-
PDF
- Split View
-
Views
-
Cite
Cite
Cheng-Jiang Wei, Shu-Chen Gu, Jie-Yi Ren, Yi-Hui Gu, Xiang-Wen Xu, Xin Chou, Xiang Lian, Xin Huang, Hai-Zhou Li, Ya-Shan Gao, Bin Gu, Tao Zan, Zhi-Chao Wang, Qing-Feng Li, The impact of host immune cells on the development of neurofibromatosis type 1: The abnormal immune system provides an immune microenvironment for tumorigenesis, Neuro-Oncology Advances, Volume 2, Issue Supplement_1, July 2020, Pages i33–i39, https://doi.org/10.1093/noajnl/vdz037
Close - Share Icon Share
Abstract
AbstractThe immune system plays an essential role in the development of tumors, which has been demonstrated in multiple types of cancers. Consistent with this, immunotherapies with targets that disrupt these mechanisms and turn the immune system against developing cancers have been proven effective. In neurofibromatosis type 1 (NF1), an autosomal dominant genetic disorder, the understanding of the complex interactions of the immune system is incomplete despite the discovery of the pivotal role of immune cells in the tumor microenvironment. Individuals with NF1 show a loss of the NF1 gene in nonneoplastic cells, including immune cells, and the aberrant immune system exhibits intriguing interactions with NF1. This review aims to provide an update on recent studies showing the bilateral influences of NF1 mutations on immune cells and how the abnormal immune system promotes the development of NF1 and NF1-related tumors. We then discuss the immune receptors major histocompatibility complex class I and II and the PD-L1 mechanism that shield NF1 from immunosurveillance and enable the immune escape of tumor tissues. Clarification of the latest understanding of the mechanisms underlying the effects of the abnormal immune system on promoting the development of NF1 will indicate potential future directions for further studies and new immunotherapies.
Neurofibromatosis type 1 (NF1), caused by mutations in the NF1 tumor suppressor gene, is a common autosomal dominant genetic disorder with an incidence of approximately 1 in 3000 individuals worldwide.1 The majority of affected individuals are predisposed to benign peripheral nerve sheath tumors arising from abnormal Schwann cells, including cutaneous neurofibromas (CNFs) and plexiform neurofibromas (PNFs).2,3 Patients with the disease suffer negative effects, both physically and psychologically. However, the medical treatment of NF1 is still disappointing, and so there is an urgent and continuing need for improved therapies for NF1.
For a long time, there was debate about whether the immune system functions as a cancer controller. Only recently has the idea become generally accepted that the immune system not only prevents tumor growth but also plays an essential role in promoting tumor growth through a process called immunoediting.4,5 Immunotherapies designed to target and disrupt these mechanisms and turn the immune system against developing cancers have been tested and shown to be effective against multiple tumors, including breast cancer,6,7 liver cancer,8 and lung cancer.9 Understanding the mechanism of the complicated role of the immune system in NF1 might also contribute to the discovery of new therapies.
However, this mechanism has been more controversial in NF1. As an autosomal dominant genetic disorder, NF1 mutations occur in nonneoplastic cells, including immune cells. NF1 encodes neurofibromin, which functions as a GTPase-activating protein (GAP) and is a negative regulator of RAS signaling. This signaling pathway is an essential regulator of cell processes that accelerate the transformation from active Ras-GTP to inactive Ras-GDP.10,11 Mutations of the NF1 gene consequently activate Ras signaling, and the hyperactivation of this in NF1-mutated immune cells contributes to an abnormal host immune system. Consistent with this, there might be a more intriguing interplay among these multiple nonneoplastic cells in the stroma (Figure 1). Together, the abnormalities in the immune microenvironment indicate more profound and complex impacts on the development of NF1 and NF1-related tumors.
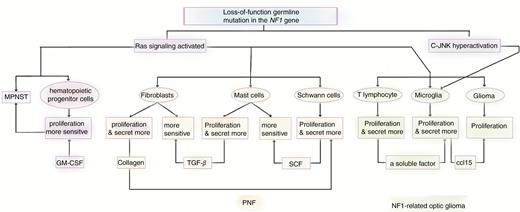
The abnormal immune system introduced by mutations in the NF1 gene promotes NF1 and NF1-related tumor initiation and development. Loss-of-function germline mutation in the NF1 genes in host immune cells introduces an abnormal immune system by activating the Ras signaling pathways. Consistent with this, interactions among multiple tumor cells and nonneoplastic cells in the NF1 microenvironment are also found. Eventually, the mutated immune system contributes to the initiation and development of NF1 and NF1-related tumors.
Previous reviews on this topic focused on only one type of immune cell or introduced immune cells into NF1 separately without underlining the pivotal impacts of mutations in the NF1 gene in immune cells. In this article, we will review the updated findings of how mutations in the NF1 gene affect the immune system both directly (such as by the activation of Ras signaling in immune cells) and indirectly (influences exerted by other mutated cells) in NF1. More importantly, we discuss the current studies about how the abnormal immune system promotes the growth and development of NF1 and NF1-related tumors. Finally, we review recent studies about abnormalities in immune receptors (major histocompatibility complex [MHC] class I and II, PD1/PD-L1) expressed on NF1 tumor cells,12,13 which are essential for the immune escape of tumors (Figure 2). Understanding these mechanisms might indicate further potential therapies for NF1 and improve treatment for NF1-related tumors.
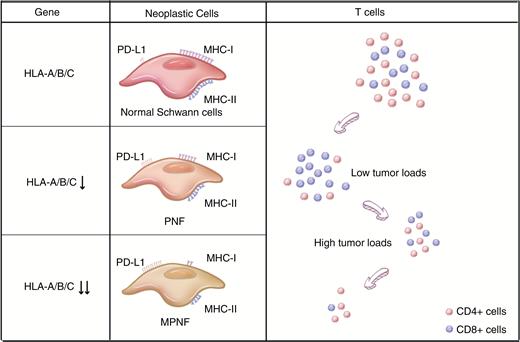
Changes in the expression of immune receptors in NF1 and abnormalities in T lymphocytes in the tumor microenvironment with NF1 development. From normal cells to neurofibroma and finally to MPNST, the ability of tumor cells to escape immunosurveillance is enhanced by degrees. On the one hand, NF1-associated Schwann cells have a gradually reduced level of MHC classes I and II compared with that of normal human Schwann cells, and this effect is caused by the downregulation of HLA-A/B/C. Consistent with this, NF1 tumor tissues show upregulated expression of PD-L1. On the other hand, there is a reduced T cell count in the NF1 microenvironment with an increased proportion of CD8+ T cells in low tumor loads and a decreased proportion in high tumor loads. CD8+ T cells are considered one of the major forces against tumor development, and the decrease in this cell type also helps the immune escape in NF1.
Discussion
It has become generally accepted recently that the immune system promotes the growth of tumors through a process called immunoediting, which comprises 3 phases: elimination, equilibrium, and escape.14,15 NF1 is frequently initiated by genetic changes and epigenetic modifications in the NF1 gene.16 However, unlike other tumors, the mutated NF1 gene also occurs in immune cells and leads to abnormalities of the immune microenvironment (Table 1). Generally, the impacts are exerted in 2 ways: (1) direct influence of the mutated NF1 gene in immune cells results in an irregular tendency toward proliferation or cell function disorders, and (2) an indirect effect is achieved through complex interactions among different NF1-mutated cells. Here, we focus on the mechanism by which the aberrant immune system in the tumor microenvironment influences NF1 and NF1-related tumors (Table 2).
| Immune cells . | Increase (+) or decrease (−) in counts . | Signaling pathway influencing the immune cells . | Other related factors in the microenvironment . | Effects of these factors . | Interaction with other cells . | Effects of the interaction . | Reference . |
|---|---|---|---|---|---|---|---|
| Hematopoietic progenitor cell | + | Promoting growth by Ras signaling | GM-CSF, IL-3, SCF | Increase after stimulation | / | / | 17–19 |
| T lymphocytes | +/− (varied among different kinds of T lymphocytes) | Ras signaling and other pathway requiring further investigations | Inflammatory cytokines such as IFNγ | Recruit CD8+ T cells | Macrophages; Tumor cells | Secretion of cytokines mediating inflammation. The proliferation of T cells varies among different stages of tumors | 20, 21 |
| Mast cells | + | Ras signaling and other signaling pathways such as c-kit | SCF | SCF | Schwann cells | NF1−/− Schwann cells increase degranulation of Nf1+/− mast cells by c-kit and mediate activation of the PIK-3 pathways | 22–24 |
| Microglia/ macrophage | + (in the optic nerves) | Neurofibromin activates a c-Jun NH2-kinase (JNK)-dependent pathway involving mixed lineage kinase (MLK) and Rac1 signaling | CX3CR1 | The decrease in CX3CR1 is related to the increase in microglia | T cells | Activated T cells secrete soluble factors to stimulate the microglia to express the Ccl15 | 25–27 |
| Immune cells . | Increase (+) or decrease (−) in counts . | Signaling pathway influencing the immune cells . | Other related factors in the microenvironment . | Effects of these factors . | Interaction with other cells . | Effects of the interaction . | Reference . |
|---|---|---|---|---|---|---|---|
| Hematopoietic progenitor cell | + | Promoting growth by Ras signaling | GM-CSF, IL-3, SCF | Increase after stimulation | / | / | 17–19 |
| T lymphocytes | +/− (varied among different kinds of T lymphocytes) | Ras signaling and other pathway requiring further investigations | Inflammatory cytokines such as IFNγ | Recruit CD8+ T cells | Macrophages; Tumor cells | Secretion of cytokines mediating inflammation. The proliferation of T cells varies among different stages of tumors | 20, 21 |
| Mast cells | + | Ras signaling and other signaling pathways such as c-kit | SCF | SCF | Schwann cells | NF1−/− Schwann cells increase degranulation of Nf1+/− mast cells by c-kit and mediate activation of the PIK-3 pathways | 22–24 |
| Microglia/ macrophage | + (in the optic nerves) | Neurofibromin activates a c-Jun NH2-kinase (JNK)-dependent pathway involving mixed lineage kinase (MLK) and Rac1 signaling | CX3CR1 | The decrease in CX3CR1 is related to the increase in microglia | T cells | Activated T cells secrete soluble factors to stimulate the microglia to express the Ccl15 | 25–27 |
| Immune cells . | Increase (+) or decrease (−) in counts . | Signaling pathway influencing the immune cells . | Other related factors in the microenvironment . | Effects of these factors . | Interaction with other cells . | Effects of the interaction . | Reference . |
|---|---|---|---|---|---|---|---|
| Hematopoietic progenitor cell | + | Promoting growth by Ras signaling | GM-CSF, IL-3, SCF | Increase after stimulation | / | / | 17–19 |
| T lymphocytes | +/− (varied among different kinds of T lymphocytes) | Ras signaling and other pathway requiring further investigations | Inflammatory cytokines such as IFNγ | Recruit CD8+ T cells | Macrophages; Tumor cells | Secretion of cytokines mediating inflammation. The proliferation of T cells varies among different stages of tumors | 20, 21 |
| Mast cells | + | Ras signaling and other signaling pathways such as c-kit | SCF | SCF | Schwann cells | NF1−/− Schwann cells increase degranulation of Nf1+/− mast cells by c-kit and mediate activation of the PIK-3 pathways | 22–24 |
| Microglia/ macrophage | + (in the optic nerves) | Neurofibromin activates a c-Jun NH2-kinase (JNK)-dependent pathway involving mixed lineage kinase (MLK) and Rac1 signaling | CX3CR1 | The decrease in CX3CR1 is related to the increase in microglia | T cells | Activated T cells secrete soluble factors to stimulate the microglia to express the Ccl15 | 25–27 |
| Immune cells . | Increase (+) or decrease (−) in counts . | Signaling pathway influencing the immune cells . | Other related factors in the microenvironment . | Effects of these factors . | Interaction with other cells . | Effects of the interaction . | Reference . |
|---|---|---|---|---|---|---|---|
| Hematopoietic progenitor cell | + | Promoting growth by Ras signaling | GM-CSF, IL-3, SCF | Increase after stimulation | / | / | 17–19 |
| T lymphocytes | +/− (varied among different kinds of T lymphocytes) | Ras signaling and other pathway requiring further investigations | Inflammatory cytokines such as IFNγ | Recruit CD8+ T cells | Macrophages; Tumor cells | Secretion of cytokines mediating inflammation. The proliferation of T cells varies among different stages of tumors | 20, 21 |
| Mast cells | + | Ras signaling and other signaling pathways such as c-kit | SCF | SCF | Schwann cells | NF1−/− Schwann cells increase degranulation of Nf1+/− mast cells by c-kit and mediate activation of the PIK-3 pathways | 22–24 |
| Microglia/ macrophage | + (in the optic nerves) | Neurofibromin activates a c-Jun NH2-kinase (JNK)-dependent pathway involving mixed lineage kinase (MLK) and Rac1 signaling | CX3CR1 | The decrease in CX3CR1 is related to the increase in microglia | T cells | Activated T cells secrete soluble factors to stimulate the microglia to express the Ccl15 | 25–27 |
The abnormal immune cell effects on neurofibromatosis type 1 and NF1-related tumors
| NF1 and NF1-related tumors . | Types of immune cells related to these tumors . | Direct influences of these immune cells on NF1-related tumors . | Related factors in these influences . | Relations between these cells and the mechanisms . | References . |
|---|---|---|---|---|---|
| PNF | Mast cells; T lymphocytes | Mast cells: 1. Potentiate fibroblasts and induce cells proliferation, blood-vessel growth and metastasis. 2. Secrete growth factors such as VEGF and MMPs, which promote angiogenesis and the development of neurofibroma. 3. SCF/c-kit axis is important in PNF. T lymphocytes: Loss of function in T lymphocytes was found. | TGF-β, VEGF, SCF | SCF secreted by Schwann cells promotes the growth of mast cells and fibroblasts. | 20–22, 28–33 |
| NF1-related optic pathway glioma | Microglia; T lymphocytes | Microglia are essential in NF1-related optic pathway glioma formation by secreting Ccl15, which stimulates glioma growth. T lymphocytes secrete a soluble factor to stimulate the proliferation and activation of microglia. | Ccl15 | Activated T cells that are stimulated by Ccl2 and Ccl12 secrete soluble factors to stimulate microglia to express Ccl15 | 26, 27, 34, 35 |
| NF1-related MPNST | Hematopoietic cells | Multiple haploinsufficient hematopoietic cells are increased in the MPNST microenvironment and accelerate the development of it. Underlying mechanisms merit further studies. | / | / | 36 |
| NF1 and NF1-related tumors . | Types of immune cells related to these tumors . | Direct influences of these immune cells on NF1-related tumors . | Related factors in these influences . | Relations between these cells and the mechanisms . | References . |
|---|---|---|---|---|---|
| PNF | Mast cells; T lymphocytes | Mast cells: 1. Potentiate fibroblasts and induce cells proliferation, blood-vessel growth and metastasis. 2. Secrete growth factors such as VEGF and MMPs, which promote angiogenesis and the development of neurofibroma. 3. SCF/c-kit axis is important in PNF. T lymphocytes: Loss of function in T lymphocytes was found. | TGF-β, VEGF, SCF | SCF secreted by Schwann cells promotes the growth of mast cells and fibroblasts. | 20–22, 28–33 |
| NF1-related optic pathway glioma | Microglia; T lymphocytes | Microglia are essential in NF1-related optic pathway glioma formation by secreting Ccl15, which stimulates glioma growth. T lymphocytes secrete a soluble factor to stimulate the proliferation and activation of microglia. | Ccl15 | Activated T cells that are stimulated by Ccl2 and Ccl12 secrete soluble factors to stimulate microglia to express Ccl15 | 26, 27, 34, 35 |
| NF1-related MPNST | Hematopoietic cells | Multiple haploinsufficient hematopoietic cells are increased in the MPNST microenvironment and accelerate the development of it. Underlying mechanisms merit further studies. | / | / | 36 |
The abnormal immune cell effects on neurofibromatosis type 1 and NF1-related tumors
| NF1 and NF1-related tumors . | Types of immune cells related to these tumors . | Direct influences of these immune cells on NF1-related tumors . | Related factors in these influences . | Relations between these cells and the mechanisms . | References . |
|---|---|---|---|---|---|
| PNF | Mast cells; T lymphocytes | Mast cells: 1. Potentiate fibroblasts and induce cells proliferation, blood-vessel growth and metastasis. 2. Secrete growth factors such as VEGF and MMPs, which promote angiogenesis and the development of neurofibroma. 3. SCF/c-kit axis is important in PNF. T lymphocytes: Loss of function in T lymphocytes was found. | TGF-β, VEGF, SCF | SCF secreted by Schwann cells promotes the growth of mast cells and fibroblasts. | 20–22, 28–33 |
| NF1-related optic pathway glioma | Microglia; T lymphocytes | Microglia are essential in NF1-related optic pathway glioma formation by secreting Ccl15, which stimulates glioma growth. T lymphocytes secrete a soluble factor to stimulate the proliferation and activation of microglia. | Ccl15 | Activated T cells that are stimulated by Ccl2 and Ccl12 secrete soluble factors to stimulate microglia to express Ccl15 | 26, 27, 34, 35 |
| NF1-related MPNST | Hematopoietic cells | Multiple haploinsufficient hematopoietic cells are increased in the MPNST microenvironment and accelerate the development of it. Underlying mechanisms merit further studies. | / | / | 36 |
| NF1 and NF1-related tumors . | Types of immune cells related to these tumors . | Direct influences of these immune cells on NF1-related tumors . | Related factors in these influences . | Relations between these cells and the mechanisms . | References . |
|---|---|---|---|---|---|
| PNF | Mast cells; T lymphocytes | Mast cells: 1. Potentiate fibroblasts and induce cells proliferation, blood-vessel growth and metastasis. 2. Secrete growth factors such as VEGF and MMPs, which promote angiogenesis and the development of neurofibroma. 3. SCF/c-kit axis is important in PNF. T lymphocytes: Loss of function in T lymphocytes was found. | TGF-β, VEGF, SCF | SCF secreted by Schwann cells promotes the growth of mast cells and fibroblasts. | 20–22, 28–33 |
| NF1-related optic pathway glioma | Microglia; T lymphocytes | Microglia are essential in NF1-related optic pathway glioma formation by secreting Ccl15, which stimulates glioma growth. T lymphocytes secrete a soluble factor to stimulate the proliferation and activation of microglia. | Ccl15 | Activated T cells that are stimulated by Ccl2 and Ccl12 secrete soluble factors to stimulate microglia to express Ccl15 | 26, 27, 34, 35 |
| NF1-related MPNST | Hematopoietic cells | Multiple haploinsufficient hematopoietic cells are increased in the MPNST microenvironment and accelerate the development of it. Underlying mechanisms merit further studies. | / | / | 36 |
Activated Mast Cells and Dysfunctional T Cells Contribute to the Growth and Development of PNF
PNF is among the most common tumor types in NF1 and contributes to multiple clinical deficits.37,38 In the immune microenvironment of PNF, abnormal mast cells have long been recognized as an essential part of tumor development. At first, mast cells were found to be related to the activation of Ras signaling and were slightly more abundant in NF1 mice than in healthy controls,28,29 which demonstrated the direct impact exerted by the NF1 gene on mast cells. Consistent with this, Nf1−/− Schwann cells secreted a large amount of stem cell factors (SCFs), recruiting the mast cells to the NF1 tumor microenvironment.22 This study further revealed through gain-of-function experiments with heterozygous mast cells that these Nf1+/− mast cells were far more sensitive to SCF stimulation and migrated faster than normal mast cells.22 Later, an abnormality in the function of Nf1+/− mast cells revealed that Schwann cells from Nf1−/− embryos promoted the increased degranulation of these cells by c-kit-mediated activation of the PIK-3 pathway.23,24 Consistent with this, these mast cells promoted the growth of PNF in multiple ways. Interactions in the NF1 stroma demonstrated that SCF-stimulated Nf1+/− mast cells were able to potentiate Nf1+/− fibroblast functions by secreting 2.5-fold more TGF-β than that of wild-type mast cells.30,31 Fibroblasts are an essential part of the NF1 microenvironment, and the collagen secreted by fibroblasts accounted for approximately half of the dry weight of the neurofibroma.30,31 Cancer-associated fibroblasts frequently promote cancer progression by inducing cell proliferation,39 inflammation,22,32 blood vessel growth, and metastasis.40,41 Interestingly, Nf1+/− fibroblasts showed the most significant ability to secrete collagen after stimulation by Nf1+/− mast cells compared with that of normal fibroblasts, which resulted from a Ras-c-abl pathway related to the Nf1 gene.22,32 Consistent with this, mast cells were also found to interact with endothelial cells by secreting growth factors such as VEGF and MMPs, which promote angiogenesis in neurofibroma.32,33 In summary, mast cells stimulate the development of NF1, especially PNF, by promoting the growth of fibroblasts and angiogenesis.
Compared with mast cell interactions, the interactions between T cells and tumor tissues are more complex and vary among the different types of these cells. In a cohort of 37 NF1 patients, a low overall T-cell count was found by global blood cell analysis.20 Interestingly, this study revealed that CD8+/CD27− and CD8+/CD57+ effector T cells in NF1 patients strongly increased when there was a low tumor load and decreased to levels below that of the control in patients with a high tumor load.20 However, an in vitro study showed that the loss of the NF1 gene in T cells resulted in enhanced Ras activation and an increase in the number of both mature and immature T cells, but the loss of NF1 also resulted in a reduction in proliferation after the T cell receptor and IL-2R stimulation.21 These 2 in vivo and in vitro studies seem contradictory, indicating a more complex interaction between the NF1 tumor microenvironment and the NF1-mutated T lymphocytes. As the activation of Ras signaling by mutations in the NF1 gene contributes to the increase in T lymphocytes in vitro, the decrease in T lymphocytes in vivo might result from the interactions among other cells in the NF1 patients or other signaling pathways that remain unclarified.
Activated Microglia Promote NF1 Low-Grade Glioma Formation and Development
Approximately 15–20% of children with NF1 are prone to the development of low-grade glial cell neoplasms (gliomas) along the optic pathway (optic pathway gliomas), which leads to 30–50% of these children developing visual impairment.34,42 Microglia have long been considered to be an essential part of the glioma microenvironment, and increased mutated microglia in the tumor microenvironment were reported to be critical cellular determinants of optic glioma proliferation both in vivo and in vitro.34,35 Hyperactivation of c-Jun NH2-terminal kinase (JNK) in the increased Nf1+/− microglia was observed, and the use of a relatively nonselective pharmacologic JNK inhibitor attenuated tumor proliferation.25 Further studies showed that reduced expression of CX3CR1, a chemokine receptor on microglia, contributed to delayed NF1-related optic pathway glioma.26,34 Recently, increases in both T cells and microglia were found in the NF1-related low-grade glioma (LGG) microenvironment.27 Moreover, no successful growth of LGG was found in immunocompromised athymic mice,27 suggesting the pivotal role of mutated T lymphocytes in the formation and development of LGG. This study also clarified parts of the mechanism by which activated T lymphocytes secrete soluble factors to stimulate microglia to express Ccl15, suggesting a T cell-microglia interaction in providing a microenvironment for NF1-related LGG engraftment.27 It was further revealed that the infiltration of T cells in the microenvironment was mediated by Ccl2 and Ccl12, which were secreted by optic glioma stem cells(o-GSCs), the cancer stem cells of murine Nf1 optic glioma.43 Consistent with this, another study comparing male and female Nf1 optic glioma mice revealed that microglia could be activated by estrogen, the primary female sex hormone, through the ERβ receptor.44 This characteristic contributed to the phenomenon that girls with NF1-related OPG were 5–10 times more likely to suffer from visual impairment than boys.45 These findings suggest multiple factors contributing to NF1-related OPG formation and development, but all of these pathways require activation microglia. The underlying molecular and cellular etiologies need to be clarified, and these findings might contribute to developing treatments to reverse visual decline.
NF1 Haploinsufficient Hematopoietic Cells Promote the Development of NF1-Related Malignant Peripheral Nerve Sheath Tumor
NF1-related malignant peripheral nerve sheath tumor (MPNST) is an aggressive peripheral nerve tumor that arises from PNF and develops in 5–10% of these patients.17 NF1-related MPNST patients showed different clinical features, such as patient age, tumor size, and clinical outcome of treatment compared with those of sporadic MPNST patients,17 and these differences might be due to the mutated tumor microenvironment of NF1 patients. Previous studies revealed that mutations in the NF1 gene led to an increase in hematopoietic progenitor cells GM-CSF stimulation.18,19 Without GM-CSF, such as in Nf1−/−Gmcsf−/− mice, there was a reduced myeloid cell count compared with that in wild-type Nf1−/− mice; this GM-CSF-induced acceleration of the proliferation of myeloid cells, however, only appeared in Nf1−/− cells.36 A recent study comparing the tumor stroma of 2 NF1-related MPNST mouse models with or without Nf1 haploinsufficiency observed an enrichment of myeloid and mast cells in MPNST with Nf1 haploinsufficiency in the tumor microenvironment, and these Nf1 haploinsufficient hematopoietic cells accelerated MPNST development.46 Further studies are needed to find the underlying mechanism of the effects of these mutated hematopoietic cells in NF1-related MPNST, and this might contribute to new therapies to treat this type of soft tissue sarcoma.
The Change in the Expression of Immune Receptors on Tumor Cells Induces the Immune Escape of NF1 and NF1-Related MPNST
MHC class I and II separately process and present antigens to effector and helper T cells. In multiple types of cancers, a reduced level of MHC expression has been found and results in a resistance to T cell attack.47,48 In MPNST, transporter-activator protein 1 (TAP1), which loads peptide antigens onto MHC class I molecules, was downregulated, and CD74, which functions in the processing and transportation of MHC class II molecules, was also reduced in Schwann cells.12 A recent study using gene analysis and flow cytometry further observed a decrease in HLA-A/B/C gene levels in all NF1-associated tumors that were examined and, interestingly, in all NF1-related cells within the tumor microenvironment.49 These results also suggest the possibility of the participation of nonneoplastic cells in the process of immune escape in NF1.
Increased levels of PD-1 and PD-L1, which are caused by the activation of multiple signaling pathways, such as the MAP kinase and Akt-mTOR pathways, function as immune suppressors to restrict the response of T cells.50,51 Recently, anti-PD-1 and anti-PD-L1 exhibited promising results in multiple types of cancers, including lung cancer,17 renal cancer,52 and breast cancer.53 In NF1, a recent study showed increased expression of PD-L1 on tumor cells, suggesting a possible strategy for immunotherapies targeting immune checkpoint inhibitors.13 The specific mechanism of the increased levels of PD-L1 and the efficiency of related immunotherapies merit further investigation.
Conclusion and Further Research
NF1 progression is not an entirely cell-autonomous process. The growth and development of NF1 and NF1-related tumors are influenced and even driven by cells of the immune system, especially mutated immune cells. Additionally, the immune system also plays a host-protective role in NF1 initiation and progression, and escape from immunosurveillance by the remodeling of multiple molecules indicates the reasonable expectation of the promising effects of related immunotherapies.
However, further studies on cellular and biochemical mechanisms are needed for a deeper understanding of the reciprocal impacts between the host immune system and NF1-related tumors. Some types of immune cells, such as B lymphocytes, have not been well studied in NF1. Moreover, an understanding of the interplay between these abnormal immune cells remains unclarified. Despite the description of some of the interactions, such as the T cell-microglia interaction, future studies are needed to complete the entire network diagram describing each part of this abnormal immune system. Research to identify these interactions is ongoing, and a better understanding should help in the design of new immunotherapies for NF1.
Funding
This work was supported by grants from Youth Doctor Collaborative Innovation Team Project (QC201803) of Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, the Shanghai Youth Top-Notch Talent Program (for Wang Zhichao), and The Shanghai Youth Talent Support Program (for Wang Zhichao).
Conflict of interest statement
None declared.
References
Author notes
These authors contributed equally to this work.




