-
PDF
- Split View
-
Views
-
Cite
Cite
Joshua A Budhu, Ugonma N Chukwueke, Sadhana Jackson, Eudocia Q Lee, J Ricardo McFaline-Figueroa, Nicole Willmarth, Mahalia Dalmage, Ichiro Kawachi, David Arons, Susan M Chang, Evanthia Galanis, Shawn L Hervey-Jumper, Patrick Y Wen, Alyx B Porter, Defining interventions and metrics to improve diversity in CNS clinical trial participation: A SNO and RANO effort, Neuro-Oncology, Volume 26, Issue 4, April 2024, Pages 596–608, https://doi.org/10.1093/neuonc/noad242
Close - Share Icon Share
Abstract
Despite major strides in cancer research and therapy, these advances have not been equitable across race and ethnicity. Historically marginalized groups (HMG) are more likely to have inadequate preventive screening, increased delays in diagnosis, and poor representation in clinical trials. Notably, Black, Hispanic, and Indigenous people represent 30% of the population but only 9% of oncology clinical trial participants. As a result, HMGs lack equitable access to novel therapies, contradicting the principle of distributive justice, as enshrined in the Belmont report, which demands the equitable selection of subjects in research involving human subjects. The lack of clinical trial diversity also leads to low generalizability and potentially harmful medical practices. Specifically, patients with brain cancer face unique barriers to clinical trial enrollment and completion due to disease-specific neurologic and treatment-induced conditions. Collectively, the intersection of these disease-specific conditions with social determinants of health fosters a lack of diversity in clinical trials. To ameliorate this disparity in neuro-oncology clinical trial participation, we present interventions focused on improving engagement of HMGs. Proposals range from inclusive trial design, decreasing barriers to care, expanding trial eligibility, access to tumor profiling for personalized medical trials, setting reasonable metrics and goals for accrual, working with patient community stakeholders, diversifying the neuro-oncology workforce, and development of tools to overcome biases with options to incentivize equity. The diversification of participation amongst neuro-oncology clinical trials is imperative. Equitable access and inclusion of HMG patients with brain tumors will not only enhance research discoveries but will also improve patient care.
Background and the Importance of Diversity in Clinical Trials
In 1993, the National Institutes of Health (NIH) Revitalization Act sought to increase the accrual of diverse subjects and published guidelines on the Inclusion of Women and Minorities as subjects in clinical research.1 However, while there has been some advancement in the percentage of women participating in clinical trials, there has been little progress with increasing diversity in racial and ethnic representation. Black, Hispanic, and Indigenous people represent 30% of the population, but only represent 9% of clinical trial participants in the United States.2,3 In all therapeutic cancer trials, White patients comprise 83.4% of trial participants.3 Specifically in therapeutic neuro-oncology trials, recent systematic reviews have demonstrated that there is ongoing and consistent underrepresentation of non-White participants.4
Ensuring diversity in clinical trials is one of the foundational ethical principles of optimizing study findings in modern-day research.5–7 The principle of distributive justice, as outlined in the Belmont report, states that the risks and benefits of research must be distributed equally.7,8 This also includes the ability to participate in clinical research, along with the equal opportunity to benefit from clinical research. Analysis using the Future Elderly Model indicates that improving clinical trial diversity and its positive impact on health disparities (HD) is an economic imperative.9,10 Inclusive research practices will also help to allay medical mistrust, especially in historically marginalized and underserved communities.9,11–13 This is particularly important, especially in an era of heightened scientific distrust.14–17 To rectify the lack of diverse representation in research, multiple medical organizations and institutions, including the National Academy of Medicine, NIH, Federal Drug Administration (FDA), and American Society of Clinical Oncology (ASCO) have recently devoted more effort and resources to diversifying clinical trials.1,2,9,18
Building upon these efforts, the Society for Neuro-Oncology (SNO) in collaboration with the Response Assessment in Neuro-Oncology (RANO) Working Group and patient advocacy organizations established a group to propose additional and nuanced initiatives for patients with CNS tumors. These recommendations are outlined in Table 1. Our patients have unique clinical presentations, symptoms, and requirements, such as an increased reliance on caregivers, impaired motor function, language difficulties, and altered behavior, which necessitates a field-specific approach to improving diversity in clinical trials. These disease-specific conditions result in additional difficulties in consenting to clinical research, as patients with higher levels of cognitive impairment are less likely to participate in clinical trials.19,20 Understanding the spectrum of disease and impact of treatment in diverse populations is essential in the pursuit of cures for neuro-oncologic diseases. Equitable access to research is fundamental to the principles of justice and equity in healthcare. This equity has been shown to result in better clinical care and generalizability of research, and is a net benefit to society both economically and socially.2,5,6,9,18,21–29
The Social-Ecological Model (SEM)—A Framework for Improving Diversity in Brain Tumor Trials
| SEM Component . | SEM Focus . | Intervention(s) . | Specific Examples . |
|---|---|---|---|
| Individual | Increase trial accessibility and patients’ knowledge | Identify opportunities to promote inclusion through adjustments in eligibility criteria | Relaxing eligibility criteria, which may exclude ethnic minorities Improve consent forms for readability and language. Consent forms should be available in different languages, especially in areas with numbers of LEP patients |
| Interpersonal | Social interactions with patients | Use peers and navigators Message construction Message delivery Change the default | Clinical trials ambassador programs through ABTA, NBTS Norm-based messaging Pop-up messages in electronic medical records |
| Organizational | Academic institutions, hospitals, pharmaceutical companies | Establish demographic enrollment criteria for every study Regulatory strategies for accrual Workforce diversification | Consider a priori strategy to meet enrollment benchmarks Strict enforcement of demographic capture Uniform approach to recruitment of URiMs in neuro-oncology |
| Community | Local community; patient advocacy | Building trust Increasing genomic capture of diverse populations | Deploy advocacy groups and medical institutions for engagement Streamlining NGS access |
| Societal | Broader policies | Lessons from the pandemic Health policy initiatives | Virtual offerings Use of direct incentives Lobbying for resources to reduce opportunity cost of trial enrollment |
| SEM Component . | SEM Focus . | Intervention(s) . | Specific Examples . |
|---|---|---|---|
| Individual | Increase trial accessibility and patients’ knowledge | Identify opportunities to promote inclusion through adjustments in eligibility criteria | Relaxing eligibility criteria, which may exclude ethnic minorities Improve consent forms for readability and language. Consent forms should be available in different languages, especially in areas with numbers of LEP patients |
| Interpersonal | Social interactions with patients | Use peers and navigators Message construction Message delivery Change the default | Clinical trials ambassador programs through ABTA, NBTS Norm-based messaging Pop-up messages in electronic medical records |
| Organizational | Academic institutions, hospitals, pharmaceutical companies | Establish demographic enrollment criteria for every study Regulatory strategies for accrual Workforce diversification | Consider a priori strategy to meet enrollment benchmarks Strict enforcement of demographic capture Uniform approach to recruitment of URiMs in neuro-oncology |
| Community | Local community; patient advocacy | Building trust Increasing genomic capture of diverse populations | Deploy advocacy groups and medical institutions for engagement Streamlining NGS access |
| Societal | Broader policies | Lessons from the pandemic Health policy initiatives | Virtual offerings Use of direct incentives Lobbying for resources to reduce opportunity cost of trial enrollment |
The Social-Ecological Model (SEM)—A Framework for Improving Diversity in Brain Tumor Trials
| SEM Component . | SEM Focus . | Intervention(s) . | Specific Examples . |
|---|---|---|---|
| Individual | Increase trial accessibility and patients’ knowledge | Identify opportunities to promote inclusion through adjustments in eligibility criteria | Relaxing eligibility criteria, which may exclude ethnic minorities Improve consent forms for readability and language. Consent forms should be available in different languages, especially in areas with numbers of LEP patients |
| Interpersonal | Social interactions with patients | Use peers and navigators Message construction Message delivery Change the default | Clinical trials ambassador programs through ABTA, NBTS Norm-based messaging Pop-up messages in electronic medical records |
| Organizational | Academic institutions, hospitals, pharmaceutical companies | Establish demographic enrollment criteria for every study Regulatory strategies for accrual Workforce diversification | Consider a priori strategy to meet enrollment benchmarks Strict enforcement of demographic capture Uniform approach to recruitment of URiMs in neuro-oncology |
| Community | Local community; patient advocacy | Building trust Increasing genomic capture of diverse populations | Deploy advocacy groups and medical institutions for engagement Streamlining NGS access |
| Societal | Broader policies | Lessons from the pandemic Health policy initiatives | Virtual offerings Use of direct incentives Lobbying for resources to reduce opportunity cost of trial enrollment |
| SEM Component . | SEM Focus . | Intervention(s) . | Specific Examples . |
|---|---|---|---|
| Individual | Increase trial accessibility and patients’ knowledge | Identify opportunities to promote inclusion through adjustments in eligibility criteria | Relaxing eligibility criteria, which may exclude ethnic minorities Improve consent forms for readability and language. Consent forms should be available in different languages, especially in areas with numbers of LEP patients |
| Interpersonal | Social interactions with patients | Use peers and navigators Message construction Message delivery Change the default | Clinical trials ambassador programs through ABTA, NBTS Norm-based messaging Pop-up messages in electronic medical records |
| Organizational | Academic institutions, hospitals, pharmaceutical companies | Establish demographic enrollment criteria for every study Regulatory strategies for accrual Workforce diversification | Consider a priori strategy to meet enrollment benchmarks Strict enforcement of demographic capture Uniform approach to recruitment of URiMs in neuro-oncology |
| Community | Local community; patient advocacy | Building trust Increasing genomic capture of diverse populations | Deploy advocacy groups and medical institutions for engagement Streamlining NGS access |
| Societal | Broader policies | Lessons from the pandemic Health policy initiatives | Virtual offerings Use of direct incentives Lobbying for resources to reduce opportunity cost of trial enrollment |
Social Determinants of Health and Health Disparities
Cancer research and discovery have increased exponentially over the past few decades. From 2009 to 2020, the FDA had 332 new approvals for anti-cancer medications, not accounting for new devices, procedures, radiotherapy, or surgical techniques.30 While these advances paint a hopeful and exciting future for cancer treatment, they have not been equitably distributed. Significant disparities still exist across cancer research, clinical care, and disease therapy.31 These disparities are also pervasive in patients with central nervous system (CNS) tumors.32 Race and socioeconomic status are independent risk factors for gross total resection, radiotherapy, and survival for meningioma.33–35 Epidemiological data suggest that socioeconomic status also affects glioblastoma survival, with patients on Medicaid or government-assisted insurance funding, having worse clinical outcomes.36 Additionally, patients with brain metastases are less likely to be offered radiotherapy, surgery, or additional treatment options based on race and income status.37–41
These HD are complex and rooted in the social, structural, and political determinants of health (SDoH).42 The SDoH make up the majority of modifiable contributors to overall health outcomes and account for much of the disparities in mortality and morbidity in cancer care.42 These determinants include, but are not limited to, socioeconomic status, access to healthcare and insurance status, community and familial support, education, and housing.43 The intersection of CNS disease-specific barriers with the SDoH make it particularly difficult for historically marginalized groups (HMG) to participate in and access clinical research. Sustainable solutions that improve the representation of diverse populations in clinical research will need to consider the SDoH. Correspondingly, the interventions described in this paper focus on addressing different aspects of the SDoH.
Interventions
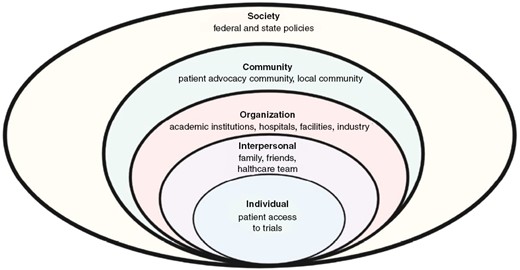
We propose practical interventions and metrics to increase diversity in clinical trials. The following interventions are poised to enhance HMG patient enrollment, in-line with nationally held benchmarks and guidelines. These guidelines and interventions can also be used to increase enrollment from other historically underrepresented groups, such as rural and elderly populations. These interventions are varied and are targeted at federal and state institutions, hospital and medical systems, researchers, care providers, advocacy groups, patients, and other stakeholders. These recommendations are based on the social-ecological model (SEM), which expands traditional individual-based interventions and also factors in SDoH and the interconnectedness of varied engaged groups.44 The five components of the SEM model are the individual, interpersonal, organizational, community, and society (Figure 1). This expands on other methods, which place much of the responsibility on the patient, rather than the overlying community and societal structure.
Individual
Identifying Opportunities to Promote Inclusion Through Adjustments in Eligibility Criteria
Overly strict eligibility criteria, without scientific justification or safety implication, restrict patient access to investigational drugs.22,23 Indeed, eligibility criteria based on laboratory test values should be carefully evaluated as they may inadvertently exclude minority populations that could otherwise meaningfully contribute and benefit from study participation. ASCO and Friends of Cancer Research (Friends) recommend that laboratory reference values should account for potential normal variations due to race, ethnicity, age, sex, and gender identity.45 Among 38 standard laboratory tests analyzed in more than 3000 healthy individuals in the National Health and Nutrition Examination Survey (NHANES), only 5 (glucose, phosphorus, potassium, total bilirubin, and uric acid) did not show significant racial/ethnic differences in distribution.46 As previously noted, Black participants had significantly higher normal ranges in creatine phosphokinase, globulin, and total protein, and lower normal ranges in hematocrit, hemoglobin, total cholesterol, triglycerides, and white blood cell count (WBC) than White participants. Patients with benign ethnic neutropenia (BEN), one of the most common causes of chronic neutropenia seen in individuals of African, Middle Eastern, and West Indian descent, are at no increased risk of infection despite their neutropenia.47 Yet, studies show that BEN may limit access to chemotherapy and even participation in clinical trials that use ANC > 1500/μL as an eligibility criterion.47,48 Adults who identify as Black have higher serum creatinine levels on average than those who do not identify as Black.49 The use of Black race in equations estimating glomerular filtration rate (GFR) is rooted in the perpetuation of racist pseudoscience.49 Efforts to eliminate the race coefficient from GFR equations are ongoing.50 Recently proposed estimated GFR equations that incorporate creatinine and cystatin C but omit race are more accurate and should be considered for eligibility determination on clinical trials.51 It is important to consider the generational impact of the SDoH, racism, and discrimination has on serum biomarkers related to trial inclusion/exclusion.52,53
Performance status for many interventional trials requires patients to be highly functioning to participate. While the intention of such strict criterion is intended to ensure fitness, safety, and integrity of the trial, this does not accurately reflect the spectrum of the population receiving treatment. Consideration of wider performance status parameters could improve inclusivity in neuro-oncology clinical trials specifically.21 Acknowledgement of language or cognitive impairment in addition to varying degrees of disability within the inclusion criteria would allow provision for greater participation while providing more broad interpretation of the study results.
Inclusive Language and Translation of Protocols and Consents
Efforts must be made to translate trial protocols to languages other than English and to languages that are commonly spoken by eligible research populations. Many trials require either a middle or high school reading level of English; however, it is not acceptable to exclude patients with low literacy or have limited English proficiency (LEP). Institutions can help to educate patients on brain tumors and clinical trials by translating medical terminology into lay friendly language and into other languages to help increase communication and understanding amongst patients and caregivers. Efforts should also be made to include health care proxies and family members in discussions, especially where culturally appropriate. This is crucial as our patients may have aphasias or difficulty processing language. Language concordance has been shown to improve the care experience and can help increase clinical trial enrollment.54,55 The importance of cultural competence and humility cannot be overstated, especially as it pertains to different HMGs perception of clinical research.2,9,56
Interpersonal
Utilizing Peers and Navigators
Social networks, patient advocacy groups, and healthcare professionals can help connect newly diagnosed patients with patient navigators or other long-term cancer patients who have experience with clinical research. Patient navigators are trained individuals who help patients navigate the healthcare system and can guide them through clinical trials and research options. Multiple patient navigator programs have shown success in increasing treatment adherence for cancer care and there is also emerging data about increasing the accrual of diverse patients.57 While there have been concerns about the cost effectiveness of patient navigation, recent data are emerging that patient navigator programs can lower health care costs.58
Patients living with cancer and former/current participants of clinical trials can also be a great resource. The organization LUNGevity has a Clinical Trials Ambassador program that connects patients with others who have experience participating in a clinical trial. This peer-to-peer connection provides direct lived experiences and information to patients and can help to allay misconceptions about risks and benefits in study involvement.59 For example, if randomized to the control arm of a trial, patients may perceive this as a lost opportunity to derive clinical benefit. It is important to clarify neuro-oncology trial design with the emphasis that most control arms are not placebo, but rather the standard of care. Connecting with a clinical trial ambassador or patient navigator can address these concerns and help in deciding if a clinical trial is the correct choice for the patient. Importantly, information about eligibility and exclusionary criteria, such as the use of anti-angiogenic agents, can be easily disseminated.
The Use of Behavioral Economics, “Nudges,” and Incentives to Promote Inclusive Practices
Behavioral economics combines psychology and economics to develop insights about human decision-making.60 In contrast to the classical model of the perfectly rational agent, behavioral economics emphasizes the shortcuts (heuristics) and biases inherent in daily decision-making. Accordingly, the goal of behavioral design is to make the “best” decision for the consumer the easiest one to make (a nudge) or the automatic one (a default option).61
These principles and methods have been used throughout medicine with success.62 For example, the Penn Medicine Nudge Unit at the University of Pennsylvania has used nudges, both for patients and clinicians, to increase generic prescription ordering, indicated cardiac rehabilitation referrals, appropriate cancer screening, and influenza vaccination compliance.63 Our field can also utilize these techniques to promote equitable care and increased diversity in clinical trials. Definitions of behavioral nudges and examples are outlined in Table 2. These techniques focus on normalizing and standardizing universal screening and eligibility of patients for clinical trials and research. Importantly, these methods can help to combat both explicit and implicit bias.64–66
| Type of Nudge . | Explanation . | Example . |
|---|---|---|
| Norm-based messaging | Uses our inherent nature to value the appropriateness of our actions to others | Messages in the clinic that disseminate normative information “Did you know that 8 out of 10 Black patients express interest in being a clinical trial participant?” |
| Message framing | Constructing the message to evoke a different response or perspective | A targeted campaign to patients emphasizing altruism in clinical research: “Participating in a clinical trial may not only help you, but can help future patients who benefit from this research” |
| Message delivery | How messages are disseminated and normalized | Wearable pins that say “Ask me about my research” or electronic health record pop-ups when accessing a historically marginalized patient’s chart asking if this patient was screened for a clinical trial |
| Change the default | People have the tendency to accept the default option | For conditions without a standard of care option, such as recurrent MGMT unmethylated glioblastoma, make clinical trials the default option, and pursue alternative treatment once trial options have been exhausted |
| Type of Nudge . | Explanation . | Example . |
|---|---|---|
| Norm-based messaging | Uses our inherent nature to value the appropriateness of our actions to others | Messages in the clinic that disseminate normative information “Did you know that 8 out of 10 Black patients express interest in being a clinical trial participant?” |
| Message framing | Constructing the message to evoke a different response or perspective | A targeted campaign to patients emphasizing altruism in clinical research: “Participating in a clinical trial may not only help you, but can help future patients who benefit from this research” |
| Message delivery | How messages are disseminated and normalized | Wearable pins that say “Ask me about my research” or electronic health record pop-ups when accessing a historically marginalized patient’s chart asking if this patient was screened for a clinical trial |
| Change the default | People have the tendency to accept the default option | For conditions without a standard of care option, such as recurrent MGMT unmethylated glioblastoma, make clinical trials the default option, and pursue alternative treatment once trial options have been exhausted |
| Type of Nudge . | Explanation . | Example . |
|---|---|---|
| Norm-based messaging | Uses our inherent nature to value the appropriateness of our actions to others | Messages in the clinic that disseminate normative information “Did you know that 8 out of 10 Black patients express interest in being a clinical trial participant?” |
| Message framing | Constructing the message to evoke a different response or perspective | A targeted campaign to patients emphasizing altruism in clinical research: “Participating in a clinical trial may not only help you, but can help future patients who benefit from this research” |
| Message delivery | How messages are disseminated and normalized | Wearable pins that say “Ask me about my research” or electronic health record pop-ups when accessing a historically marginalized patient’s chart asking if this patient was screened for a clinical trial |
| Change the default | People have the tendency to accept the default option | For conditions without a standard of care option, such as recurrent MGMT unmethylated glioblastoma, make clinical trials the default option, and pursue alternative treatment once trial options have been exhausted |
| Type of Nudge . | Explanation . | Example . |
|---|---|---|
| Norm-based messaging | Uses our inherent nature to value the appropriateness of our actions to others | Messages in the clinic that disseminate normative information “Did you know that 8 out of 10 Black patients express interest in being a clinical trial participant?” |
| Message framing | Constructing the message to evoke a different response or perspective | A targeted campaign to patients emphasizing altruism in clinical research: “Participating in a clinical trial may not only help you, but can help future patients who benefit from this research” |
| Message delivery | How messages are disseminated and normalized | Wearable pins that say “Ask me about my research” or electronic health record pop-ups when accessing a historically marginalized patient’s chart asking if this patient was screened for a clinical trial |
| Change the default | People have the tendency to accept the default option | For conditions without a standard of care option, such as recurrent MGMT unmethylated glioblastoma, make clinical trials the default option, and pursue alternative treatment once trial options have been exhausted |
Message Construction
Norm-based messaging is an approach that utilizes our inherent nature to value the appropriateness of our actions as compared to others. For example, outside the realm of healthcare, utility companies motivate consumers to become more energy-efficient by displaying (on the monthly bill) comparisons between the electricity and natural gas use of individual households compared to their more efficient neighbors.
One important reason for low participation rates of HMG in oncology trials is ascribed to HCPs failure to invite Black patients based on inaccurate perceptions of widespread mistrust. An example of norm-based messaging to correct such implicit bias is to promote messages such as “Did you know that 8 out of 10 Black patients express interest in being a clinical trial participant?”
Behavioral design can be also applied to message framing. A well-known example is how the hotel industry cut costs by convincing guests that their bedsheets did not need to be changed daily as a way of “saving the environment.” Similarly, enhancing clinical trial diversity could benefit from appealing to the altruism of patients, for example, emphasizing the potential gains to other patients like them (as opposed to narrow self-interest).
Message Delivery
Messages can be transmitted via any medium, from paper flyers in the hospital staircase to the electronic health record (EHR). An example of this type of messaging was during the COVID pandemic and vaccination of high-risk groups. Interventions such as personalized text-based reminders were shown to increase percentages of both vaccinations and appointments.67
For neuro-oncology, we can use pop-up messages in the EHR for all patients who are eligible for clinical trials. When accessing a patient’s chart, a message may read: “Have you screened [patient name] for a clinical trial? Research shows that patients of all races enroll in clinical trials when given the same opportunities and choices.”68 These types of messages reinforce social norms while also counteracting implicit bias, such as the false belief that HMGs are less likely to be interested in clinical research. Consistent and standardized reminders can help to ensure that every patient is given appropriate care and the opportunity to participate in clinical research. Another way that this could be emphasized would be by adding similar messaging to treatment guidelines and algorithms, for example, the National Comprehensive Cancer Network (NCCN) guidelines for glioblastoma could include the disclaimer that patients of all races and ethnicities value participation in clinical research.
These messages can also be disseminated to our patient population electronically. For example, many institutions now use text-based reminders for appointments. Messages emphasizing the benefits of clinical trials and research while also normalizing it (eg, providing context about the number of patients who engage in clinical trials and the benefits of research) can be targeted for all our eligible patients, especially those from marginalized groups. Messages should be clear, concise, and not so frequent to produce message or alarm fatigue. Thoughtful placement of these messages, as not to interrupt normal care or workflow, should be used.69
Another related intervention is to emphasize the importance of clinical trials in our field on a daily and innocuous basis. Pins, buttons, or badges with “Ask me about my research,” or “Have you considered a clinical trial,” can be worn by providers. Similar messaging can be included in clinic settings and the hospital. These types of messages normalize the concept of cancer research as an everyday, common practice, as part of the oncologist’s toolkit, and not as a measure of last resort.
Change the Default
Another behavioral economics concept is the default effect, which is the tendency to accept the default option. This has been used in a variety of domains. A notable example is increasing organ donation. Countries in which the default option is organ donation or where opt-in choices are switched to opt-out choices (Select this box to be an organ donor → Select this box to NOT be an organ donor) have significantly increased rates of organ donation.70,71
We can use this technique, particularly in cases where there is no established standard of care such as recurrent glioblastoma, to change the default choice to clinical trial enrollment. The inclusion of clinical trials as first-line therapy in the NCCN guidelines is an example of this. As a field, we can build on this by making clinical trial enrollment the default option. Only if the patient is ineligible or unable to participate in a clinical trial should other treatment options be considered. All institutional and practice guidelines should be updated to reflect clinical trial participation as a default setting.
Organizational
Establish Demographic Specific Enrollment Criteria for Every Study
Patient enrollment should match either the disease incidence or mortality rate, in-line with established benchmarks.4 The FDA has created guidelines for industry in support of enhancing the diversity in clinical trial enrollment.18 Similar support of the home institution is necessary in keeping with the priority of national agencies to reduce this disparity. As such, every participating site should include an enrollment plan in which investigators have an a priori strategy that will be taken to meet enrollment benchmarks—with language of inclusionary practices for recruitment, enrollment, and retention of participants from underrepresented groups. Within study teams, it may be beneficial to embed patient or advocacy representatives who will undoubtedly have an eye towards inclusive enrollment practices.
Incorporated into the design, should also be well-defined criteria for permitting exemptions to inclusive trials. For example, certain genetic and inherited diseases may not benefit from including diverse patient populations. Unless evidence is provided to the contrary, including diverse patient populations should be the norm. Additionally, studies that are unable to meet these inclusive standards should include a statement of generalizability when published. Specifically, if only White patients are enrolled and followed on study, then it must be stated in published results “these findings may not reflect the outcome for other patient populations, including HMGs and thus these results are inconclusive.” Table 3 outlines potential metrics for success over the next decade (Table 3).
| By 1 year | Mandate from Neuro-Oncology journals that race/ethnicity data must be included or commented on as a criterion for manuscript review/acceptance |
| By 5 years | Race/ethnicity demographics reported in ≥33% of all brain tumor trials Consortia trials (ie, Alliance) should have HMG representation data consistent with available epidemiologic data |
| By 10 years | Race/ethnicity demographic data reported in ≥50% of all brain tumor trials (consortia and industry-sponsored) |
| By 1 year | Mandate from Neuro-Oncology journals that race/ethnicity data must be included or commented on as a criterion for manuscript review/acceptance |
| By 5 years | Race/ethnicity demographics reported in ≥33% of all brain tumor trials Consortia trials (ie, Alliance) should have HMG representation data consistent with available epidemiologic data |
| By 10 years | Race/ethnicity demographic data reported in ≥50% of all brain tumor trials (consortia and industry-sponsored) |
| By 1 year | Mandate from Neuro-Oncology journals that race/ethnicity data must be included or commented on as a criterion for manuscript review/acceptance |
| By 5 years | Race/ethnicity demographics reported in ≥33% of all brain tumor trials Consortia trials (ie, Alliance) should have HMG representation data consistent with available epidemiologic data |
| By 10 years | Race/ethnicity demographic data reported in ≥50% of all brain tumor trials (consortia and industry-sponsored) |
| By 1 year | Mandate from Neuro-Oncology journals that race/ethnicity data must be included or commented on as a criterion for manuscript review/acceptance |
| By 5 years | Race/ethnicity demographics reported in ≥33% of all brain tumor trials Consortia trials (ie, Alliance) should have HMG representation data consistent with available epidemiologic data |
| By 10 years | Race/ethnicity demographic data reported in ≥50% of all brain tumor trials (consortia and industry-sponsored) |
IRB and FDA Strategies to Increase Accrual
Clinical trial regulatory bodies, including the FDA and IRBs, must be active agents in both advocating for and ensuring brain tumor trials are accruing diverse patient populations. Despite the current requirements that clinical trials collect and report demographics, only 63% of FDA oncology drug approval trials between 2000 and 2018 and 20% of neuro-oncology trials between 2000 and 2019 reported racial demographics.4,24 While NCI and NIH-funded trials have strict reporting requirements and mandates on racial representation in trials, industry-sponsored trials do not have reporting requirements for early-stage clinical studies. Recognizing this limitation, Congress recently passed legislation allowing the FDA to codify guidance on diversity action patients for enrolling patients from HMGs. Drug sponsors will be required to submit a diversity action plan detailing the steps taken to ensure inclusive recruiting practices and a good faith effort in promoting diversity for the trial. This guidance also provides additional guidance for IRBs to set and enforce equitable standards, especially with non-governmental drug sponsors.28
Once demographic data are collected, these findings would be permitted for full report on the clinicaltrials.gov website; further motivating other institutions to follow the same inclusionary practices. Use of these regulatory bodies would thus ensure representation was equitable and diverse, as a conduit to future clinical trial design and implementation of findings. We understand that challenges exist with enforcing such regulatory requirements, thus continuous and strong engagement across the clinical team in research support (eg, nursing, data management, protocol navigators) will be imperative to ensuring rigorous inclusive standards are met.
Inclusion of community-based groups, patient advocacy groups, and health equity experts in protocol writing can also be helpful in promoting inclusionary practices. Community-based participatory research (CBPR) programs have shown synergistic collaborations between researchers and community members and can serve as a framework for therapeutic clinical trials. This can include colearning and capacity building with both researchers and community members, addressing power imbalances by including community members in decision-making and IRBs, and engaging in cooperative and collegial partnerships.
Diversification of the Workforce
Diversification of the workforce is crucial to mitigating HD and addresses many issues that contribute to the lack of representativeness in clinical trials, including building trust with HMGs, improving cultural and linguistic competency, direct community engagement and outreach, and decreasing biases. The definition of populations underrepresented in medicine and research (URiM) is now broadly defined as including any population in medicine that is underrepresented relative to its population in the United States. Historically, the following groups have been characterized as URiM: Black/African American, Hispanic/Latino, Indigenous, and Hawaiian/Pacific Islanders. In the United States, those who identify as Hispanic/Latino represent 18.5% of the U.S. population; however, only 5.8% of physicians and 3% of oncologists. A similar trend is noted for Black/African Americans, who represent 13.5% of the U.S. population; however, only 5% and 2.3% of physicians and oncologists, respectively.72
As the leading organization for clinicians and researchers in neuro-oncology, the mission of SNO is to “promote advances in neuro-oncology through education and research.” In 2020, the SNO president and executive leadership commissioned its Women and Diversity committee to conduct a survey of SNO membership. In addition to characterizing the academic efforts and career aims of the members, this survey also sought to understand the demographics of its wider membership. Among 386 members who completed the survey, 12% self-identified as Hispanic/Latino and 5% as African American.73
As these numbers are further interrogated, there are opportunities for intervention and improvement, namely through examining the trainee pathway. ASCO published their report tracking supply and demand in Oncology in their strategic plan. At the time, Black/African Americans accounted for only 2.3% of US Oncology fellows, while Hispanic/Latino were 5.3%.72 As there has been a trend toward medical neuro-oncologists being trained as neurologists, in examining the demographics in Neurology, underrepresented groups are only 12% of adult neurology residents.74
We ask our organizations and workplaces to place an increased emphasis on diversity. This relates not only to researchers and health care professionals but to all staff involved in health care. There is overwhelming data that diversity increases health outcomes in HMG and also increases business performance and interpersonal relationships of employees.9,75–78 As a field, we can also utilize our national meetings and convenings to help increase diversity, engage in outreach, and build trust. During annual SNO meetings, we can partner with community organizations to raise awareness about CNS cancer and use our influence to encourage health equity and inclusion. These can include patient-facing sessions, partnering with community centers and charitable organizations for a day of service, or a mentorship day for local school children. Given the longstanding structural impediments that lead to lack of diversity in medicine, we also need to make a concerted effort across all educational groups to encourage diverse candidates to consider a career in neuro-oncology. There exist opportunities to collaborate with premedical and undergraduate medical students through affinity groups including the Student National Medical Association, the Latino Medical Student Association, and through institutional partnerships, which primarily educate, and train URiM students.
Partnership With Industry
Industry has tremendous influence in shaping the interventions available for the future of cancer care, and consequently, their responsibility in promoting inclusive practices and addressing disparities in healthcare is significant. One opportunity is to increase both diversity in the workforce and to train investigators to conduct inclusive research. For example, the Bristol Meyers Squibb Foundation has partnered with National Medical Fellowships (NMF, an organization established in 1946 with a specific charge of increasing physicians from underrepresented backgrounds to address access and disparities in healthcare) to create the Robert A. Winn Diversity in Clinical Trials Career Development Award (Winn CDA).79 The grant was established in 2021 to increase diversity in clinical trials and to train diverse principal investigators on how to conduct inclusive clinical research. NMF has also partnered with other pharmaceutical companies to create additional programs to increase diversity in clinical trials.
Society for Neuro-Oncology has partnered with Novocure to create the SNO/Novocure Travel Scholarship for URiM medical students through the committee for Women and Diversity established in 2021. This award provides funding for ten 3rd or 4th year medical students to attend the SNO annual meeting, during which time the scholars interact and meet current SNO members and participate in programming through the Women and Diversity Committee. This program is now in its 3rd year. As we continue to partner with industry, it will be important to track the outcomes of scholars in these initiatives, such as those who ultimately enter fields leading to neuro-oncology (Neurology, Neurosurgery, Radiation Oncology, Medical Oncology, etc.).
In addition to their role in providing tools to bolster the workforce, industry has the capacity to promote equity through offering resources and services that would empower patients from diverse backgrounds. For instance, resources may be provided to ensure patient-facing materials including consents are translated into the language of choice allowing more broad participation. This is often an expense that is absorbed by the primary investigator and their institution. Barriers to participation in industry-sponsored trials also include transportation, lodging, and additional out-of-pocket costs including food, childcare, and missed compensation for the patient and caregiver. While keeping in compliance with ethical considerations, compensation to alleviate these additional expenses should be taken into consideration in industry-sponsored trials. While the responsibility in promoting equity and inclusion within cancer care is reliant on all participants in healthcare, industry partners have the opportunity to not only leverage their unique role in healthcare but also to provide accountability to ensure every patient can benefit from research and new treatments.
Community Level
Building Trust
There is a well-documented and well-deserved mistrust of the medical system especially among HMGs.4,15,16,24,29 Community organizations can work with patients and the medical community to promote healthy and good practices. This includes aspects of medical trust and mistrust, and how we can build better relationships and trust with patients. For example, researchers can utilize tenets of CBPR when conducting research to obtain feedback and partnership from community members. CBPR emphasizes shared power and decision-making, with community members as active members of the research team. This transparency and power sharing, especially when designing trials and recruiting participants, can bridge the gap between the established research field and the communities we serve. In some cases, when medical mistrust is longstanding between the community and medical establishment, restorative justice can be useful.80 Restorative justice focuses on repairing harm through different mediation techniques without solely blaming or punishing the offender. These tenets can be very useful when repairing years of either medical or institutional mistrust.
Patients in underserved communities may lack awareness that a trial exists for their brain tumor type. Advocacy groups, medical institutions, pharmaceutical sponsors, and local health departments can help share trial information with underserved communities to educate and empower patients to ask questions and seek out trials when interested. The Brain Tumor Network, National Brain Tumor Society, and American Brain Tumor Association are patient advocacy groups that help connect patients with clinical trial opportunities. Advocacy groups can also help by gathering data, such as race, ethnicity, and zip code data, from the patients they serve to ensure that they are reaching diverse populations. This will be instrumental in building a better approach to filling the gaps in awareness and engagement within those patient populations.27
Increasing Genomic Capture of Diverse Populations
Expanding from the local community to the worldwide community, we also need to focus on increasing the representation of diverse groups in tissue sampling, biobanking, and next-generation sequencing (NGS). A recent analysis of 4 major cancer genomic projects revealed that 73.3% of all data across cancers was derived from White patients, and that the proportion of patient samples lacking ethnic or racial data (11.6%) far outweighed the representation of verified underrepresented populations (8.9% African American, 5.6% Asian, and 0.6% Other).81 Moreover, analysis of cancer-related genome-wide association studies spanning over 6 million samples found over 90% of samples were from White patients and 5% from Asian patients, with less than 1% of samples coming from other racial groups.82 This effect trickles down to the bench, with 46% of cancer cell lines in the NCI Patient-Derived Model Registry having no race or ethnicity data at all and with 38% of the remainder derived from White, followed by 12% from Asian, 4% from Black, and less than 1% from Hispanic patients. In our sphere, the TCGA data set for glioblastoma encompasses 617 samples from 596 individuals of which 85% are White, and only 9% are Black, 2% are Asian, and the remainder are of unknown race. Thus, attempts to identify biologically important differences by ancestry that impact survival in glioblastoma is marred by lack of available data and research materials. The rarity of many CNS tumors along with the lack of racial diversity of available samples leads to an underdeveloped understanding of both biological differences due to ancestry and how the SDoH and discrimination can lead to epigenetic changes and differences at the cellular level.
Remedying the lack of diversity in biobanking, tissue sampling, and NGS will require concerted efforts and partnerships. As NGS becomes more common and standard of care, every effort should be made to provide tumor sequencing for HMG patients, even if not specifically tied to a therapeutic opportunity in the short term. Many insurances, including many Medicaid plans, now provide coverage for NGS. Medicare, federally funded insurance for elderly population, also recently approved the coverage of NGS for stage III and IV cancers (this also includes CNS grade 3 and 4 tumors), including glioblastoma.83
When insurance coverage is limited, for example, in states that have not approved Medicaid coverage of NGS, strategic partnerships between research institutions and community sites can help provide support. Academic medical centers (AMC), community hospitals, health centers, and private practices can work together with industry to establish collaborative partnerships, which can have mutually beneficial outcomes. For example, a private practice serving the community can enter with a partnership with the local AMC, obtaining easier and streamlined NGS access and clinical trial options for patients. The AMC also benefits from adding another potential patient to their institutional biobank. These can be modeled after the community outreach and engagement program of NCI comprehensive cancer centers. Additionally, NGS companies can work to expand platforms and accessibility in HMG communities. This will become increasingly important as more targeted therapies are developed, and the absence of NGS may preclude patients from these trials and therapies.
Societal Level
Virtual Care, Decentralization, and Lessons From the Pandemic
The challenges that have led to inequitable representation of diverse populations in neuro-oncologic care are multifaceted, including systemic barriers that limit access.84 Proximity and access to a dedicated cancer center and/or tertiary referral center is one of the barriers limiting inclusion of diverse patient populations in cancer clinical trials. The onset of the COVID-19 pandemic resulted in delays to treatment, clinical trial enrollment, and increased angst for patients and providers.85 Simultaneously, it also expedited the use of telemedicine in the care of cancer patients, allowing for improved access and reducing barriers that include transportation, distance, time off work, childcare, and other socioeconomic factors that have the propensity to limit care.86,87
Patients with neuro-oncologic disease often have deficits that impair cognition and mobility, requiring significant caregiver support. Virtual neuro-oncologic care not only promotes inclusion from a socioeconomic perspective but also allows extended family access to the virtual visit, enhancing patient care through broad caregiver support. Virtual tumor boards, formulation of treatment plans, and screening for clinical trials not only provided access to empower local providers to care for their patients but also allowed opportunity for patients who desire second opinion without requiring travel.88–90 Through expanded virtual offerings, integrated health programs saw an exponential increase in participation and demonstrated feasibility and appeal to patients to receive care in this manner.91 While studies have been done within neuro-oncology to support the use of telemedicine for this patient population,92 work is ongoing to identify optimal utilization of virtual care, particularly, as the state licensing requirements that were lifted during the Public Health Emergency are reenacted.93 While limitations of telemedicine include access to high-speed internet, video capability, and potentially privacy, it is intended to augment and not meant to replace the in-person encounters that remain the gold standard of care. Despite these limitations, the benefits of improved access for rural and underserved populations are evident and allow increased opportunity for equity and inclusion in neuro-oncologic care.
For in-person visits that cannot be substituted, every effort must be made to provide or subsidize transportation for patients. Recent studies have shown that significant costs, including parking fees, can result in financial toxicity for patients.94 Research stakeholders should subsidize these costs, either with coverage of transportation costs or by partnership with rideshare companies to provide free or discounted transportation. This is particularly important for neuro-oncology patients, who may have trouble driving, due to cognitive or neurologic deficits.
Patient and Provider Incentives to Promote Inclusivity and Health Policy Initiatives
Enrolling patients with limited resources to clinical trials may require additional time and resources. Lengthy conversations about clinical research, including are often nonreimbursable. This can place pressure on the clinical and research teams, driving research teams to prioritize patients who require the least amount of effort for enrollment. This inherently creates a feedback loop, as patients with higher health literacy, higher socioeconomic status, fluent in English, and better reimbursement schemes (as opposed to those who are on Medicaid or uninsured), are more likely to be familiar with clinical research and more likely to be targeted by clinical research teams. To create parity, direct incentives should be employed. These can include increased relative value units for patient encounters requiring an interpreter, or specific current procedural terminology codes for increased time related to patient encounters. Other incentives such as increased protected or administrative time can be built into clinic schedules to facilitate participation.
Increased incentives for patients can also help encourage trial participation. Lower-income patients are less likely to enroll in clinical trials and there should be dedicated funding to mitigate additional burden experienced by patients from HMGs.95 Routine expenses related to clinical trial activities should be reimbursed. An important piece of legislation, the Clinical Trial Treatment Act, went into effect on January 1, 2022, which required Medicaid organizations to cover routine costs associated with clinical trials.96 Going one step further, need-based renumeration for participation could cover lost wages, transportation, childcare costs, and housing. Transportation and lodging barriers for women with young children, caregivers, and patients that would be otherwise unable to participate is a known strategy to boost study enrollment. Proposed legislation with these provisions, such as the DIVERSE Trials Act, has so far failed to pass.97 Expanded legislation and policy are invaluable in providing the tools and resources for patients and providers. These programs should not be limited to government-backed insurance plans—private insurers should also cover routine costs associated with clinical trials. As a profession, we can partner together to advocate for these changes at both the state and federal levels. The SNO public policy committee, together with other organizations such as ASCO or the American Academy of Neurology (AAN), can work collaboratively with patients, patient advocacy groups, industry, and communities to lobby for laws focused on improving access to clinical trials and treatment.
Recognizing the importance of access to clinical trials and dedicated cancer centers, New York State approved legislation in 2023, which specified that Medicaid managed care plans will have to provide access to NCI-designated cancer centers.98 We encourage other states to follow suit and ensure that Medicaid beneficiaries have access to cancer centers with robust clinical trial offerings. Insurance barriers for second opinions and clinical trials screening visits contribute to additional delays and ultimately a decreased pool of diverse candidates, which unfairly affects those with limited insurance and resource options the most. Specifically for neuro-oncology, access issues often lead to difficulties enrolling patients in upfront trials.99 Many trials require central tissue review, surgery, or radiation to be performed at the sponsoring institution. Additionally, insurance delays, prior authorizations, and approvals, can lead to delays that make enrolling in a clinical trial very difficult. Flexible and emergency insurance authorizations and expanded telehealth access can help make consultations for NGS, clinical trials, biobanking much easier for both patients and providers.
Institutional and local programs can be instrumental. One such example is the Integrated Cancer Care Access Network, a program based out of MSKCC, which provides resources such as financial assistance, legal help, transportation, and community assistance to at-risk patients.100 Notably, this program has expanded to feature many public and community hospitals. This network has been successful in improving cancer care adherence and could be pivoted to promote the accrual of diverse research subjects. Similarly, other groups, such as patient advocacy groups, can help provide or compile resources that counteract the financial and resource burdens that can accompany enrollment in a clinical trial. This can also include direct grant funding or renumeration. While there may be increased upfront costs, the future proceeds from diversification of clinical research will pay untold dividends in the future.9
Summary
The social determinants of health contribute to HD, which in turn negatively affects all patients with cancer. Increasing the representation, diversity, and inclusive practices of clinical research will help to diminish these HD and optimize clinical care. Using the SEM, we can provide meaningful interventions based on the stakeholder interests and research/clinical objectives. As our neuro-oncology field continues to perform cutting-edge research into the physiology and treatment of these tumors, we have an obligation to ensure all these advances are distributed equitably. It is imperative that we improve clinical trial and clinical research diversity by using a multidisciplinary approach and engaging all stakeholders. With an intentional commitment to inclusion and health equity, we will be doing our part to “first do no harm” and provide better healthcare for all patients.
Conflict of interest statement
J.A. B. and U.N.C. both received the Robert A. Winn Diversity in Clinical Trials Career Development Award, which is mentioned as an intervention in the manuscript. No other authors report conflicts of interest.
Funding
This work was supported by the Nicholls-Biondi Diversity Clinical Scientist Program at Memorial Sloan Kettering Cancer Center.
References
Author notes
Joshua A Budhu and Ugonma N Chukwueke are co-first authors.
- brain tumors
- cancer
- demography
- ethnic group
- hispanics or latinos
- languages
- medical oncology
- diagnosis
- neoplasms
- cancer research
- subject selection
- incentives
- health disparity
- equity
- community
- workforce
- neurologic oncology
- clinical research
- prevention
- partnerships
- social determinants of health
- health equity
- indigenous peoples
- malignant brain neoplasms




