-
PDF
- Split View
-
Views
-
Cite
Cite
Yazmin Odia, Matthew D Hall, Timothy Francis Cloughesy, Patrick Y Wen, Isabel Arrillaga-Romany, Doured Daghistani, Minesh P Mehta, Rohinton S Tarapore, Samuel C Ramage, Joshua E Allen, Selective DRD2 antagonist and ClpP agonist ONC201 in a recurrent non-midline H3 K27M-mutant glioma cohort, Neuro-Oncology, Volume 26, Issue Supplement_2, April 2024, Pages S165–S172, https://doi.org/10.1093/neuonc/noae021
Close - Share Icon Share
Abstract
Diffuse midline glioma, H3 K27-altered (H3 K27M-altered DMG) are invariably lethal, disproportionately affecting the young and without effective treatment besides radiotherapy. The 2016 World Health Organization (WHO) Central Nervous System (CNS) Tumors Classification defined H3 K27M mutations as pathognomonic but restricted diagnosis to diffuse gliomas involving midline structures by 2018. Dordaviprone (ONC201) is an oral investigational small molecule, DRD2 antagonist, and ClpP agonist associated with durable responses in recurrent H3 K27M-mutant DMG. Activity of ONC201 in non-midline H3 K27M-mutant diffuse gliomas has not been reported.
Patients with recurrent non-midline H3 K27M-mutant diffuse gliomas treated with ONC201 were enrolled in 5 trials. Eligibility included measurable disease by Response Assessment in Neuro-Oncology (RANO) high-grade glioma, Karnofsky/Lansky performance score ≥60, and ≥90 days from radiation. The primary endpoint was overall response rate (ORR).
Five patients with cerebral gliomas (3 frontal, 1 temporal, and 1 parietal) met inclusion. One complete and one partial response were reported by investigators. Blinded independent central review confirmed ORR by RANO criteria for 2, however, 1 deemed nonmeasurable and another stable. A responding patient also noted improved mobility and alertness.
H3 K27M-mutant diffuse gliomas occasionally occur in non-midline cerebrum. ONC201 exhibits activity in H3 K27M-mutant gliomas irrespective of CNS location.
The diffuse midline glioma, H3 K27-altered diagnosis is restricted to midline locations per World Health Organization 2021 classification.
H3 K27M mutation occurs infrequently in non-midline gliomas.
ONC201 may exhibit single-agent activity in non-midline H3 K27-mutant gliomas.
In the 2021 World Health Organization (WHO) classification, diffuse midline glioma, H3 K27-altered is defined by tumor location and presence of H3 K27 alterations. This mutation occurs infrequently in non-midline central nervous system gliomas. This case series reports outcomes for 5 patients with recurrent, non-midline H3 K27-mutant gliomas treated with ONC201 as part of 5 prospective clinical trials. The results suggest ONC201 may exhibit single-agent activity in non-midline H3 K27-mutant gliomas. Further study of non-midline H3 K27M-mutant tumors is recommended to characterize survival and drug activity.
The H3 K27M mutation occurs in approximately 80% of pediatric and 15%–58% of adult diffuse midline gliomas (DMG),1,2 with the majority centered in the thalamus or brainstem. Since the 2016 WHO classification of central nervous system (CNS) tumors, DMGs exhibiting the H3 K27M mutation were recognized as a distinct grade IV glioma category regardless of histological features.3 In the 2021 WHO classification of CNS tumors, the diagnosis was redefined as DMG, H3 K27-altered (H3 K27M-altered DMG), restricted to infiltrative gliomas and midline locations, from thalami to brainstem to spinal cord. Surgical resection is generally limited due to anatomic constraints, and the standard of care remains radiotherapy alone.4 Dozens of clinical trials of radiation dose escalation or modifications, radiosensitizing agents, and cytotoxic and targeted drugs have failed to improve outcomes. As a result, H3 K27M-mutant DMGs remain uniformly fatal, with a median survival of 11–15 months.5,6
The updated 2021 WHO classification now excludes non-midline gliomas from the H3 K27M-altered DMG diagnosis. While the H3 K27M mutation is associated with poor prognosis in DMGs, its incidence, biological and clinical impact, and prognostic significance in the extremely rare non-midline gliomas remain unclear.7,8 Due to their hemispheric location, in contrast to midline gliomas, these non-midline gliomas may be more amenable to resection, which could yield superior survival. Second, non-midline tumors may possess a distinct pattern of concurrent genetic alterations and molecular aberrations distinct from brainstem and thalamic lesions. Together, these factors may contribute to different patterns of response and survival in non-midline H3 K27M-mutant gliomas compared to classical H3 K27M-altered DMG located within midline structures.
ONC201 is an investigational small molecular antitumor agent that crosses the blood-brain barrier and is both a selective dopamine DRD2/3 antagonist, resulting in dual inactivation of Akt and ERK and TRAIL-mediated apoptosis, and a caseinolytic protease P agonist resulting in mitochondrial dysfunction in cancer cells.9,10 In a phase 2 trial, ONC201 did not improve progression-free survival in recurrent glioblastomas in adults who were not molecularly stratified, but an initial series of patients harboring the H3 K27M mutation demonstrated sustained clinical and radiographic responses.11–13 First results from the completed phase 1 trial in pediatrics were recently published,14 along with favorable clinical outcomes including a complete response among expanded access cases.12 ONC201 is now being tested in a randomized phase 3 trial for newly diagnosed H3 K27M-mutant diffuse glioma [NCT05580562].
The purpose of this series was to evaluate the clinical efficacy of ONC201 monotherapy in recurrent H3 K27M-mutant gliomas in non-midline locations in patients evaluable for objective response by Response Assessment for Neuro-Oncology (RANO) criteria.
Methods
Cohort
Patients in our cohort were selected from 5 prospective ONC201 studies (ONC006 [NCT02525692], ONC013 [CT03295396], ONC014 [NCT03416530, pediatric], ONC016 [compassionate use program], and ONC018 [NCT03134131, expanded access program]). Each protocol received institutional review board approval. Pediatric and adult patients (≥2 years of age) with recurrent H3 K27M-mutant diffuse glioma were previously screened for an integrated analysis of monotherapy efficacy objective response by RANO high-grade gliomas (HGG) criteria of ONC201 in recurrent H3 K27M-mutant DMGs, but our patient series were excluded from prior analyses15 due to tumors with non-midline location. All patients in our non-midline cohort were enrolled up to February 26, 2020, the same enrollment and data cutoff for the primary analysis in patients with midline disease.16 Eligibility criteria included age ≥2 years, Karnofsky/Lansky performance score (KPS/LPS) ≥60, and prior radiation therapy with a washout of ≥90 days prior to first dordaviprone dose. Additional washout requirements included 23 days for temozolomide, 42 days for antibodies, and 28 days for other anticancer therapies. Patients were required to have progressive H3 K27M-mutant non-midline glioma that was measurable per RANO-HGG criteria (assessed by investigator). Patients were excluded if they had leptomeningeal spread, cerebrospinal fluid dissemination, diffuse intrinsic pontine glioma (DIPG), or primary spinal disease. All imaging and outcome data for patients in this study were prospectively collected.
Treatment
All patients received oral ONC201 monotherapy at 625 mg (5 capsules, 125 mg each) once weekly for all adults. For age <18 years, the dose was scaled by weight (kg): 125 mg (1 capsule) for 10–12.4 kg, 250 (2 capsules) for 12.5–27.4 kg, 275 mg (3 capsules) for 27.5–42.4 kg, 500 mg (4 capsules) for 42.5–52.4 kg, and 625 mg (5 capsules) for 52.5 kg or greater. For patients with swallowing difficulty, capsules opened and diluted in OraSweet solution or Gatorade were permitted as specified by the respective protocol amendment.
Assessments
Contrast-enhanced MRI scans were performed at baseline and then every 2 months during treatment with ONC201. The study investigators from each institution measured tumor dimensions using RANO criteria for HGG17 for baseline and all subsequent study MRIs, using T1 postcontrast and T2 or FLAIR sequences. Blinded independent central review (BICR) using dual reader with adjudication-assessed responses by RANO-HGG and RANO criteria for low-grade gliomas (RANO-LGG) was also performed for each patient as part of an integrated analyses, but our cohort was excluded from prior reports due to its non-midline location. Toxicities were prospectively collected at each treatment visit using Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Endpoints
Objective response rate (ORR) as best response was evaluated according to RANO-HGG criteria18 by investigator assessment and by BICR. In addition, analyses included ORR by RANO-LGG criteria19 by BICR.
Results
Five patients from 3 different institutions were identified as having non-midline tumors by BICR. Demographics are provided in Table 1 and their clinical summary detailed in Table 2. Median age was 33 (range 13–38) years old. The patients were predominantly of female sex (4/5, 80%) and white race (3/5, 60%). Primary tumor location was frontal lobe in 3 (60%), temporal in 1 (20%), and parietal in 1 (20%); 3 of 5 patients (60%) had multifocal disease at enrollment. H3 K27M mutation was confirmed by immunohistochemistry in 4/5 (80%), while 1 (20%) case was identified by next-generation sequencing (NGS) detecting an H3.1 K27M mutation. All patients were enrolled at their first tumor recurrence at a median of 28 (range 23–68) weeks from end of initial radiotherapy. Most patients were not on corticosteroids; 1 patient was on 24 mg of dexamethasone at enrollment. The median Karnofsky Performance Scale (KPS) function score at study enrollment was 90 (range 70–100).
Patients With Non-Midline H3 K27M-Mutant Diffuse Gliomas: Demographics and Baseline Characteristics
| Characteristics . | Cohort (N = 5) . |
|---|---|
| Age, y, median (range) | 33 (13–38) |
| Female sex, n (%) | 4 (80.0) |
| Race, n (%) | |
| White | 3 (60) |
| Black | 1 (20) |
| Asian | 1 (20) |
| Weight, kg, median (range) | 60 (54–102) |
| KPS score, median (range) | 90 (70–100) |
| Primary tumor location, n (%) | |
| Frontal lobe | 3 (60) |
| Temporal lobe | 1 (20) |
| Parietal lobe | 1 (20) |
| Multifocal disease, n (%) | |
| Yes | 3 (60) |
| No | 2 (40) |
| H3 K27M mutation detection, n (%) | |
| Immunohistochemistry | 4 (80) |
| Next-generation sequencing | 1 (20)a |
| Number of recurrences, median (range) | 1 (1–1) |
| Time from prior radiotherapy, wk, median (range) | 28 (23–68) |
| Dexamethasone dose, mg, median (range) | 0 (0–24) |
| Reirradiation, n (%) | 2 (40) |
| Characteristics . | Cohort (N = 5) . |
|---|---|
| Age, y, median (range) | 33 (13–38) |
| Female sex, n (%) | 4 (80.0) |
| Race, n (%) | |
| White | 3 (60) |
| Black | 1 (20) |
| Asian | 1 (20) |
| Weight, kg, median (range) | 60 (54–102) |
| KPS score, median (range) | 90 (70–100) |
| Primary tumor location, n (%) | |
| Frontal lobe | 3 (60) |
| Temporal lobe | 1 (20) |
| Parietal lobe | 1 (20) |
| Multifocal disease, n (%) | |
| Yes | 3 (60) |
| No | 2 (40) |
| H3 K27M mutation detection, n (%) | |
| Immunohistochemistry | 4 (80) |
| Next-generation sequencing | 1 (20)a |
| Number of recurrences, median (range) | 1 (1–1) |
| Time from prior radiotherapy, wk, median (range) | 28 (23–68) |
| Dexamethasone dose, mg, median (range) | 0 (0–24) |
| Reirradiation, n (%) | 2 (40) |
Abbreviation: KPS = Karnofsky performance status.
aPatient had tumor with H3.1 K27 mutation.
Patients With Non-Midline H3 K27M-Mutant Diffuse Gliomas: Demographics and Baseline Characteristics
| Characteristics . | Cohort (N = 5) . |
|---|---|
| Age, y, median (range) | 33 (13–38) |
| Female sex, n (%) | 4 (80.0) |
| Race, n (%) | |
| White | 3 (60) |
| Black | 1 (20) |
| Asian | 1 (20) |
| Weight, kg, median (range) | 60 (54–102) |
| KPS score, median (range) | 90 (70–100) |
| Primary tumor location, n (%) | |
| Frontal lobe | 3 (60) |
| Temporal lobe | 1 (20) |
| Parietal lobe | 1 (20) |
| Multifocal disease, n (%) | |
| Yes | 3 (60) |
| No | 2 (40) |
| H3 K27M mutation detection, n (%) | |
| Immunohistochemistry | 4 (80) |
| Next-generation sequencing | 1 (20)a |
| Number of recurrences, median (range) | 1 (1–1) |
| Time from prior radiotherapy, wk, median (range) | 28 (23–68) |
| Dexamethasone dose, mg, median (range) | 0 (0–24) |
| Reirradiation, n (%) | 2 (40) |
| Characteristics . | Cohort (N = 5) . |
|---|---|
| Age, y, median (range) | 33 (13–38) |
| Female sex, n (%) | 4 (80.0) |
| Race, n (%) | |
| White | 3 (60) |
| Black | 1 (20) |
| Asian | 1 (20) |
| Weight, kg, median (range) | 60 (54–102) |
| KPS score, median (range) | 90 (70–100) |
| Primary tumor location, n (%) | |
| Frontal lobe | 3 (60) |
| Temporal lobe | 1 (20) |
| Parietal lobe | 1 (20) |
| Multifocal disease, n (%) | |
| Yes | 3 (60) |
| No | 2 (40) |
| H3 K27M mutation detection, n (%) | |
| Immunohistochemistry | 4 (80) |
| Next-generation sequencing | 1 (20)a |
| Number of recurrences, median (range) | 1 (1–1) |
| Time from prior radiotherapy, wk, median (range) | 28 (23–68) |
| Dexamethasone dose, mg, median (range) | 0 (0–24) |
| Reirradiation, n (%) | 2 (40) |
Abbreviation: KPS = Karnofsky performance status.
aPatient had tumor with H3.1 K27 mutation.
| . | ONC006 DFCI-52 . | ONC006 MCC-10010 . | ONC006 MCC-10011 . | ONC006 UCLA-20002 . | ONC014 MCI-PED-10 . |
|---|---|---|---|---|---|
| Age (y) | 28 | 33 | 38 | 37 | 13 |
| Sex | Male | Female | Female | Female | Female |
| Race | White | Asian | White | White | Black/African American |
| Weight (kg) | 77.3 | 54 | 57.3 | 59.6 | 101 |
| Initial diagnosis | Glioblastoma | Diffuse glioma H3 K27M | Glioblastoma | Glioblastoma | Diffuse glioma H3 K27M |
| Primary site(s) | Frontal lobe | Parietal lobe, corona radiata | Frontal lobe, temporal lobe | Frontal lobe | Frontal lobe |
| K27M assay type | NGS | IHC | IHC | IHC | IHC |
| Number of recurrences | 1 | 1 | 1 | 2 | 1 |
| Target lesions | 1 | 2 | 2 | 1 | 2 |
| KPS/LPS (baseline) | 100 | 70 | 100 | 90 | 70 |
| Prior surgery | Partial resection | Partial resection | Partial resection | Gross total resection | Partial resection (2) |
| Prior therapies | TMZ | TMZ | TMZ | TMZ lomustine | TMZ |
| Prior radiation | Y | Y | Y | Y | Y |
| . | ONC006 DFCI-52 . | ONC006 MCC-10010 . | ONC006 MCC-10011 . | ONC006 UCLA-20002 . | ONC014 MCI-PED-10 . |
|---|---|---|---|---|---|
| Age (y) | 28 | 33 | 38 | 37 | 13 |
| Sex | Male | Female | Female | Female | Female |
| Race | White | Asian | White | White | Black/African American |
| Weight (kg) | 77.3 | 54 | 57.3 | 59.6 | 101 |
| Initial diagnosis | Glioblastoma | Diffuse glioma H3 K27M | Glioblastoma | Glioblastoma | Diffuse glioma H3 K27M |
| Primary site(s) | Frontal lobe | Parietal lobe, corona radiata | Frontal lobe, temporal lobe | Frontal lobe | Frontal lobe |
| K27M assay type | NGS | IHC | IHC | IHC | IHC |
| Number of recurrences | 1 | 1 | 1 | 2 | 1 |
| Target lesions | 1 | 2 | 2 | 1 | 2 |
| KPS/LPS (baseline) | 100 | 70 | 100 | 90 | 70 |
| Prior surgery | Partial resection | Partial resection | Partial resection | Gross total resection | Partial resection (2) |
| Prior therapies | TMZ | TMZ | TMZ | TMZ lomustine | TMZ |
| Prior radiation | Y | Y | Y | Y | Y |
| . | ONC006 DFCI-52 . | ONC006 MCC-10010 . | ONC006 MCC-10011 . | ONC006 UCLA-20002 . | ONC014 MCI-PED-10 . |
|---|---|---|---|---|---|
| Age (y) | 28 | 33 | 38 | 37 | 13 |
| Sex | Male | Female | Female | Female | Female |
| Race | White | Asian | White | White | Black/African American |
| Weight (kg) | 77.3 | 54 | 57.3 | 59.6 | 101 |
| Initial diagnosis | Glioblastoma | Diffuse glioma H3 K27M | Glioblastoma | Glioblastoma | Diffuse glioma H3 K27M |
| Primary site(s) | Frontal lobe | Parietal lobe, corona radiata | Frontal lobe, temporal lobe | Frontal lobe | Frontal lobe |
| K27M assay type | NGS | IHC | IHC | IHC | IHC |
| Number of recurrences | 1 | 1 | 1 | 2 | 1 |
| Target lesions | 1 | 2 | 2 | 1 | 2 |
| KPS/LPS (baseline) | 100 | 70 | 100 | 90 | 70 |
| Prior surgery | Partial resection | Partial resection | Partial resection | Gross total resection | Partial resection (2) |
| Prior therapies | TMZ | TMZ | TMZ | TMZ lomustine | TMZ |
| Prior radiation | Y | Y | Y | Y | Y |
| . | ONC006 DFCI-52 . | ONC006 MCC-10010 . | ONC006 MCC-10011 . | ONC006 UCLA-20002 . | ONC014 MCI-PED-10 . |
|---|---|---|---|---|---|
| Age (y) | 28 | 33 | 38 | 37 | 13 |
| Sex | Male | Female | Female | Female | Female |
| Race | White | Asian | White | White | Black/African American |
| Weight (kg) | 77.3 | 54 | 57.3 | 59.6 | 101 |
| Initial diagnosis | Glioblastoma | Diffuse glioma H3 K27M | Glioblastoma | Glioblastoma | Diffuse glioma H3 K27M |
| Primary site(s) | Frontal lobe | Parietal lobe, corona radiata | Frontal lobe, temporal lobe | Frontal lobe | Frontal lobe |
| K27M assay type | NGS | IHC | IHC | IHC | IHC |
| Number of recurrences | 1 | 1 | 1 | 2 | 1 |
| Target lesions | 1 | 2 | 2 | 1 | 2 |
| KPS/LPS (baseline) | 100 | 70 | 100 | 90 | 70 |
| Prior surgery | Partial resection | Partial resection | Partial resection | Gross total resection | Partial resection (2) |
| Prior therapies | TMZ | TMZ | TMZ | TMZ lomustine | TMZ |
| Prior radiation | Y | Y | Y | Y | Y |
All patients tolerated ONC201 therapy well without any grade 3–4 treatment-related toxicities. No dose modifications or dose-limiting toxicities were observed. No serious adverse events were reported while on ONC201 therapy. For all patients receiving ONC201 as a monotherapy across all studies, there have been infrequent reports of patients with skin or allergic adverse reactions. These have generally been mild to moderate in severity and only one patient discontinued treatment (due to grade 1 rash).
Two of the 5 patients showed partial responses by RANO-HGG criteria (ORR, 40%; Table 3) as assessed by the site investigator: 1 complete response and 1 partial response. According to RANO-HGG criteria by BICR, 1 patient who responded to the investigator assessment was deemed nonmeasurable at baseline. Another responding patient by investigator assessment had best tumor shrinkage of 52.6% at the last imaging assessment; while achieving a >50% decrease in target lesion product of diameters necessary for partial response, the BICR review also identified a new lesion at that timepoint, so the best response deemed by BICR was stable disease (Figure 1A and B). Both patients had an objective response by RANO-LGG criteria per BICR and remained on ONC201 treatment for 14 and 41.1 months (ongoing); the remaining 3 patients had treatment durations of 2.1, 2.5, and 3.5 months. Responses were associated with clinical benefits of increased mobility in the adult case and improved level of alertness in the pediatric case. Figure 1 depicts measurements over time as per RANO-HGG (A) and RANO-LGG (B) criteria.
Best Response by BICR and Investigator to ONC201 Treatment by RANO Criteria
| Criteria . | BICR . | Investigator . | |
|---|---|---|---|
| RANO-HGG . | RANO-LGG . | RANO-HGG . | |
| Evaluable patients (N) | 5 | 5 | 5 |
| CR | 0 | 0 | 1 |
| PR | 0 | 2 | 1 |
| SD | 3 | 2 | 2 |
| PD | 2 | 0 | 1 |
| NE | 0 | 1 | 0 |
| ORR | 0% | 40% | 40% |
| DCR | 60% | 80% | 80% |
| Criteria . | BICR . | Investigator . | |
|---|---|---|---|
| RANO-HGG . | RANO-LGG . | RANO-HGG . | |
| Evaluable patients (N) | 5 | 5 | 5 |
| CR | 0 | 0 | 1 |
| PR | 0 | 2 | 1 |
| SD | 3 | 2 | 2 |
| PD | 2 | 0 | 1 |
| NE | 0 | 1 | 0 |
| ORR | 0% | 40% | 40% |
| DCR | 60% | 80% | 80% |
Abbreviations: BICR = Blind Independent Central Review; CR = complete response; DCR = durable control rate (CR + PR + SD); HGG = high-grade glioma; LGG = lower grade glioma; NE = not evaluable; ORR = overall response rate (CR + PR); PD = progressive disease; PR = partial response; RANO = Response Assessment for Neuro-Oncology; SD = stable disease.
Best Response by BICR and Investigator to ONC201 Treatment by RANO Criteria
| Criteria . | BICR . | Investigator . | |
|---|---|---|---|
| RANO-HGG . | RANO-LGG . | RANO-HGG . | |
| Evaluable patients (N) | 5 | 5 | 5 |
| CR | 0 | 0 | 1 |
| PR | 0 | 2 | 1 |
| SD | 3 | 2 | 2 |
| PD | 2 | 0 | 1 |
| NE | 0 | 1 | 0 |
| ORR | 0% | 40% | 40% |
| DCR | 60% | 80% | 80% |
| Criteria . | BICR . | Investigator . | |
|---|---|---|---|
| RANO-HGG . | RANO-LGG . | RANO-HGG . | |
| Evaluable patients (N) | 5 | 5 | 5 |
| CR | 0 | 0 | 1 |
| PR | 0 | 2 | 1 |
| SD | 3 | 2 | 2 |
| PD | 2 | 0 | 1 |
| NE | 0 | 1 | 0 |
| ORR | 0% | 40% | 40% |
| DCR | 60% | 80% | 80% |
Abbreviations: BICR = Blind Independent Central Review; CR = complete response; DCR = durable control rate (CR + PR + SD); HGG = high-grade glioma; LGG = lower grade glioma; NE = not evaluable; ORR = overall response rate (CR + PR); PD = progressive disease; PR = partial response; RANO = Response Assessment for Neuro-Oncology; SD = stable disease.
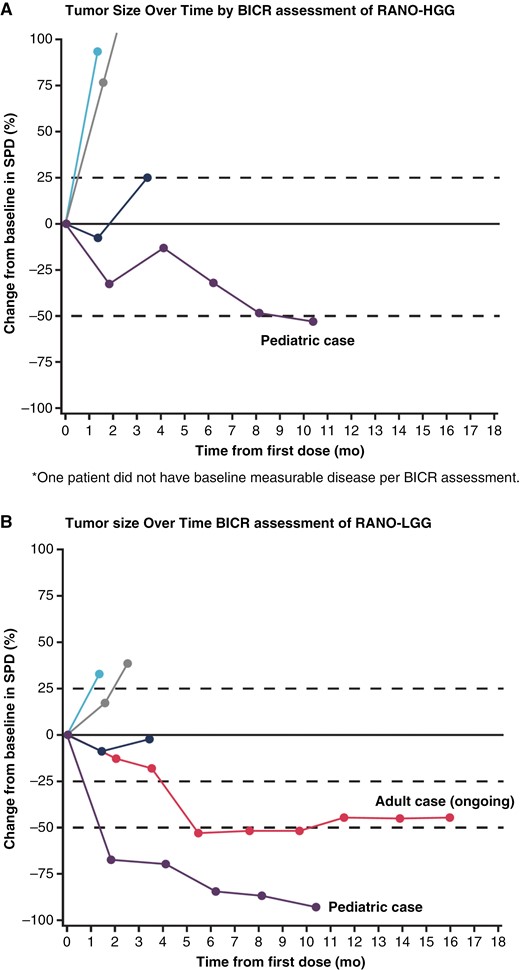
Patients with recurrent non-midline H3 K27M-mutant diffuse gliomas: change in tumor measurement over time by RANO criteria. Tumor measurements over time based on (A) RANO high-grade glioma (RANO-HGG)* and (B) RANO low-grade glioma (RANO-LGG) criteria. Enrollment cutoff: February 27, 2020; data cutoff: May 31, 2021. Only patients with measurable target-enhancing lesions at both baseline and post-baseline assessments as assessed by the local investigator were included. BICR = blinded independent central review; RANO = response assessment for neuro-oncology; SPD = sum of products of perpendicular diameters (target-enhancing lesions per BICR).*One patient did not have baseline measurable disease per BICR assessment.
Figure 2A details a 38-year-old female patient with a primary tumor location in the frontal lobe consistent with a malignant astrocytoma, harboring an H3 K27M mutation, IDH-wildtype, MGMT promoter unmethylated, ATRX mutational loss, and PIK3CA mutation by NGS. Initial standard chemoradiotherapy ended in August 2018 but was complicated by severe and prolonged thrombocytopenia that precluded adjuvant therapy with temozolomide. The patient received tumor treating fields following chemoradiotherapy until tumor progression was noted in September 2019. The patient was enrolled by December 2019, achieved complete response by RANO-HGG by September 2020 (9 months) as per investigator assessment, and remained disease-free as of April 2023 (on treatment at 41.1 months). This patient did not require corticosteroids and remained functionally intact (KPS 100%) between December 2019 and April 2023. The course was complicated by transient ONC201-related allergy controlled with antihistamine therapy. ONC201-related nausea was initially managed with single, then required dual antiemetic therapy. No serious (grade 3 or higher) toxicities were noted.
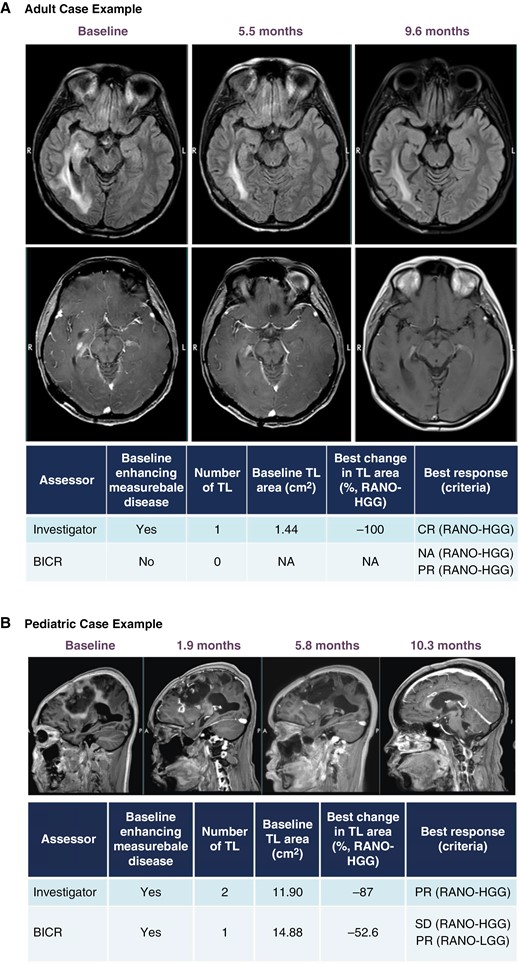
ONC201 responder case studies. Brain MRI over time shown for an adult case with complete response (A) and pediatric case with partial response* (B) treated with ONC201. *For the pediatric case (2B), the best change in TL area achieved >50% reduction necessary for PR; however, a new lesion at this timepoint detected by BICR resulted in an overall best response of SD by HGG-RANO. BICR = blinded independent central review; CR = complete response; HGG = high-grade glioma; LGG = neuro-oncology low-grade glioma; NA = not applicable; PR = partial response; RANO = response assessment for neuro-oncology; TL = target lesion.
Figure 2B details a 14-year-old female patient with a primary tumor location in the frontal lobe consistent with a malignant astrocytoma, harboring an H3 K27M mutation, MGMT promoter unmethylated, TP53 mutation, and ATRX mutational loss. After initial chemoradiotherapy ending in August 2019, tumor progression was noted after 3 cycles of adjuvant temozolomide. She was enrolled on trial by February 2020, achieved maximal (partial) response by 10 months on therapy, but ultimately progressed after 12 months since start of therapy. She did not require corticosteroids and remained functionally stable (KPS 60%) while on therapy with ONC201. Her hemiparesis improved from maximal assist to independence with standing and transfers. No drug-related toxicities were noted throughout the course of ONC201 therapy.
Discussion
H3 K27-altered DMG is a uniformly lethal disease that has no proven effective or approved medical therapies to date. Radiotherapy can prolong survival and provide some symptomatic relief, but offers only a modest survival benefit. There are ongoing trials for this diagnosis, including but not limited to the ONC201 phase 3 ACTION study for non-DIPG, non-spinal H3 K27M diffuse glioma [NCT05580562], GD2 CAR-T cell trial for DIPG [NCT04196413] evaluating new agents and various focused ultrasound trials investigator novel modalities.
The purpose of this series was to evaluate the clinical efficacy of monotherapy ONC201 in recurrent H3 K27M-mutant non-midline gliomas enrolled across multiple early phase trials in patients evaluable for objective response by RANO criteria. This paper also questions the exclusion of non-midline diffuse gliomas from the WHO diagnosis of DMG, H3 K27-altered. This series highlights that the H3 K27M mutational status, rather than anatomy, dictates response to treatment of ONC201 and argues for a molecular rather than anatomical basis of treatment selection. Furthermore, as testing for H3 K27M is standard of care for patients with midline gliomas but is not always standard in non-midline tumors, this manuscript describes the potential value of testing patients with non-midline disease, especially those proven to be IDH-wildtype, as responses to ONC201 were similar regardless of the location.
ONC201 demonstrated efficacy in integrated analyses of patients treated with monotherapy, both via antagonism of DRD2 and ClpP agonism.16,20,21 Early RNA-seq studies showed the H3 K27M mutation led to downstream upregulation of DRD2 expression, which ONC201 antagonizes. DRD2 expression by RNA-seq is elevated in histone H3 K27M-mutant glioma tumors compared with adult and pediatric H3 wildtype or G34 gliomas (P = .02). ONC201 is more potently cytotoxic to histone H3 K27M-mutant (median IC50 ~0.6 µM, n = 5 lines) compared with histone H3 wildtype or G34 variant glioma cultures (median IC50 ~1.5 µM, n = 7 lines; P <.01; Chi et al. 2017). DRD2 expression was significantly increased in H3 K27M mutant glioblastoma archival tumors relative to H3 K27M wildtype adult/pediatric gliomas.21 Response to ONC201 for H3 K27M-altered DMG also correlated to high DRD2 protein expression.20 The mechanism of ONC201 was further elucidated by Venneti et al., where radiographic response was associated with increased expression of key tricarboxylic acid cycle-related genes assessed in tumor sequencing. ONC201 treatment specifically increased levels of the l-enantiomer of 2-hydroxyglutarate levels in cultured H3 K27M-mutant DMG cells and patient CSF samples. Increased l-2-HG levels corresponded with increases in repressive H3K27me3 in vitro and in human tumors accompanied by epigenetic downregulation of cell cycle regulation and neuroglial differentiation genes. Overall, ONC201 demonstrated efficacy in H3 K27M-mutant DMG by disrupting integrated metabolic and epigenetic pathways and reversing pathognomonic H3K27me3 reduction.13
Further, Kawakibi et al. suggested that patients with thalamic H3 K27M-mutant DMG treated with ONC201 developed more frequent radiographic responses with significantly longer overall survival compared to other midline tumor locations. In this retrospective analysis, median survival was not reached for thalamic tumors at a median follow-up of 22 months compared to a historical median survival of 12.5 months (P = .0001).22 Interestingly, the comparison of Venetti et al. focused on nonrecurrent patients with primary thalamic versus brainstem, H3 K27M-mutant DMG. In this series, similar responses were observed between thalamic and brainstem locations.15 In contrast, as previously reported by Arrillaga-Romany et al. in 2021, in the recurrent, H3 K27M-mutated DMG assessed by the BICR integrated analysis, the thalamic location was associated with a higher response rate.16 The predictive value of anatomical location or co-mutations of H3 K27M-altered gliomas remains unclear. These differences in response rates and OS may be explained by both the differential expression of DRD2 and its signal transduction antagonistic family member, DRD5, throughout the CNS.
Midline gliomas with H3 K27M mutations also frequently harbor co-mutations in TP53 and EGFR, which are generally associated with poor prognosis.23 Patients with H3 K27M-mutant DMG treated with ONC201 that also exhibited either EGFR mutations or elevated EGFR expression as a marker of resistance were associated with resistance to therapy and inferior OS.22 The degree to which the poor prognosis of H3 K27M-altered DMG is related to the inability to resect these predominantly midline tumors relative to the biologic nature of the H3 K27 and H3K27me3 alterations remains unclear. Non-midline H3 K27M-mutant gliomas do not fit the diagnostic criterion for H3 K27-altered DMG as defined by the 2021 WHO classification of CNS tumors, but share the presence of its pathognomonic mutation.7 To date, the prognosis of non-midline gliomas harboring H3 K27M mutations has not previously been reported and should not be assumed to be the same as those in midline locations.
This case series presents the first evaluation of recurrent non-midline H3 K27-mutant gliomas in a small cohort treated with ONC201 monotherapy as part of 5 prospective clinical trials. After ~4 years and >350 patients enrolled on ONC201 studies, only 5 non-midline glioma patients were identified. The varying study enrollment requirements, however, make it difficult to extrapolate the true frequency of non-midline H3 K27M-mutant diffuse glioma. Nonetheless, these clearly represent an ultra-rare group among the K27M-mutant gliomas, which are predominantly midline in location. Another factor limiting the identification of H3 K27M-mutated non-midline glioma is testing frequency and biases. While midline gliomas are routinely tested for H3 K27M, non-midline gliomas are less frequently tested. Routine testing of non-midline gliomas for K27M mutations and inclusion in ACTION and future ONC201 studies may be critical to conclusively demonstrating activity in non-midline gliomas.
Funding
This article appears as part of the supplement “H3 K27M-Mutant Glioma: Disease State Overview,” sponsored by Chimerix, Inc. M.D.H. received grant support from Florida Department of Health [8LA04 and 22L01].
Conflict of interest statement
Y.O.: DSMB (unpaid) for Actuate Therapeutics, Oncoceutics, GammaTile; Trial support by BMS, Novocure, Chimerix, Karyopharm, VBI Therapeutics, MIMIVAX, and CNS Pharmaceuticals. No financial conflicts of interests. M.D.H.: Honorarium from IBA, Treasurer PTCOG-NA (unpaid), Pediatric Subcommittee Co-Chair PTCOG (unpaid), and PCG Executive Council Representative for MCI (unpaid). M.P.M.: Consulting fees from Karyopharm, Kazia Therapeutics, Sapience, Zap, Mevion, Xoft; Board of Directors Oncoceutics; Stock in Chimerix. P.W.: Research Support: Astra Zeneca, Black Diamond, Bristol Meyers Squibb, Celgene, Chimerix, Eli Lily, Erasca, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Servier, Vascular Biogenics, VBI Vaccines, Advisory Board/Consultant: Astra Zeneca, Black Diamond, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, and VBI Vaccine. R.S.T., S.C.R., and J.E.A.: Employees and shareholders of Chimerix.
Supplement sponsorship
This article appears as part of the supplement “H3 K27M-mutant Glioma: Disease State Overview,” sponsored by Chimerix, Inc.
Authorship statement
Experimental design, implementation, or analysis and interpretation of the data: Y.O., P.Y.W., I.A.-R., D.D., M.P.M., R.S.T., S.C.R., and J.E.A. Writing of the manuscript: Y.O., M.D.H., R.S.T., S.C.R., and J.E.A. Reviewed and revised the manuscript, as well as read and approved the final version: Y.O., M.D.H., T.F.C., P.Y.W., I.A.-R., D.D., M.P.M., R.S.T., S.C.R., and J.E.A.
Data Availability
All data will be made available upon reasonable request by contacting Chimerix, Inc.




