-
PDF
- Split View
-
Views
-
Cite
Cite
Kazuhiko Mishima, Ryo Nishikawa, Yoshitaka Narita, Junki Mizusawa, Minako Sumi, Tomoyuki Koga, Nobuyoshi Sasaki, Manabu Kinoshita, Motoo Nagane, Yoshiki Arakawa, Koji Yoshimoto, Ichiyo Shibahara, Naoki Shinojima, Kenichiro Asano, Takao Tsurubuchi, Hikaru Sasaki, Akio Asai, Takashi Sasayama, Yasutomo Momii, Atsushi Sasaki, Shigeo Nakamura, Masaru Kojima, Jun-ichi Tamaru, Kazuhiro Tsuchiya, Miho Gomyo, Kayoko Abe, Manabu Natsumeda, Fumiyuki Yamasaki, Hiroshi Katayama, Haruhiko Fukuda, Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C, Neuro-Oncology, Volume 25, Issue 4, April 2023, Pages 687–698, https://doi.org/10.1093/neuonc/noac246
Close - Share Icon Share
Abstract
The goal was to determine whether the addition of temozolomide (TMZ) to the standard treatment of high-dose methotrexate (HD-MTX) and whole-brain radiotherapy (WBRT) for primary central nervous system lymphoma (PCNSL) improves survival.
An open-label, randomized, phase III trial was conducted in Japan, enrolling immunocompetent patients aged 20–70 years with histologically confirmed, newly diagnosed PCNSL. After administration of HD-MTX, patients were randomly assigned to receive WBRT (30 Gy) ± 10 Gy boost (arm A) or WBRT ± boost with concomitant and maintenance TMZ for 2 years (arm B). The primary endpoint was overall survival (OS).
Between September 29, 2014 and October 15, 2018, 134 patients were enrolled, of whom 122 were randomly assigned and analyzed. At the planned interim analysis, 2-year OS was 86.8% (95% confidence interval [CI]: 72.5–94.0%) in arm A and 71.4% (56.0–82.2%) in arm B. The hazard ratio was 2.18 (95% CI: 0.95–4.98), with the predicted probability of showing the superiority of arm B at the final analysis estimated to be 1.3%. The study was terminated early due to futility. O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status was measured in 115 tumors, and it was neither prognostic nor predictive of TMZ response.
This study failed to demonstrate the benefit of concomitant and maintenance TMZ in newly diagnosed PCNSL.
No benefit of concomitant and maintenance TMZ was shown in newly diagnosed PCNSL.
MGMT status was neither prognostic nor predictive for TMZ treatment in PCNSL.
TMZ is an oral alkylating agent that penetrates the blood–brain barrier with moderate toxicity, and it has shown anti-tumor activities in PCNSL in single-arm studies. We conducted a randomized, phase III study comparing HD-MTX with WBRT ± boost and HD-MTX with WBRT ± boost with concomitant and maintenance TMZ. Patients in the TMZ arm did not show any survival advantage in the planned interim analysis, and this study was terminated early because of futility. MGMT promoter methylation, which is known to be prognostic and predictive in glioblastoma, was neither prognostic nor predictive for TMZ treatment in newly diagnosed PCNSL.
Primary central nervous system lymphoma (PCNSL) is an aggressive, extranodal non-Hodgkin lymphoma that could affect any part of the neuroaxis, including the eyes, brain, leptomeninges, or spinal cord, and it accounts for about 3–5% of all primary intracranial tumors.1 Whole-brain radiation therapy (WBRT) was historically chosen as the standard modality to treat PCNSL, given the multifocal and highly infiltrative nature of the tumor. Although the response rate to this treatment is approximately 90%, WBRT alone without chemotherapy is known to be inadequate, since the median survival time is as short as 12 to 18 months, and the 5-year survival is as low as 18–35%.2 The introduction of high-dose methotrexate (HD-MTX) has improved the median survival time to 33 months, which made HD-MTX followed by WBRT the standard of care for patients with newly diagnosed PCNSL.3 However, despite the high initial complete response (CR) rate obtained by HD-MTX with WBRT, more than 50% of the patients experience relapse within the first 2 years after diagnosis.4 It may be worth noting that 30–40-Gy WBRT is not currently the standard of care in patients above 60 years of age due to increased neuro-toxicity.5
The therapeutic effect of chemotherapies is limited due to the lack of drugs that efficiently penetrate the blood–brain barrier (BBB) and the diffusely infiltrating growth of PCNSL. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), which is the standard therapy for systemic lymphoma, in combination with WBRT, did not show any survival benefit compared to WBRT alone.6 The BBB, which hinders the penetration of most of these drugs (rituximab, doxorubicin, and vincristine), is thought to be the major reason. It is also known that the prognosis of PCNSL remains poor even after complete remission with induction chemotherapy and WBRT.7 Maintenance treatments that prolong remission, delay relapse, and maintain tumor dormancy may improve outcomes in PCNSL. Thus, we hypothesized that combining HD-MTX and WBRT with a chemotherapeutic agent that is effective against PCNSL, capable of penetrating the BBB with acceptable toxicity, and is suitable as maintenance chemotherapy would lead to the development of a new treatment strategy.
Temozolomide (TMZ) is an oral alkylating agent that penetrates the BBB with moderate toxicity.8 TMZ is currently the standard chemotherapeutic drug and possible radiosensitizing agent to treat glioblastoma.9 It improves survival of patients with glioblastoma by 2.5 months relative to radiotherapy (RT) alone when used in concurrent TMZ with RT and then 6 adjuvant cycles of TMZ, according to the results of a phase III study, EORTC 26981.10 TMZ is also active in newly diagnosed and recurrent PCNSLs. Glass et al. reported a phase I/II study adding TMZ with induction and post-irradiation therapy for newly diagnosed PCNSL and showed an objective response rate of 85.7% and 2-year survival rate of 80.0%.11 Rubenstein et al. also reported, although the results were published after the initiation of the present study, that induction therapy using HD-MTX with TMZ together with rituximab achieved an objective response rate of 77%.12 Reni et al. reported a response rate of 31%, a median overall survival (OS) of 3.9 months, and a median progression-free survival (PFS) of 2.8 months for recurrent PCNSL.13
We hypothesized that adding concomitant TMZ to WBRT after HD-MTX and post-irradiation maintenance TMZ would result in improved disease control of PCNSL. It was also assumed that residual PCNSL cells in the tumor regression phase after RT contained a minimal subpopulation of PCNSL cells in the proliferation phase. Thus, adding TMZ, a cell cycle-non-specific DNA alkylating agent, for treatment maintenance was considered theoretically appropriate, similar to that for the standard regimen for glioblastoma.14,15
Based on the rationale shown above, we planned and conducted a randomized, phase III trial as a Japan Clinical Oncology Group (JCOG) study, named JCOG1114C, to test the hypothesis that adding concomitant and maintenance TMZ chemotherapy to standard treatment with HD-MTX and WBRT improves survival for patients with newly diagnosed PCNSL. The relationship between the TMZ treatment effect and the methylation status of the promoter region of the MGMT gene, whose product is a molecular determinant of TMZ sensitivity in glioblastoma, was also evaluated.16
Patients and Methods
Study Design and Participants
This study was designed as a randomized, open-label, phase III trial. It involved 30 Japanese hospitals participating in the Brain Tumor Study Group of the JCOG. Newly diagnosed, non-immunocompromised, PCNSL patients were enrolled at the first registration.
Eligibility criteria for the first registration included: age 20–70 years; histologically proven diagnosis of diffuse large B-cell lymphoma; disease exclusively localized within the CNS (with or without intraocular lymphoma); no evidence of lymphomatous meningitis; no evidence of lymphomatosis cerebri; postoperative date of tumor biopsy or removal between 3 and 35 days; an Eastern Cooperative Oncology Group performance status score of 0–2 (ECOG PS 3 allowed if due to neurological deficits); no prior chemotherapy or RT for any cancers; and adequate organ function. Exclusion criteria are described in the Supplementary Material. Eligibility criteria for the second registration are described in the Supplementary Material. Diagnostic histopathological materials of all registered cases were referred for central review. All patients provided written, informed consent once eligibility was confirmed and after they had reviewed the protocol’s contents. This trial conformed to the Declaration of Helsinki, and the study protocol was approved by the institutional review boards of the participating institutions.
Procedures
Patients who passed the first registration received 3 cycles of HD-MTX (3.5 g/m2 as an intravenous infusion over 3 hours) on day 1 of a 14-day cycle. Patients received adequate hydration, urinary alkalinization, and folinic acid rescue 24 hours after MTX. The first cycle of HD-MTX had to start within 7 days after the first registration. If renal or hepatic dysfunction or cytopenia developed, HD-MTX treatment was postponed, and the MTX dose was subsequently reduced according to the guidelines in the protocol.
Response to HD-MTX treatment was assessed by contrast-enhanced brain MRI 8-14 days after the first and second cycles of HD-MTX, and by contrast-enhanced brain MRI, CSF cytology, slit-lamp examination (necessary for patients with ocular involvement), and the use of corticosteroids, 8–21 days after the third cycle of MTX. The International PCNSL Collaborative Group Response Criteria were used for response assessment.17 All assessed brain MRIs with measurable tumors (diameter ≥ 1 cm) at baseline were centrally reviewed.
Patients who received at least one cycle of HD-MTX were randomly assigned in a 1 to 1 ratio at the second registration to receive WBRT at a dose of 30 Gy for cases without intraparenchymal tumor after HD-MTX and 30-Gy WBRT + 10-Gy local boost for cases with positive intraparenchymal tumor as a control (arm A), or WBRT ± boost with concomitant and maintenance TMZ for 2 years as an experimental treatment (arm B). TMZ concomitantly administered with RT was consecutive-day dosing of 75 mg/m2, starting from the first day of RT and ending on the last day of RT. Four to 5 weeks after the RT, TMZ maintenance (100–200 mg/m2/day, days 1–5, every 4 weeks) was started for 2 years (from the start of HD-MTX). The dosing and schedule of TMZ administration are similar to those for glioblastoma.10
Patients with the absence of visible intraparenchymal tumor after HD-MTX treatment received 15 fractions of 2-Gy WBRT. WBRT included the lower edge of the first segment of the cervical spinal cord and the posterior two-thirds of the orbits. The eyeball was included for irradiation for cases with ocular involvement. An integrated boost of 5 fractions of 2 Gy was administered to the tumor area(s) for patients with the presence of visible intraparenchymal tumor after HD-MTX treatment. Quality assurance reviews were performed at the Radiotherapy Support Center under the supervision of the JCOG Radiotherapy Committee.
All adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 before every cycle. In arm A, protocol treatment was completed if RT was completed. In arm B, protocol treatment was completed if maintenance TMZ chemotherapy was completed. After completion of protocol treatment, the disease was assessed every 3 months.
The effect of treatment on neurocognitive functions was assessed by the Mini-Mental State Examination (MMSE), which was performed before the first registration, within 30 days after WBRT, every 6 months for 2 years, and then every 12 months for 2 to 5 years.
Outcomes
The primary endpoint of this study was OS, defined as the time from randomization at the second registration to death from any cause or to the last date of contact with the surviving patient. Secondary endpoints were response rate after HD-MTX, response rate after RT, CR rate after HD-MTX, CR rate after RT, PFS, the incidence of adverse events, the incidence of early death, the incidence of treatment-related death, the incidence of grade 4 non-hematological adverse events, the proportion of non-worsening MMSE, the proportion of HD-MTX completion, the proportion of RT completion, and cycles of maintenance TMZ chemotherapy. All eligible cases at the second registration with measurable lesions were subject to analyses of the response rate and the CR rate after HD-MTX and RT (or concomitant TMZ and RT), respectively. PFS was from the date of randomization at the second registration to the first date of first disease progression or death from any cause.
Random Assignment
Randomization was done at the JCOG Data Center with the use of the minimization method with random component balancing for the institution, ECOG performance status at the second registration (0–1 vs. 2–3), age at the first registration (≤ 60 vs. ≥ 61 years), and the presence or absence of intraparenchymal tumor after HD-MTX. Patients, attending physicians, and individuals assessing outcomes and analyzing data were not involved in treatment allocation.
MGMT Testing with Pyrosequencing
Serial 5-μm-thick sections of formalin-fixed and paraffin-embedded (FFPE) tumor tissues and/or fresh-frozen tumor tissues were collected at the Kyorin University Faculty of Medicine for MGMT analysis. Unstained FFPE slides collected for central pathology diagnosis were also used from patients in whom those samples were available. DNA extraction procedures are described in the Supplementary Material. The methylation rate of 10 CpG sites (CpG74-CpG83) of the MGMT promoter was quantitatively analyzed with pyrosequencing as in another JCOG study (JCOG1910),18–20 given that hypermethylation of the MGMT promoter is associated with good outcomes in patients with newly diagnosed glioblastoma treated with TMZ.21,22 The extent of methylation was dichotomized to either hypermethylated or hypomethylated using the median value as the cutoff.
Statistical Analysis
Two-year OS in arm A was set as 65% based on reports of HD-MTX-based chemotherapy and WBRT of 62–69%.7,23–25 Since the highest 2-year OS in the previous reports on HD-MTX plus RT for PCNSLs was 70%, a 10% increase in the 2-year OS was anticipated, resulting in expected 2-year OS of 80% in the experimental arm. With a one-sided significance level of 5%, 3-year accrual, and 3-year follow-up, the required sample size to attain 80% power was 60 patients per arm, 120 eligible patients in total (60 observed events in total). Considering several ineligible cases, the total target sample size was set to 130 randomized patients.
The first interim analysis was planned for the date on which half of the planned sample size had been enrolled, and the second interim analysis was scheduled for the date on which the entire planned number of patients had been enrolled. Results of the interim analysis were reviewed by the JCOG Data and Safety Monitoring Committee, and investigators were masked to the results. The prespecified stopping rules for futility were as follows: if the survival curve for arm B was below that for arm A (ie, HR > 1.0), early termination of the study owing to futility would be considered, taking into account various factors such as the toxicity profile and the precision of the estimated HR.
OS and PFS curves were estimated by the Kaplan–Meier method. Treatment arms were compared by the stratified log-rank test. A hazard ratio (HR) of the treatment effect and its confidence interval (CI) was calculated using a stratified Cox proportional hazards model. Prespecified subgroup analyses were performed according to performance status, age, residual enhancing tumor after HD-MTX, sex, and ocular disease. Others were conducted as post hoc analyses. All analyses were performed by the JCOG Data Center using SAS 9.4 (SAS Institute). This trial was registered with the Japan Registry of Clinical Trials, number jRCTs031180207.
Results
Between September 20, 2014 and August 24, 2018, a total of 134 patients were enrolled at the first registration (Figure 1); of these patients, one did not receive treatment. Therefore, 133 patients received at least one cycle of HD-MTX, 114 patients completed 3 cycles of HD-MTX, and 6 patients terminated HD-MTX due to acute toxic effects, 11 due to progressive disease, and two due to other reasons.
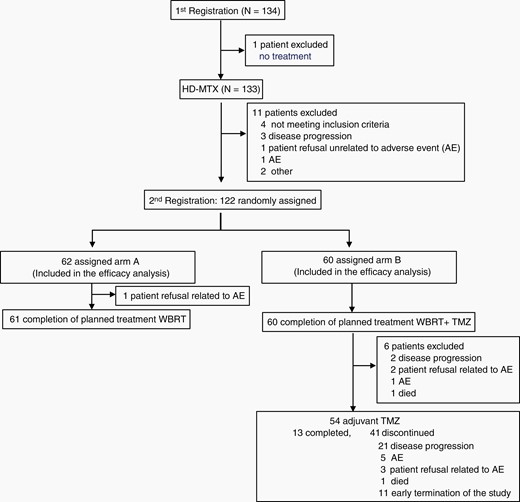
CONSORT diagram showing the distribution and treatment of patients in the JCOG1114C study. AE, adverse event.
Of the 133 HD-MTX-treated patients, 11 were excluded before the second registration (Figure 1). Therefore, 122 patients underwent randomization, of whom 62 and 60 patients were assigned to the WBRT ± boost (arm A) and the WBRT ± boost with concomitant TMZ + maintenance TMZ (arm B), respectively, and efficacy and safety were analyzed (Figure 1). Baseline characteristics were well balanced between the arms (Table 1). All tumors were proven to be diffuse large B-cell lymphoma on central pathological review.
| . | Arm A (n = 62) . | Arm B (n = 60) . | Total (n = 122) . |
|---|---|---|---|
| Age, y | |||
| Median | 63 | 62 | 62 |
| Range | 35–70 | 34–70 | 34–70 |
| Sex | |||
| Male | 40 (64.5%) | 35 (58.3%) | 75 (61.5%) |
| Female | 22 (35.5%) | 25 (41.7%) | 47 (38.5%) |
| ECOG PS | |||
| 0 | 9 (14.5%) | 3 (5.0%) | 12 (9.8%) |
| 1 | 21 (33.9%) | 28 (46.7%) | 49 (40.2%) |
| 2 | 16 (25.8%) | 13 (21.7%) | 29 (23.8%) |
| 3 | 16 (25.8%) | 16 (26.7%) | 32 (26.2%) |
| Lesion at first registration | |||
| Single | 27 (43.5%) | 30 (50.0%) | 57 (46.7%) |
| Multiple | 35 (56.5%) | 30 (50.0%) | 65 (53.3%) |
| Ocular disease | |||
| No | 55 (88.7%) | 53 (88.3%) | 108 (88.5%) |
| Yes | 7 (11.3%) | 7 (11.7%) | 14 (11.5%) |
| Intraparenchymal tumor after HD-MTX | |||
| Absent | 14 (22.5%) | 12 (20%) | 26 (21.3%) |
| Present | 48 (77.4%) | 48 (80%) | 96 (78.7%) |
| Response to HD-MTX by central review | n = 52 | n = 49 | n = 101 |
| CR/CRu | 14/52 (26.9%) | 9/49 (18.4%) | 23/101 (22.8%) |
| PR | 25/52 (48.1%) | 24/49 (49.0%) | 49/101(48.5%) |
| SD | 7/52 (13.5) | 6/49 (12.2) | 13/101 (12.9) |
| PD | 6/52 (11.5) | 10/49 (20.4) | 16/101 (15.8) |
| MGMT status | n = 60 | n = 55 | n = 115 |
| Hypermethylated | 29/60 (48.3) | 29/55 (52.7) | 58/115 (50.4) |
| Hypomethylated | 31/60 (51.7) | 26/55 (47.3) | 57/115 (49.6) |
| . | Arm A (n = 62) . | Arm B (n = 60) . | Total (n = 122) . |
|---|---|---|---|
| Age, y | |||
| Median | 63 | 62 | 62 |
| Range | 35–70 | 34–70 | 34–70 |
| Sex | |||
| Male | 40 (64.5%) | 35 (58.3%) | 75 (61.5%) |
| Female | 22 (35.5%) | 25 (41.7%) | 47 (38.5%) |
| ECOG PS | |||
| 0 | 9 (14.5%) | 3 (5.0%) | 12 (9.8%) |
| 1 | 21 (33.9%) | 28 (46.7%) | 49 (40.2%) |
| 2 | 16 (25.8%) | 13 (21.7%) | 29 (23.8%) |
| 3 | 16 (25.8%) | 16 (26.7%) | 32 (26.2%) |
| Lesion at first registration | |||
| Single | 27 (43.5%) | 30 (50.0%) | 57 (46.7%) |
| Multiple | 35 (56.5%) | 30 (50.0%) | 65 (53.3%) |
| Ocular disease | |||
| No | 55 (88.7%) | 53 (88.3%) | 108 (88.5%) |
| Yes | 7 (11.3%) | 7 (11.7%) | 14 (11.5%) |
| Intraparenchymal tumor after HD-MTX | |||
| Absent | 14 (22.5%) | 12 (20%) | 26 (21.3%) |
| Present | 48 (77.4%) | 48 (80%) | 96 (78.7%) |
| Response to HD-MTX by central review | n = 52 | n = 49 | n = 101 |
| CR/CRu | 14/52 (26.9%) | 9/49 (18.4%) | 23/101 (22.8%) |
| PR | 25/52 (48.1%) | 24/49 (49.0%) | 49/101(48.5%) |
| SD | 7/52 (13.5) | 6/49 (12.2) | 13/101 (12.9) |
| PD | 6/52 (11.5) | 10/49 (20.4) | 16/101 (15.8) |
| MGMT status | n = 60 | n = 55 | n = 115 |
| Hypermethylated | 29/60 (48.3) | 29/55 (52.7) | 58/115 (50.4) |
| Hypomethylated | 31/60 (51.7) | 26/55 (47.3) | 57/115 (49.6) |
CR, complete response; CRu, complete response unconfirmed; ECOG PS, Eastern Cooperative Oncology Group performance status; HD-MTX, high-dose methotrexate; MGMT, O6-methylguanine-DNA methyltransferase; PD, progressive disease; PR, partial response; SD, stable disease.
| . | Arm A (n = 62) . | Arm B (n = 60) . | Total (n = 122) . |
|---|---|---|---|
| Age, y | |||
| Median | 63 | 62 | 62 |
| Range | 35–70 | 34–70 | 34–70 |
| Sex | |||
| Male | 40 (64.5%) | 35 (58.3%) | 75 (61.5%) |
| Female | 22 (35.5%) | 25 (41.7%) | 47 (38.5%) |
| ECOG PS | |||
| 0 | 9 (14.5%) | 3 (5.0%) | 12 (9.8%) |
| 1 | 21 (33.9%) | 28 (46.7%) | 49 (40.2%) |
| 2 | 16 (25.8%) | 13 (21.7%) | 29 (23.8%) |
| 3 | 16 (25.8%) | 16 (26.7%) | 32 (26.2%) |
| Lesion at first registration | |||
| Single | 27 (43.5%) | 30 (50.0%) | 57 (46.7%) |
| Multiple | 35 (56.5%) | 30 (50.0%) | 65 (53.3%) |
| Ocular disease | |||
| No | 55 (88.7%) | 53 (88.3%) | 108 (88.5%) |
| Yes | 7 (11.3%) | 7 (11.7%) | 14 (11.5%) |
| Intraparenchymal tumor after HD-MTX | |||
| Absent | 14 (22.5%) | 12 (20%) | 26 (21.3%) |
| Present | 48 (77.4%) | 48 (80%) | 96 (78.7%) |
| Response to HD-MTX by central review | n = 52 | n = 49 | n = 101 |
| CR/CRu | 14/52 (26.9%) | 9/49 (18.4%) | 23/101 (22.8%) |
| PR | 25/52 (48.1%) | 24/49 (49.0%) | 49/101(48.5%) |
| SD | 7/52 (13.5) | 6/49 (12.2) | 13/101 (12.9) |
| PD | 6/52 (11.5) | 10/49 (20.4) | 16/101 (15.8) |
| MGMT status | n = 60 | n = 55 | n = 115 |
| Hypermethylated | 29/60 (48.3) | 29/55 (52.7) | 58/115 (50.4) |
| Hypomethylated | 31/60 (51.7) | 26/55 (47.3) | 57/115 (49.6) |
| . | Arm A (n = 62) . | Arm B (n = 60) . | Total (n = 122) . |
|---|---|---|---|
| Age, y | |||
| Median | 63 | 62 | 62 |
| Range | 35–70 | 34–70 | 34–70 |
| Sex | |||
| Male | 40 (64.5%) | 35 (58.3%) | 75 (61.5%) |
| Female | 22 (35.5%) | 25 (41.7%) | 47 (38.5%) |
| ECOG PS | |||
| 0 | 9 (14.5%) | 3 (5.0%) | 12 (9.8%) |
| 1 | 21 (33.9%) | 28 (46.7%) | 49 (40.2%) |
| 2 | 16 (25.8%) | 13 (21.7%) | 29 (23.8%) |
| 3 | 16 (25.8%) | 16 (26.7%) | 32 (26.2%) |
| Lesion at first registration | |||
| Single | 27 (43.5%) | 30 (50.0%) | 57 (46.7%) |
| Multiple | 35 (56.5%) | 30 (50.0%) | 65 (53.3%) |
| Ocular disease | |||
| No | 55 (88.7%) | 53 (88.3%) | 108 (88.5%) |
| Yes | 7 (11.3%) | 7 (11.7%) | 14 (11.5%) |
| Intraparenchymal tumor after HD-MTX | |||
| Absent | 14 (22.5%) | 12 (20%) | 26 (21.3%) |
| Present | 48 (77.4%) | 48 (80%) | 96 (78.7%) |
| Response to HD-MTX by central review | n = 52 | n = 49 | n = 101 |
| CR/CRu | 14/52 (26.9%) | 9/49 (18.4%) | 23/101 (22.8%) |
| PR | 25/52 (48.1%) | 24/49 (49.0%) | 49/101(48.5%) |
| SD | 7/52 (13.5) | 6/49 (12.2) | 13/101 (12.9) |
| PD | 6/52 (11.5) | 10/49 (20.4) | 16/101 (15.8) |
| MGMT status | n = 60 | n = 55 | n = 115 |
| Hypermethylated | 29/60 (48.3) | 29/55 (52.7) | 58/115 (50.4) |
| Hypomethylated | 31/60 (51.7) | 26/55 (47.3) | 57/115 (49.6) |
CR, complete response; CRu, complete response unconfirmed; ECOG PS, Eastern Cooperative Oncology Group performance status; HD-MTX, high-dose methotrexate; MGMT, O6-methylguanine-DNA methyltransferase; PD, progressive disease; PR, partial response; SD, stable disease.
According to the central review for response assessment, 14/52 (26.9%: 95% CI: 15.6%–41.0%) patients achieved CR or unconfirmed CR (CRu, defined as minimal residual tumor on MRI17) in arm A with HD-MTX treatment, a higher proportion than in arm B (9/49 [18.4%: 95% CI: 8.8%–32.0%]), whereas PR and SD proportions were similar between the arms (PR: 48.1% [arm A] vs. 49.0% [arm B], SD: 13.5% [arm A] vs. 12.2% [arm B]). In arm B, 10/49 (20.4%) patients showed PD, more than in arm A (6/52 [11.5%]).
Protocol treatment was terminated for one patient in arm A during WBRT due to the patient’s refusal of further treatment. There was no termination of protocol treatment in arm B during RT plus concomitant TMZ. Defined maintenance TMZ was not delivered for 6 patients (Figure 1). Therefore, 54 of 60 (90%) patients started maintenance TMZ treatment. In the maintenance treatment period, protocol treatment was terminated due to adverse events (one developed grade 2 neutropenia at cycle 16, one grade 4 retinal detachment at cycle 8, one grade 3 lung infection at cycle 9, one grade 4 delirium at cycle 5, and one grade 3 anorexia at cycle 1), disease progression (21 patients), patient’s refusal with adverse events (3 patients), death due to unknown cause (1 patient), and early study termination (11 patients). Thirteen patients completed the planned 2-year maintenance treatment. The median number of cycles of TMZ maintenance was 14.5 (interquartile range: 6–20).
Response rates to RT were evaluated by central review (Supplementary Table 1). CR/CRu was achieved in 29/52 (55.8%) in arm A and 27/49 (55.1%) in arm B. Overall response rates (CR/CRu + PR) after RT were 96.2% and 100% in arms A and B, respectively. Response profiles were almost identical between the arms.
Toxicity
During HD-MTX, grade 3 or 4 adverse events occurred in 69 (51.9%) of 133 patients. Lymphopenia was the most frequent grade 3 or 4 adverse event, occurring in 30 (22.6%) patients. Other common grade 3 or 4 adverse events were neutropenia in 12 (9%), leucopenia in 6 (4.5%), increased ALT in 17 (12.8%), hyponatremia in 10 (7.5%), hypopotassemia in 6 (4.5%), and urinary tract infection in 4 (3%). No treatment-related deaths were recorded during this period.
Table 2 shows adverse events that occurred during RT. The most common grade 3 or 4 adverse events were lymphopenia (18 [30%] of 60 patients in arm B and 7 [11.5%] of 61 patients in arm A). Treatment termination due to adverse events during RT was not observed in both arms.
| . | Arm A, N = 61 . | . | . | Arm B, N = 60 . | . | . |
|---|---|---|---|---|---|---|
| Adverse Event . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . |
| Hematological | ||||||
| Leukopenia | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| Anemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Neutropenia | 1 | 0 | 1.6 | 2 | 0 | 3.3 |
| Lymphopenia | 6 | 1 | 11.5 | 16 | 2 | 30.0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-hematological | ||||||
| AST elevation | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| ALT elevation | 0 | 1 | 1.6 | 0 | 1 | 1.7 |
| Hyponatremia | 0 | 0 | 0 | 2 | 0 | 3.3 |
| Hyperkalemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Hypokalemia | 0 | 0 | 0 | 3 | 0 | 5.0 |
| Nausea | 0 | − | 0 | 1 | − | 1.7 |
| Vomiting | 0 | 0 | 0 | 1 | 0 | 1.7 |
| . | Arm A, N = 61 . | . | . | Arm B, N = 60 . | . | . |
|---|---|---|---|---|---|---|
| Adverse Event . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . |
| Hematological | ||||||
| Leukopenia | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| Anemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Neutropenia | 1 | 0 | 1.6 | 2 | 0 | 3.3 |
| Lymphopenia | 6 | 1 | 11.5 | 16 | 2 | 30.0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-hematological | ||||||
| AST elevation | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| ALT elevation | 0 | 1 | 1.6 | 0 | 1 | 1.7 |
| Hyponatremia | 0 | 0 | 0 | 2 | 0 | 3.3 |
| Hyperkalemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Hypokalemia | 0 | 0 | 0 | 3 | 0 | 5.0 |
| Nausea | 0 | − | 0 | 1 | − | 1.7 |
| Vomiting | 0 | 0 | 0 | 1 | 0 | 1.7 |
ALT: alanine aminotransferase; AST, aspartate aminotransferase; TMZ, temozolomide; WBRT, whole brain radiation therapy.
| . | Arm A, N = 61 . | . | . | Arm B, N = 60 . | . | . |
|---|---|---|---|---|---|---|
| Adverse Event . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . |
| Hematological | ||||||
| Leukopenia | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| Anemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Neutropenia | 1 | 0 | 1.6 | 2 | 0 | 3.3 |
| Lymphopenia | 6 | 1 | 11.5 | 16 | 2 | 30.0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-hematological | ||||||
| AST elevation | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| ALT elevation | 0 | 1 | 1.6 | 0 | 1 | 1.7 |
| Hyponatremia | 0 | 0 | 0 | 2 | 0 | 3.3 |
| Hyperkalemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Hypokalemia | 0 | 0 | 0 | 3 | 0 | 5.0 |
| Nausea | 0 | − | 0 | 1 | − | 1.7 |
| Vomiting | 0 | 0 | 0 | 1 | 0 | 1.7 |
| . | Arm A, N = 61 . | . | . | Arm B, N = 60 . | . | . |
|---|---|---|---|---|---|---|
| Adverse Event . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . | Grade 3 . | Grade 4 . | Grade 3 or 4 (%) . |
| Hematological | ||||||
| Leukopenia | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| Anemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Neutropenia | 1 | 0 | 1.6 | 2 | 0 | 3.3 |
| Lymphopenia | 6 | 1 | 11.5 | 16 | 2 | 30.0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-hematological | ||||||
| AST elevation | 1 | 0 | 1.6 | 1 | 0 | 1.7 |
| ALT elevation | 0 | 1 | 1.6 | 0 | 1 | 1.7 |
| Hyponatremia | 0 | 0 | 0 | 2 | 0 | 3.3 |
| Hyperkalemia | 0 | 0 | 0 | 1 | 0 | 1.7 |
| Hypokalemia | 0 | 0 | 0 | 3 | 0 | 5.0 |
| Nausea | 0 | − | 0 | 1 | − | 1.7 |
| Vomiting | 0 | 0 | 0 | 1 | 0 | 1.7 |
ALT: alanine aminotransferase; AST, aspartate aminotransferase; TMZ, temozolomide; WBRT, whole brain radiation therapy.
Regarding adverse events during the maintenance phase of arm B, the most common grade 3 or 4 adverse events were lymphopenia in 24 [44.4%] of 54 patients, fatigue in 5 [9.3%] of 54 patients, leukopenia in 4 [7.4%] of 54 patients, and neutropenia in 2 [3.7%] of 54 patients (Supplementary Table 2). This high incidence of severe lymphopenia during the concomitant phase during RT may reflect this combination treatment of TMZ with WBRT, not local RT as in glioblastoma.26 The high incidence of severe lymphopenia during the maintenance phase may reflect the longer duration of TMZ administration compared to 6 cycles in glioblastoma.27
Efficacy
At the data cut-off date of June 2019, the second interim analysis was conducted after the completion of patient accrual (median follow-up from the second registration, 1.63 years). The 2-year OS was 86.8% (95% CI: 72.5–94.0%) for patients assigned to arm A and 71.4% (95% CI: 56.0–82.2%) for those assigned to arm B (HR, 2.18; 95% CI: 0.95–4.98; one-sided P = .97 by the stratified log-rank test; Supplementary Figure S1A). The predicted probability for showing the superiority of arm B at the final analysis was estimated to be 1.3%.28 These results led to the early termination of this study according to the prespecified stopping criteria on the basis of futility based on the recommendation of the Data and Safety Monitoring Committee. The median PFS was 2.6 (95% CI: 1.8 to not estimated) years in arm A and 1.8 (95% CI: 1.1–2.8) years in arm B (HR 1.54: 95% CI: 0.88–2.70; Supplementary Figure S1B).
An updated analysis was conducted for the 122 patients based on the data as of November 2021 (median follow-up, 3.5 years). The 2-year OS was 86.7% (95% CI: 75.2–93.1%) for patients assigned to arm A and 76.2% (95% CI: 63.1–85.1%) for those assigned to arm B (Figure 2A). Median PFS was 3.5 years for arm A (95% CI: 2.3–4.7 years) and 2.3 years for arm B (95% CI: 1.2–2.8 years; Figure 2B). Survival of the patients with CR/CRu after induction HD-MTX was analyzed separately (Table 3, Supplementary Figure S3). PFS and OS for CR/CRu patients in arms A and B were quite identical.
| Factor . | . | Arm . | Events/No. . | 2-y OS (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|
| Age, y | ≤60 | A | 7/25 | 88.0 (67.3–96.0) | 1 |
| B | 8/25 | 76.0 (54.2–88.4) | 1.315 (0.476–3.632) | ||
| ≥61 | A | 16/37 | 85.8 (69.2–93.8) | 1 | |
| B | 14/35 | 76.4 (58.3–87.5) | 1.010 (0.492–2.074) | ||
| PS at second registration | 0–1 | A | 15/44 | 88.1 (73.7–94.9) | 1 |
| B | 13/44 | 81.8 (66.8–90.4) | 0.901 (0.429–1.896) | ||
| 2–3 | A | 8/18 | 83.3 (56.8–94.3) | 1 | |
| B | 9/16 | 59.6 (30.8–79.6) | 1.838 (0.701–4.817) | ||
| Sex | Male | A | 13/40 | 92.1 (77.5–97.4) | 1 |
| B | 14/35 | 76.4 (58.3–87.5) | 1.393 (0.653–2.970) | ||
| Female | A | 10/22 | 77.3 (53.7–89.8) | 1 | |
| B | 8/25 | 75.8 (53.8–88.3) | 0.750 (0.295–1.908) | ||
| Ocular disease | – | A | 22/55 | 86.9 (74.4–93.5) | 1 |
| B | 20/53 | 72.9 (58.6–83.0) | 1.075 (0.586–1.972) | ||
| + | A | 1/7 | 85.7 (33.4–97.9) | 1 | |
| B | 2/7 | 100.0 (100.0–100.0) | 1.901 (0.172–20.996) | ||
| Lesion at the first registration | Single | A | 11/27 | 88.5 (68.4–96.1) | 1 |
| B | 10/30 | 76.5 (57.0–88.1) | 0.941 (0.398–2.223) | ||
| Multiple | A | 12/35 | 85.3 (68.2–93.6) | 1 | |
| B | 12/30 | 76.2 (56.5–87.9) | 1.334 (0.598–2.976) | ||
| RPA subgroup | 1 | A | 3/8 | 75.0 (31.5–93.1) | 1 |
| B | 1/5 | 80.0 (20.4–96.9) | 0.518 (0.054–4.989) | ||
| 2 | A | 13/38 | 91.7 (76.3–97.2) | 1 | |
| B | 10/33 | 78.2 (59.7–89.0) | 0.927 (0.406–2.117) | ||
| 3 | A | 7/16 | 81.3 (52.5–93.5) | 1 | |
| B | 11/22 | 72.2 (48.2–86.5) | 1.490 (0.574–3.869) | ||
| Response to HD-MTX by central review | PR, SD, PD | A | 15/38 | 89.3 (73.8–95.8) | 1 |
| B | 15/40 | 74.3 (57.5–85.3) | 1.164 (0.568–2.384) | ||
| CR/CRu | A | 4/14 | 76.9 (44.2–91.9) | 1 | |
| B | 2/9 | 77.8 (36.5–93.9) | 0.798 (0.146–4.360) | ||
| MGMT | Hypomethylated | A | 14/31 | 83.3 (64.5–92.7) | 1 |
| B | 9/26 | 72.2 (50.4–85.7) | 0.888 (0.384–2.053) | ||
| Hypermethylated | A | 9/29 | 89.7 (71.3–96.5) | 1 | |
| B | 12/29 | 78.9 (58.8–89.9) | 1.432 (0.601–3.409) |
| Factor . | . | Arm . | Events/No. . | 2-y OS (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|
| Age, y | ≤60 | A | 7/25 | 88.0 (67.3–96.0) | 1 |
| B | 8/25 | 76.0 (54.2–88.4) | 1.315 (0.476–3.632) | ||
| ≥61 | A | 16/37 | 85.8 (69.2–93.8) | 1 | |
| B | 14/35 | 76.4 (58.3–87.5) | 1.010 (0.492–2.074) | ||
| PS at second registration | 0–1 | A | 15/44 | 88.1 (73.7–94.9) | 1 |
| B | 13/44 | 81.8 (66.8–90.4) | 0.901 (0.429–1.896) | ||
| 2–3 | A | 8/18 | 83.3 (56.8–94.3) | 1 | |
| B | 9/16 | 59.6 (30.8–79.6) | 1.838 (0.701–4.817) | ||
| Sex | Male | A | 13/40 | 92.1 (77.5–97.4) | 1 |
| B | 14/35 | 76.4 (58.3–87.5) | 1.393 (0.653–2.970) | ||
| Female | A | 10/22 | 77.3 (53.7–89.8) | 1 | |
| B | 8/25 | 75.8 (53.8–88.3) | 0.750 (0.295–1.908) | ||
| Ocular disease | – | A | 22/55 | 86.9 (74.4–93.5) | 1 |
| B | 20/53 | 72.9 (58.6–83.0) | 1.075 (0.586–1.972) | ||
| + | A | 1/7 | 85.7 (33.4–97.9) | 1 | |
| B | 2/7 | 100.0 (100.0–100.0) | 1.901 (0.172–20.996) | ||
| Lesion at the first registration | Single | A | 11/27 | 88.5 (68.4–96.1) | 1 |
| B | 10/30 | 76.5 (57.0–88.1) | 0.941 (0.398–2.223) | ||
| Multiple | A | 12/35 | 85.3 (68.2–93.6) | 1 | |
| B | 12/30 | 76.2 (56.5–87.9) | 1.334 (0.598–2.976) | ||
| RPA subgroup | 1 | A | 3/8 | 75.0 (31.5–93.1) | 1 |
| B | 1/5 | 80.0 (20.4–96.9) | 0.518 (0.054–4.989) | ||
| 2 | A | 13/38 | 91.7 (76.3–97.2) | 1 | |
| B | 10/33 | 78.2 (59.7–89.0) | 0.927 (0.406–2.117) | ||
| 3 | A | 7/16 | 81.3 (52.5–93.5) | 1 | |
| B | 11/22 | 72.2 (48.2–86.5) | 1.490 (0.574–3.869) | ||
| Response to HD-MTX by central review | PR, SD, PD | A | 15/38 | 89.3 (73.8–95.8) | 1 |
| B | 15/40 | 74.3 (57.5–85.3) | 1.164 (0.568–2.384) | ||
| CR/CRu | A | 4/14 | 76.9 (44.2–91.9) | 1 | |
| B | 2/9 | 77.8 (36.5–93.9) | 0.798 (0.146–4.360) | ||
| MGMT | Hypomethylated | A | 14/31 | 83.3 (64.5–92.7) | 1 |
| B | 9/26 | 72.2 (50.4–85.7) | 0.888 (0.384–2.053) | ||
| Hypermethylated | A | 9/29 | 89.7 (71.3–96.5) | 1 | |
| B | 12/29 | 78.9 (58.8–89.9) | 1.432 (0.601–3.409) |
CI, confidence interval; CR, complete response; CRu, complete response unconfirmed; HD-MTX, high-dose methotrexate; HR, hazard ratio; MGMT, O6-methylguanine-DNA methyltransferase; OS, overall survival; PD, progressive disease; PR, partial response; PS, performance status; RPA, recursive partitioning analysis; SD, stable disease
| Factor . | . | Arm . | Events/No. . | 2-y OS (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|
| Age, y | ≤60 | A | 7/25 | 88.0 (67.3–96.0) | 1 |
| B | 8/25 | 76.0 (54.2–88.4) | 1.315 (0.476–3.632) | ||
| ≥61 | A | 16/37 | 85.8 (69.2–93.8) | 1 | |
| B | 14/35 | 76.4 (58.3–87.5) | 1.010 (0.492–2.074) | ||
| PS at second registration | 0–1 | A | 15/44 | 88.1 (73.7–94.9) | 1 |
| B | 13/44 | 81.8 (66.8–90.4) | 0.901 (0.429–1.896) | ||
| 2–3 | A | 8/18 | 83.3 (56.8–94.3) | 1 | |
| B | 9/16 | 59.6 (30.8–79.6) | 1.838 (0.701–4.817) | ||
| Sex | Male | A | 13/40 | 92.1 (77.5–97.4) | 1 |
| B | 14/35 | 76.4 (58.3–87.5) | 1.393 (0.653–2.970) | ||
| Female | A | 10/22 | 77.3 (53.7–89.8) | 1 | |
| B | 8/25 | 75.8 (53.8–88.3) | 0.750 (0.295–1.908) | ||
| Ocular disease | – | A | 22/55 | 86.9 (74.4–93.5) | 1 |
| B | 20/53 | 72.9 (58.6–83.0) | 1.075 (0.586–1.972) | ||
| + | A | 1/7 | 85.7 (33.4–97.9) | 1 | |
| B | 2/7 | 100.0 (100.0–100.0) | 1.901 (0.172–20.996) | ||
| Lesion at the first registration | Single | A | 11/27 | 88.5 (68.4–96.1) | 1 |
| B | 10/30 | 76.5 (57.0–88.1) | 0.941 (0.398–2.223) | ||
| Multiple | A | 12/35 | 85.3 (68.2–93.6) | 1 | |
| B | 12/30 | 76.2 (56.5–87.9) | 1.334 (0.598–2.976) | ||
| RPA subgroup | 1 | A | 3/8 | 75.0 (31.5–93.1) | 1 |
| B | 1/5 | 80.0 (20.4–96.9) | 0.518 (0.054–4.989) | ||
| 2 | A | 13/38 | 91.7 (76.3–97.2) | 1 | |
| B | 10/33 | 78.2 (59.7–89.0) | 0.927 (0.406–2.117) | ||
| 3 | A | 7/16 | 81.3 (52.5–93.5) | 1 | |
| B | 11/22 | 72.2 (48.2–86.5) | 1.490 (0.574–3.869) | ||
| Response to HD-MTX by central review | PR, SD, PD | A | 15/38 | 89.3 (73.8–95.8) | 1 |
| B | 15/40 | 74.3 (57.5–85.3) | 1.164 (0.568–2.384) | ||
| CR/CRu | A | 4/14 | 76.9 (44.2–91.9) | 1 | |
| B | 2/9 | 77.8 (36.5–93.9) | 0.798 (0.146–4.360) | ||
| MGMT | Hypomethylated | A | 14/31 | 83.3 (64.5–92.7) | 1 |
| B | 9/26 | 72.2 (50.4–85.7) | 0.888 (0.384–2.053) | ||
| Hypermethylated | A | 9/29 | 89.7 (71.3–96.5) | 1 | |
| B | 12/29 | 78.9 (58.8–89.9) | 1.432 (0.601–3.409) |
| Factor . | . | Arm . | Events/No. . | 2-y OS (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|
| Age, y | ≤60 | A | 7/25 | 88.0 (67.3–96.0) | 1 |
| B | 8/25 | 76.0 (54.2–88.4) | 1.315 (0.476–3.632) | ||
| ≥61 | A | 16/37 | 85.8 (69.2–93.8) | 1 | |
| B | 14/35 | 76.4 (58.3–87.5) | 1.010 (0.492–2.074) | ||
| PS at second registration | 0–1 | A | 15/44 | 88.1 (73.7–94.9) | 1 |
| B | 13/44 | 81.8 (66.8–90.4) | 0.901 (0.429–1.896) | ||
| 2–3 | A | 8/18 | 83.3 (56.8–94.3) | 1 | |
| B | 9/16 | 59.6 (30.8–79.6) | 1.838 (0.701–4.817) | ||
| Sex | Male | A | 13/40 | 92.1 (77.5–97.4) | 1 |
| B | 14/35 | 76.4 (58.3–87.5) | 1.393 (0.653–2.970) | ||
| Female | A | 10/22 | 77.3 (53.7–89.8) | 1 | |
| B | 8/25 | 75.8 (53.8–88.3) | 0.750 (0.295–1.908) | ||
| Ocular disease | – | A | 22/55 | 86.9 (74.4–93.5) | 1 |
| B | 20/53 | 72.9 (58.6–83.0) | 1.075 (0.586–1.972) | ||
| + | A | 1/7 | 85.7 (33.4–97.9) | 1 | |
| B | 2/7 | 100.0 (100.0–100.0) | 1.901 (0.172–20.996) | ||
| Lesion at the first registration | Single | A | 11/27 | 88.5 (68.4–96.1) | 1 |
| B | 10/30 | 76.5 (57.0–88.1) | 0.941 (0.398–2.223) | ||
| Multiple | A | 12/35 | 85.3 (68.2–93.6) | 1 | |
| B | 12/30 | 76.2 (56.5–87.9) | 1.334 (0.598–2.976) | ||
| RPA subgroup | 1 | A | 3/8 | 75.0 (31.5–93.1) | 1 |
| B | 1/5 | 80.0 (20.4–96.9) | 0.518 (0.054–4.989) | ||
| 2 | A | 13/38 | 91.7 (76.3–97.2) | 1 | |
| B | 10/33 | 78.2 (59.7–89.0) | 0.927 (0.406–2.117) | ||
| 3 | A | 7/16 | 81.3 (52.5–93.5) | 1 | |
| B | 11/22 | 72.2 (48.2–86.5) | 1.490 (0.574–3.869) | ||
| Response to HD-MTX by central review | PR, SD, PD | A | 15/38 | 89.3 (73.8–95.8) | 1 |
| B | 15/40 | 74.3 (57.5–85.3) | 1.164 (0.568–2.384) | ||
| CR/CRu | A | 4/14 | 76.9 (44.2–91.9) | 1 | |
| B | 2/9 | 77.8 (36.5–93.9) | 0.798 (0.146–4.360) | ||
| MGMT | Hypomethylated | A | 14/31 | 83.3 (64.5–92.7) | 1 |
| B | 9/26 | 72.2 (50.4–85.7) | 0.888 (0.384–2.053) | ||
| Hypermethylated | A | 9/29 | 89.7 (71.3–96.5) | 1 | |
| B | 12/29 | 78.9 (58.8–89.9) | 1.432 (0.601–3.409) |
CI, confidence interval; CR, complete response; CRu, complete response unconfirmed; HD-MTX, high-dose methotrexate; HR, hazard ratio; MGMT, O6-methylguanine-DNA methyltransferase; OS, overall survival; PD, progressive disease; PR, partial response; PS, performance status; RPA, recursive partitioning analysis; SD, stable disease
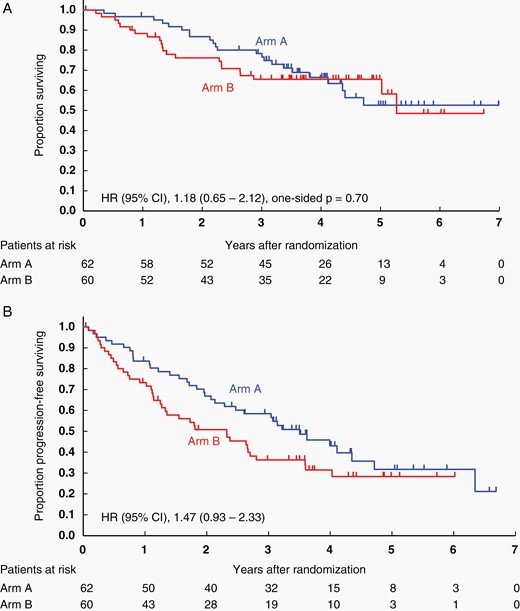
Kaplan–Meier curves for the intention-to-treat population. (A) OS and (B) PFS at the updated analysis (data cutoff date, November 25, 2021). OS, overall survival; PFS, progression-free survival.
Furthermore, the methylation status of the promoter region of MGMT was compared retrospectively in arms A and B. Data on MGMT promoter methylation status determined by pyrosequencing was available in 115 patients (86% of the first registered patients). Of the 115 evaluated tumors, 107 (93.0%) harbored > 10% methylation of the MGMT promoter, and the median value was 19.5%. The methylation level was < 10% in all normal controls. The proportion of hypermethylated tumors (MGMT promoter methylation > 19.5%) was similar between the arms (Table 1). Patients with MGMT-hypermethylated tumors did not show superior OS to patients with MGMT-hypomethylated tumors (Figure 3A). MGMT promoter methylation was not prognostic for OS and PFS (PFS shown in Supplementary Figure S2A). No survival benefit (OS and PFS) was observed for concomitant and maintenance TMZ in either of the cohorts with MGMT-hypermethylated tumors (HR, 1.43; 95% CI: 0.60–3.41; Figure 3B, PFS shown in Supplementary Figure S2B) or with MGMT-hypomethylated tumors (HR, 0.89; 95% CI: 0.38–2.05; Figure 3C, PFS shown in Supplementary Figure S2C), although the sample size was too small to draw any definite conclusions. No association between MGMT promoter methylation status and OS or PFS was observed when analyzed with continuous or other cutoff values for MGMT methylation (data not shown).
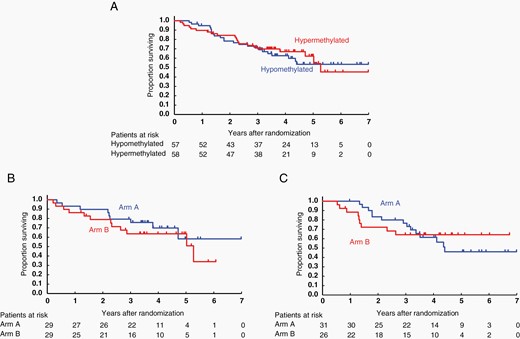
OS with respect to MGMT promoter methylation. (A) Kaplan–Meier curves of MGMT promoter hypermethylated versus hypomethylated tumors in the whole cohort suggesting that MGMT promoter methylation is not a prognostic factor. (B) Kaplan–Meier curves of patients with MGMT promotor hypermethylated tumors. There is no survival advantage or disadvantage in either arm A or arm B, suggesting that MGMT promoter methylation is not predictive for TMZ treatment. (C) Kaplan–Meier curves of patients with MGMT promoter hypomethylated tumors. MGMT, O6-methylguanine-DNA methyltransferase.
Subgroup analyses and comparisons of OS between the treatment arms were performed by sex (male vs. female), age (≤ 60 vs. ≥ 61 years), residual enhanced tumor after HD-MTX (absent vs. present), and the MSKCC RPA class (class 1 vs. 2 vs. 3).29 No subgroups that would benefit from concomitant and maintenance TMZ (arm B) were identified (Table 3).
Table 3 also shows OS for CR/CRu patients, which was not significantly different. Kaplan–Meier curves also showed no difference in survival of CR/CRu patients (Supplementary Figure S3).
Discussion
In this randomized, phase III study, the addition of concomitant and maintenance TMZ to standard treatment of HD-MTX and WBRT did not improve OS and PFS in patients with newly diagnosed PCNSL. The study results were released early without long-term follow-up based on the recommendation of the JCOG Data and Safety Monitoring Committee as a consequence of the interim analysis. Given the lack of clinical benefit in the TMZ treatment arm, TMZ was discontinued in all patients who remained on protocol-mandated therapy. This study is the first randomized trial of WBRT with/without radiation boost together with concomitant and maintenance TMZ after HD-MTX.
The negative results in this trial were unexpected, since a previous report from Glass et al. demonstrated that induction chemotherapy with HD-MTX, rituximab, and TMZ followed by WBRT and 10 months of post-irradiation maintenance TMZ for PCNSL in the RTOG-0227, phase II study, prolonged 2-year PFS and OS compared with historical controls from the RTOG-9310 trial.7,11 Since TMZ has been used in clinical trials30,31 and is thought to be an important drug for therapeutic development in PCNSL, the finding that TMZ was ineffective in the consolidation and maintenance therapy of PCNSL in the present study is both disappointing and intriguing.
Several explanations can be proposed for these unexpected results. First, the most probable cause of the discrepancy in the survival outcomes was likely due to TMZ treatment-related adverse events, such as severe lymphopenia. Grade 3 or 4 lymphopenia was seen in 30% of patients during WBRT + concomitant TMZ and in 44.4% of patients during maintenance TMZ. Lymphopenia might be a biomarker of impaired host immunity in cancer patients, since it was shown that a decreased low absolute lymphocyte count is associated with a poor prognosis in patients with lymphoma and other cancers.32,33 Although the underlying mechanisms and reasons for the association of lymphopenia with a worse outcome in terms of OS and PFS in PCNSL patients are not well known, lymphopenia might be associated with insufficient production of cytokines, which stimulate chemokine production, leading to reduced anti-tumor immunity.34
The second possible cause might be the unbalanced CR rate between the arms after induction HD-MTX (the higher CR rate of 26.9% in arm A than that of 18.4% in arm B). It has been shown that patients with CR at the end of induction HD-MTX (± ifosfamide) had a better OS than those who did not achieve CR in a randomized, phase III trial.4 However, this is a weak hypothesis, because there were no remarkable additive effects of TMZ proven in the subgroup of patients with CR after HD-MTX.
MGMT gene promoter methylation is a robust predictor of response and survival in glioblastoma patients treated with TMZ. The previous systemic diffuse large B-cell lymphoma studies reported that MGMT promoter methylation is a marker of good prognosis through increased sensitivity to alkylating agents conferred by MGMT inactivation.35 Recent retrospective studies demonstrated that MGMT promoter methylation status correlates with the response of PCNSL to TMZ.36 In the present study, MGMT promoter methylation status was not prognostic for OS in newly diagnosed PCNSL. In addition, there were no benefits of TMZ treatment in the subgroup analysis regarding the methylation status of the MGMT promoter, suggesting that MGMT methylation is not predictive of TMZ efficacy in newly diagnosed PCNSL, which contradicts the findings for glioblastoma. There could potentially be factors other than MGMT promoter methylation that affect TMZ sensitivity in PCNSL. Leshchenko et al. reported that TMZ resistance of diffuse large B-cell lymphoma cell lines was not dependent on MGMT promoter methylation status but was associated with hypermethylation and downregulation of a set of genes, including SMAD1, IGLL1, and TOP2B. In their study, the use of the demethylating agent decitabine reverted the repression of these genes, resulting in synergistic effects with TMZ.37 The use of decitabine in combination with TMZ could be considered in future studies.
In conclusion, in newly diagnosed PCNSL, the addition of concomitant and maintenance TMZ to standard treatment of HD-MTX and WBRT did not improve survival. TMZ has no role as concomitant therapy with WBRT and maintenance use after WBRT in this disease. Additional follow-up will be performed to evaluate long-term OS, neurocognition, QoL, and secondary malignancy.
Acknowledgments
The authors thank Dr. Koichi Ichimura for his valuable guidance, comments on MGMT analysis and interpretation of the results and Saki Shimizu for her excellent technical assistance. Partial results of this trial were previously presented at the 2020 American Society of Clinical Oncology annual meeting. The authors also thank the patients and their families and the investigators of the JCOG Brain Tumor Study Group for participating in this study, and the members of the JCOG Data Center and JCOG Operations Office for their support in data management (Ms. Shoko Todo, Ms. Chikako Aibara) and study coordination (Dr. Keisuke Kanato, Ms. Mayumi Hayakawa).
In Japan, TMZ has already been granted marketing approval for GBM, but not for PCNSL, based on the Pharmaceutical Affairs Law. This study was conducted under the Advanced Medical Care System of MHLW, which enabled us to conduct a clinical trial with reimbursement of routine medical care cost by the National Health Insurance System even if the trial included off-label use of a drug. The authors are grateful to Merck Sharp & Dohme Corp./MSD K.K. for provision of temozolomide. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp./MSD K.K.
Funding
This study was supported by the National Cancer Center Research and Development Funds (23-A-20, 26-A-4, 29-A-3, 2020-J-3); Grant-in-Aid for Clinical Cancer Research (H26-088) from the Ministry of Health, Labour and Welfare of Japan (MHLW); AMED under Grant Numbers JP16ck0106091 and JP19ck0106341; JSPS KAKENHI Grant Number 20K09375; and a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp./MSD K.K.
Conflict of Interest Statement
K.M. received research funding from Ono, Chugai, MSD, Eisai, Teijin, Medical U and A, AbbVie, Otsuka, Daiichi-Sankyo, and Nihon Medi-Physics; honoraria from Ono, Chugai, and Eisai. R.N. received research funding from Chugai, Eisai, AbbVie, Toray, and MSD; consulting fee with Novocure; honoraria from Chugai, MSD, Nippon Kayaku, Ono, Eisai, and Daiichi-Sankyo. Y.N. received research funding from Ono, Chugai, Eisai, AbbVie, Daiichi-Sankyo, Taiho, Nihon Medi-Physics, and Sumitomo; honoraria from Ono, Chugai, Novocure, UCB, Nippon-Kayaku, and Eisai. J.M. received honoraria from Chugai and Taiho. NSa received honoraria from Ono and Daiichi-Sankyo. M.Ki. received research funding from Chugai, Eisai, Otsuka, Daiichi-Sankyo, Mitsubishi-Tanabe, Stryker, and Medtronic; honoraria from Ono, Chugai, Eisai, Daiichi-Sankyo, BrainLab, Integra Japan, and Novocure. MNag received research funding from AbbVie, Eisai, MSD, Chugai, Daiichi-Sankyo, Pfizer, Kyowa-Kirin, Nippon Kayaku, Tsumura, Shionogi, Otsuka, Astellas, Teijin, Bayer, and Ono; consulting fees from AbbVie, Daiichi-Sankyo, RIEMSER, and Ono; honoraria from Chugai, Novocure, Daiichi-Sankyo, Ono, Nippon Kayaku, Sumitomo-Dainippon, Eisai, and Kyowa-Kirin. Y.A. received funding from Philips, Otsuka, Chugai, Nihon Medi-Physics, Daiichi-Sankyo, Stryker, Eisai, Japan Blood Products Organization, Ono, Taiho, Sumitomo-Dainippon, Astellas, and Sanofi; honoraria from Nippon Kayaku, Novocure, UCB Japan, Ono, BrainLab, Merck, Chugai, Eisai, Daiichi-Sankyo, Carl Zeiss, and Nihon Medi-Physics. H.S. received research funding from Ono and Eisai. AA received research funding from Chugai, Takeda, Eisai, MSD, Tanabe-Mitsubishi, Daiichi-Sankyo, Pfizer, Otsuka, and AstraZeneca. T.S. received research funding from Eisai, Otsuka, Kowa, Asahikasei, Daiichi-Sankyo, and Nihon Medi-Physics. M.Nat. received honoraria from Chugai, Daiichi-Sankyo, Eisai, and Novocure. H.F. received honoraria from Chugai and m3.com. The other authors declare no conflict of interest.
Authorship
Conception and design: K.M., R.N., T.K., Y.N., J.M., M.S., H.K., and H.F. Collection and assembly of data: K.M., R.N., Y.N., M.Ki., M.Nag., Y.A., K.Y., I.S., N.Sh., K.A., T.T., H.S., A.A., T.S., and Y.M. Data analysis and interpretation: K.M., R.N., Y.N., J.M., M.S., H.K., and H.F. Central histological diagnosis: A.S., S.N., M.Ko., and J.T. Central MRI diagnosis: K.T., M.G., K.A., M.Nat., and F.Y. Central molecular diagnosis: N.Sa. and M.Nag. Manuscript writing: All authors. No unpublished papers cited.




