grade 2 | Ref | – | Ref | – | Ref | – | Ref | – |
| Grade 3 | 1.88 (0.75–4.69) | .18 | 3.73 (1.37–10.2) | .01 | 2.02 (0.79–5.18) | .15 | 3.78 (1.37–10.4) | .01 |
| Grade 4 | 2.83 (1.19–6.72) | .02 | 4.80 (1.94–11.9) | .001 | 2.66 (1.11–6.38) | .03 | 3.19 (1.15–8.81) | .03 |
| Time to recurrence (months) | 0.03 (0.00–347.4) | .46 | – | – | 0.91 (0.83–0.995) | .04 | NS | NS |
| Prior Chemoradiotherapy (vs RT alone) | 0.60 (0.25–1.43) | .25 | – | – | 0.49 (0.17–1.37) | .17 | NS | NS |
| dDF (vs LF/fDF) | 2.45 (0.93–6.43) | .07 | NS | NS | 2.19 (0.76–6.33) | .15 | NS | NS |
| Salvage surgery/LITT | 0.36 (0.19–0.69) | .002 | 0.31 (0.16–0.60) | .001 | 0.40 (0.21–0.78) | .007 | 0.36 (0.18–0.73) | .005 |
| Nonalkylating therapy | 3.24 (1.58–6.63) | .001 | NS | NS | 3.33 (1.62–6.84) | .001 | 2.78 (1.11–6.92) | .03 |
| Early reRT | 0.48 (0.23–1.01) | .054 | 0.43 (0.19–0.95) | .04 | 0.49 (0.22–1.11) | .09 | NS | NS |
RT = radiation therapy; LF = local failure within prior RT field; fDF = focal distant failure outside of prior RT field; dDF = disseminated distant failure; LITT = laser interstitial thermal therapy; reRT = reirradiation.
Univariable and Multivariable Cox Regression Analysis for Factors Associated with Worse PFS and OS for IDH-Mutant Astrocytoma
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P value . | HR (95% CI) . | P-value . |
| Median age | 0.97 (0.95–1.00) | .046 | NS | NS | 0.98 (0.96–1.01 | .20 | NS | NS |
| Male | 1.59 (0.83–3.06) | .16 | NS | NS | 2.05 (1.01–4.17) | .047 | 3.05 (1.35–6.87) | .007 |
| Nonwhite race | 2.56 (0.97–6.77) | .06 | NS | NS | 2.87 (1.08–7.65) | .04 | NS | NS |
| Grade | .06 | .003 | .09 | .03 | ||||
| Grade 2 | Ref | – | Ref | – | Ref | – | Ref | – |
| Grade 3 | 1.88 (0.75–4.69) | .18 | 3.73 (1.37–10.2) | .01 | 2.02 (0.79–5.18) | .15 | 3.78 (1.37–10.4) | .01 |
| Grade 4 | 2.83 (1.19–6.72) | .02 | 4.80 (1.94–11.9) | .001 | 2.66 (1.11–6.38) | .03 | 3.19 (1.15–8.81) | .03 |
| Time to recurrence (months) | 0.03 (0.00–347.4) | .46 | – | – | 0.91 (0.83–0.995) | .04 | NS | NS |
| Prior Chemoradiotherapy (vs RT alone) | 0.60 (0.25–1.43) | .25 | – | – | 0.49 (0.17–1.37) | .17 | NS | NS |
| dDF (vs LF/fDF) | 2.45 (0.93–6.43) | .07 | NS | NS | 2.19 (0.76–6.33) | .15 | NS | NS |
| Salvage surgery/LITT | 0.36 (0.19–0.69) | .002 | 0.31 (0.16–0.60) | .001 | 0.40 (0.21–0.78) | .007 | 0.36 (0.18–0.73) | .005 |
| Nonalkylating therapy | 3.24 (1.58–6.63) | .001 | NS | NS | 3.33 (1.62–6.84) | .001 | 2.78 (1.11–6.92) | .03 |
| Early reRT | 0.48 (0.23–1.01) | .054 | 0.43 (0.19–0.95) | .04 | 0.49 (0.22–1.11) | .09 | NS | NS |
| . | UVA for PFS . | . | MVA for PFS . | . | UVA for OS . | . | MVA for OS . | . |
|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P value . | HR (95% CI) . | P-value . |
| Median age | 0.97 (0.95–1.00) | .046 | NS | NS | 0.98 (0.96–1.01 | .20 | NS | NS |
| Male | 1.59 (0.83–3.06) | .16 | NS | NS | 2.05 (1.01–4.17) | .047 | 3.05 (1.35–6.87) | .007 |
| Nonwhite race | 2.56 (0.97–6.77) | .06 | NS | NS | 2.87 (1.08–7.65) | .04 | NS | NS |
| Grade | .06 | .003 | .09 | .03 | ||||
| Grade 2 | Ref | – | Ref | – | Ref | – | Ref | – |
| Grade 3 | 1.88 (0.75–4.69) | .18 | 3.73 (1.37–10.2) | .01 | 2.02 (0.79–5.18) | .15 | 3.78 (1.37–10.4) | .01 |
| Grade 4 | 2.83 (1.19–6.72) | .02 | 4.80 (1.94–11.9) | .001 | 2.66 (1.11–6.38) | .03 | 3.19 (1.15–8.81) | .03 |
| Time to recurrence (months) | 0.03 (0.00–347.4) | .46 | – | – | 0.91 (0.83–0.995) | .04 | NS | NS |
| Prior Chemoradiotherapy (vs RT alone) | 0.60 (0.25–1.43) | .25 | – | – | 0.49 (0.17–1.37) | .17 | NS | NS |
| dDF (vs LF/fDF) | 2.45 (0.93–6.43) | .07 | NS | NS | 2.19 (0.76–6.33) | .15 | NS | NS |
| Salvage surgery/LITT | 0.36 (0.19–0.69) | .002 | 0.31 (0.16–0.60) | .001 | 0.40 (0.21–0.78) | .007 | 0.36 (0.18–0.73) | .005 |
| Nonalkylating therapy | 3.24 (1.58–6.63) | .001 | NS | NS | 3.33 (1.62–6.84) | .001 | 2.78 (1.11–6.92) | .03 |
| Early reRT | 0.48 (0.23–1.01) | .054 | 0.43 (0.19–0.95) | .04 | 0.49 (0.22–1.11) | .09 | NS | NS |
RT = radiation therapy; LF = local failure within prior RT field; fDF = focal distant failure outside of prior RT field; dDF = disseminated distant failure; LITT = laser interstitial thermal therapy; reRT = reirradiation.
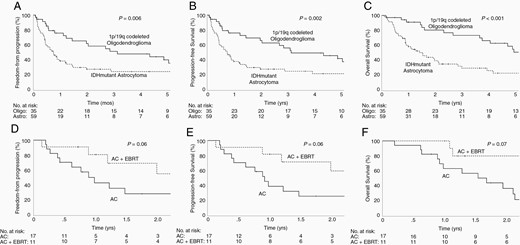
(A–C) Freedom-from progression (FFP), (PFS), and (OS) of recurrent 1p/19q codeleted oligodendroglioma (Oligo) versus IDH-mutant astrocytoma (Astro). (D-F) FFP, PFS, and OS of the subset of Astro patients with local failure > 2 years after prior RT who received either alkylating chemotherapy alone (AC) or AC plus repeat external beam radiation therapy (AC+EBRT).
Reirradiation With Temozolomide
Eighteen patients (7 Oligo and 11 Astro) received repeat EBRT (6 IMRT, 12 PBT) in combination with AC to a previously irradiated region of LR (14 early reRT and 4 delayed reRT). All received TMZ with reRT: 13 patients with concurrent and adjuvant TMZ, and 5 with only adjuvant TMZ. The median time from the initial RT to reRT was 11.3 years (range: 2.4–26.2). The median RT dose was 37.8 Gy (range: 35–54 Gy). Most regimens were standard fractionation at 1.8–2 Gy/fr, but two patients were treated with hypofractionated EBRT (40.05 Gy at 2.67 Gy/fr and 35 Gy at 3.5 Gy/fr). After a median follow-up of 13.3 months from the start of reRT, the 2-yr FFP was 61%, 2-yr PFS was 71%, and 2-year OS was 75%. There were 3 grade 3 sRN and 1 grade 2 sRN, but none when cumulative EQD2 was < 100 Gy (Figure 3A, B). The median time to RN was 9.9 months (range 6.8–11.7). There was no reported optic neuropathy or brainstem necrosis. All but one patient had available dosimetric data from the reRT course for dose-volume histogram analysis, and 14 patients also had composite RT plans. The median PTV was 61 cm3 (range: 12–456 cm3). The median cumulative EQD2 to the brain was 95 Gy (range: 86–107). The median maximum dose to the optic apparatus from the second course RT was 7 Gy (range: 0–27), with only 1 patient receiving dose >25 Gy (Figure 3C), and 2 patients received >80 Gy of maximum cumulative dose to the optic apparatus (Figure 3D). The median maximum dose to the brainstem was 7 Gy (range: 0–37 Gy), with only 1 patient receiving dose > 35 Gy (Figure 3E), and 2 patients received 90 Gy of maximum cumulative dose to the brainstem (Figure 3F).
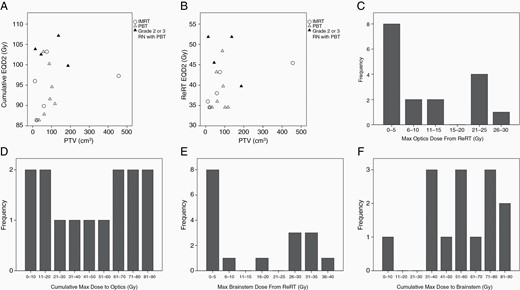
(A–B) Scatter plot of tumor volume versus reirradiation (reRT) or cumulative EQD2 (biologic equivalent dose at 2 Gy, assuming a/b = 3 for brain tissue) for a cohort of 18 patients treated with repeat external beam radiation therapy (EBRT) with temozolomide. (C–D) Maximum reRT or cumulative dose to optic structures. (E–F) Maximum reRT or cumulative dose to the brainstem.
Discussion
This study reports our institutional experience treating radiation-relapsed Astro and Oligo. Recurrent Astro exhibited significantly worse clinical outcomes than recurrent Oligo. More than 50% of lower grade Astro or Oligo transformed to higher grade disease at recurrence. Approximately half of the cohort received salvage surgery before SST, and approximately half of those with LF >2 years after initial RT also received reRT. Notably, Astro appeared to benefit from local therapy, including salvage surgery and early reRT. The most common type of initial SST was AC, which was associated with improved OS than non-AC. Early reRT appeared to be more commonly offered for recurrent Astro, fDF, or LF >2 years after the initial RT. Due to selection bias, the benefit of reRT could not be ascertained from this retrospective study. However, selective reRT using EBRT in combination with TMZ appears feasible with an acceptable risk of sRN.
Our study demonstrates markedly different survival outcomes between radiation-relapsed Oligo and Astro patients. While many studies have established the superior disease control and survival of Oligo over Astro in the upfront setting, our report contributes to the emerging literature suggesting that this difference persists at recurrence.3 Miller et al recently described their institutional outcomes of recurrent IDH-mutant gliomas and observed higher PFS for Oligo than Astro (median PFS of 4.2 years vs 2.6 years, respectively).13 Thus, future clinical trials of recurrent IDH-mutant gliomas should consider stratifying for these two molecular cohorts.
Salvage surgery remains an important consideration for the management of recurrent IDH-mutant gliomas. Our results indicate that approximately 50% of lower grade Astro or Oligo may have progressed to higher grade at recurrence, so repeat sampling through biopsy or resection should be strongly considered in establishing an accurate staging. Ramakrishna et al have previously reported their experience of 52 grade 2 gliomas who had reoperation after recurrence and demonstrated that the extent of resection (EOR) of the reoperation was prognostic for OS and PFS. Sixty percent of their cohort were radiation-relapsed gliomas, but molecular classification including IDH and 1p/19q status was not known.14 Our findings are overall consistent with their observation but also suggest that recurrent Astro may derive a more significant benefit from salvage surgery than Oligo, which echoes the observations that the EOR appears to have a more considerable prognostic impact for newly diagnosed Astro than Oligo.15–17 This discrepancy may be due to the more indolent natural history and enhanced sensitivity of Oligo to adjuvant therapies compared to Astro.16,17 Whether this discrepancy should influence the surgical approach of recurrent IDH-mutant gliomas may deserve further investigation. We also observed an emerging trend of LITT as another surgical approach to treat recurrent IDH-mutant gliomas. LITT is minimally invasive and may affect the blood-brain barrier and tumor micro-environment, so its role in managing recurrent IDH-mutant gliomas also deserves further investigation.18–20
In the current study, AC appears to be the more popular and more effective SST than non-AC. Previous studies have demonstrated objective response rates (ORR) of 50–60% for TMZ and PCV.21,22 Taal et al. previously described a cohort of 58 radiation-relapsed low-grade astrocytomas (86% IDH-mutant) treated with salvage TMZ and observed an ORR of 54%.21 Van Den Bent et al previously reported a multi-institutional retrospective study of 52 radiation-relapsed histological oligodendrogliomas or mixed oligoastrocytomas (IDH and 1p/19q status not available) treated with salvage PCV and observed an ORR of 63%.22 Multiple prospective studies have also demonstrated promising PFS outcomes by rechallenging with TMZ for unselected high-grade gliomas who have relapsed after definitive RT and TMZ.23–25 Approximately 10% of patients in our study received bevacizumab which has recently been shown to be not active for recurrent IDH-mutant gliomas in the TAVAREC study. The TAVAREC study was a randomized phase II study comparing TMZ alone vs TMZ plus bevacizumab for recurrent grade II-III astrocytomas without 1p/19q codeletions (65% with confirmed IDH mutation). The addition of bevacizumab to TMZ failed to improve OS, PFS, or quality of life.26 Altogether, these data suggest that TMZ or lomustine/PCV should be considered first-line SST for relapsed IDH-mutant gliomas whenever possible, and the use of bevacizumab should be limited. Future clinical trials testing novel agents for recurrent IDH-mutant gliomas may need to consider combining with AC or testing as second-line salvage therapy after progression on AC.
The role of reRT for recurrent IDH-mutant gliomas remains undefined. Existing evidence is mostly limited to small single-institution retrospective studies of low-grade gliomas without detailed molecular classification, limiting the comparison to the present investigation.9 Careful selection of patients may achieve efficacy and limit treatment toxicity, as may efforts to reduce total reRT doses, minimize target volumes, and prolong intervals between prior RT and reRT.27 The initial results of RTOG 1205, a randomized phase II trial of hypofractionated reRT and bevacizumab versus bevacizumab alone for recurrent (mostly IDH-wildtype) GBM, showed that reRT improved PFS at 6 months but not OS.28 Another small randomized study comparing reRT plus chemotherapy has demonstrated improved PFS for bevacizumab-refractory and mostly IDH-wildtype GBM (only 8% IDH-mutant) compared to chemotherapy alone.29 Similarly, we observed early reRT was associated with improved PFS for the Astro patients on MVA. Notably, repeat EBRT with TMZ was perhaps the most common reRT strategy and appeared feasible with an acceptable rate of sRN. However, selection bias may have confounded the observed association with improved survival outcomes, such as that the subset of Astro receiving AC+EBRT had longer disease-free interval since the initial RT and higher proportion with salvage surgery than those receiving AC alone (Supplementary Table S1). Thus, prospective clinical trials would be needed to confirm the benefit of reRT for radiation-relapsed IDH-mutant glioma. Outside of clinical trial setting and when administered with AC such as TMZ, we recommend careful patient selection (such as PTV < 100 cm3) and standard fractionated EBRT to a dose of 36–45 Gy while limiting the cumulative EQD2 to below 100 Gy.
The primary limitations of this study include its retrospective design from a single institution and the diverse treatment approaches over a lengthy period. The sample size is likely limited to detect small effect sizes, especially for the subset analyses. Given the retrospective nature and lack of randomized control, selection bias by the treating physicians to choose surgery, AC, or reRT may also confound the survival benefit observed as evident in Supplementary Table S1. Thus, the observed associations between treatment approaches with clinical outcomes should be considered hypothesis-generating and warrant further validation from prospective studies. Due to the irregular shape and often diffuse border of these recurrent tumors and the confounding appearances of treatment-related changes, tumor size and volume are not included in the multivariable analysis, and they may have a confounding effect on treatment selection and prognosis. Furthermore, the toxicities of multiple lines of alkylating chemotherapy may play an important role in treatment selection and are not captured in this retrospective analysis, so future prospective studies should also investigate the impact of salvage chemotherapy on patient reported outcomes and quality of life. Overall, this study supports that radiation-relapsed IDH-mutant gliomas should be considered for salvage surgery whenever possible and then receive AC as first-line SST. ReRT may be considered in combination with AC for selected case (focal disease and >2 years since the initial RT) to improve local control, but its benefit on survival would need to be clarified in prospective studies. Future studies to identify additional active systemic agents for relapsed IDH-mutant gliomas who have progressed on salvage AC would be an important area of unmet need.
Funding
None declared.
Conflict of interest statement. J.H. reports research support from Pfizer, outside the submitted work. J.L.C. reports research support from NeoImmuneTech Inc., Incyte Corporation, and Merck, outside the submitted work. The laboratory of M.G.C. has received funding support from NeoimmuneTech Inc. and M.G.C. receives royalties from UpToDate, outside the submitted work. M.G.C. has received research support from Orbus Pharmaceuticals for a clinical trial for patients with anaplastic oligodendrogliomas, outside the submitted work. G.P.D. is a cofounder of Immunovalent and a member of the Scientific Advisory Board for Ziopharm Oncology, outside the submitted work. A.H.K. is a consultant for Monteris Medical and received research grants from Monteris Medical, Stryker, and Collagen Matrix, outside the submitted work. M.R.C. reports funding from IMRIS Inc. for an unrestricted educational grant to support an iMRI database and outcomes analysis project, the IMRIS Muliticenter intraoperative MRI Neurosurgery Database (I-MiND), The Head for the Cure Foundation, Mrs. Carol Rossfeld and The Alex & Alice Aboussie Family Charitable Foundation, an unrestricted educational grant from the Subcortical Surgery Grant Program, and research support from Barbara and George Holtzman, outside the submitted work. E.C.L. reports stock ownership: Neurolutions, General Sensing, Osteovantage, Pear Therapeutics, Face to Face Biometrics, Immunovalent, Caeli Vascular, Acera, Sora Imaging Solutions, Inner Cosmos; consultant for Monteris Medical, E15, Acera, Alcyone, Intellectual Ventures, Medtronic Inc., Neurolutions, Osteovantage, Pear Therapeutics, Inc., Sante Ventures, and Microbot; Scientific Advisory Board for Pear Therapeutics, Microbot; licensing from Intellectual Property: Neurolutions, Osteovantage, Caeli Vascular; and licensing/product development agreements or royalties for inventions/IP: Cerovations, Intellectual Ventures, outside the submitted work. The remaining authors declare no conflict of interest.
Authorship Statement. Study design: S.R. and J.H. Data collection: S.M., S.R., J.L.C., M.G.C., T.M.J., G.A., C.D.A., M.R.C., E.C.L., J.L.D., G.P.D., A.H.K., and J.H. Analysis and interpretation: S.M., S.R., J.L.C., M.G.C., T.M.J., G.A., C.D.A., M.R.C., E.C.L., J.L.D., G.P.D., A.H.K., and J.H. Writing: S.M., S.R., and J.H. Manuscript review and approval: S.M., S.R., J.L.C., M.G.C., T.M.J., G.A., C.D.A., M.R.C., E.C.L., J.L.D., G.P.D., A.H.K., and J.H.
References
Author notes
These authors contributed equally to this work.
- radiation therapy
- astrocytoma
- chemotherapy regimen
- heterogeneity
- glioma
- isocitrates
- oligodendroglioma
- oligonucleotides
- oxidoreductase
- salvage therapy
- surgical procedures, operative
- surgery specialty
- treatment outcome
- temozolomide
- systemic therapy
- kaplan-meier survival curve
- cox proportional hazards models
- progression-free survival
- re-irradiation




