-
PDF
- Split View
-
Views
-
Cite
Cite
Angela C Hirbe, Xiaochun Zhang, Sonika Dahiya, Abigail Godec, John Chrisinger, Yu Tao, Jingqin Luo, David H Gutmann, β–III-spectrin immunohistochemistry as a potential diagnostic tool with high sensitivity for malignant peripheral nerve sheath tumors, Neuro-Oncology, Volume 20, Issue 6, June 2018, Pages 858–860, https://doi.org/10.1093/neuonc/noy038
Close - Share Icon Share
The most common malignancy affecting adults with neurofibromatosis type 1 (NF1) is malignant peripheral nerve sheath tumor (MPNST), an aggressive sarcoma that typically develops from benign plexiform neurofibromas.1,2 Establishing an accurate diagnosis is one of the greatest challenges in managing NF1-MPNST. MPNSTs can be difficult to distinguish from benign counterparts (plexiform neurofibromas), precursor lesions (low-grade [LG] MPNSTs and atypical neurofibromas), and other sarcomas.3 Recent studies have identified loss of H3K27 trimethylation in 34%–54% of MPNSTs and only 7%–14% of other sarcomas.4,5 While this is a promising biomarker, alone it lacks discriminatory power, since loss of H3K27 trimethylation is observed in only 50% of MPNSTs.
To discover additional MPNST molecular changes, we leveraged whole exome sequencing to identify β–III-spectrin as a frequently mutated gene in MPNSTs. The detection of β–III-spectrin expression in >90% of MPNSTs, but not in normal peripheral nerve or benign neurofibromas,6 raised the possibility that β–III-spectrin staining might serve as a diagnostic biomarker for MPNSTs.
To explore this possibility, β–III-spectrin immunostaining was performed on formalin-fixed paraffin-embedded sections of atypical neurofibromas (n = 9), LG-MPNSTs (n = 7), high-grade (HG) MPNSTs (n = 13), and other soft tissue sarcomas, including leiomyosarcomas (n = 13), undifferentiated pleiomorphic sarcomas (n = 13), synovial sarcomas (n = 10), and fibrosarcomas (n = 5). Cases were identified in the anatomic pathology archives at Washington University (2011–2017). Immunohistochemistry (IHC) staining was performed using rabbit polyclonal β–III-spectrin antibody (H-70; Santa Cruz Biotechnology). Images were acquired at 400x magnification using an Olympus BX51 camera. Samples were called positive if >90% of cells demonstrated strong staining and negative if staining was weak/moderate in fewer than 50% of the cells.
Strong β–III-spectrin immunoreactivity was detected in all HG-MPNSTs, 57% of LG-MPNSTs, 0 atypical neurofibromas, and only 5% of other sarcoma subtypes (Fig. 1A). All cases were reexamined by 2 pathologists (S.D. and J.C.) to confirm the diagnosis rendered clinically. Interestingly, of the 4 LG-MPNSTs that were scored as positive, 2 were thought to represent HG-MPNST by one of the pathologists based on a mitotic index of 10/10 high power field (HPF) and 13/10 HPF; both recurred as HG-MPNST (Fig. 1B), suggesting that β–III-spectrin immunoreactivity may be predictive of transformation. While the remaining LG-MPNSTs and atypical neurofibromas fell into the “negative” category, they exhibited weak staining in 25%–50% of cells. However, for other sarcomas, 95% (39/41) of tumors were called negative (30/41 were completely negative and 9/41 showed only weak to moderate staining in <50% of the cells). Sensitivity and specificity were calculated for β–III-spectrin IHC positivity as a diagnostic test for HG-MPNST versus LG-MPNSTs, atypical neurofibromas, and other sarcomas. A linear logistic regression model was used to generate the receiver operating characteristics curves, and the overall diagnostic ability of β–III-spectrin IHC was gauged by the area under the curve. In this regard, there was a high sensitivity in distinguishing HG-MPNSTs from LG-MPNSTs, atypical neurofibromas, and other sarcomas (Fig. 1C).
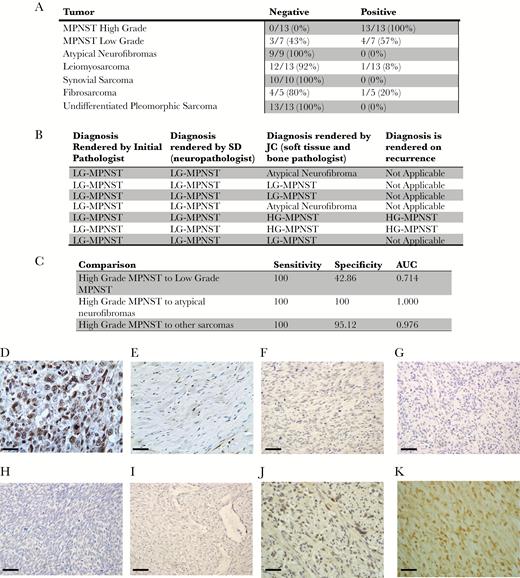
β–III-spectrin immunostaining in MPNSTs and other sarcomas. (A) Positive β–III-spectrin immunoreactivity was detected in 100% (13/13) of HG-MPNSTs, 25% (4/16 tumors) of LG-MPNSTs or atypical neurofibromas, and 5% (2/41 tumors) of the other sarcomas. (B) Two of 7 LG-MPNSTs were called HG-MPNST by one of the reviewing pathologists based on a mitotic count of >10/10 HPF. Both of these cases displayed β–III-spectrin immunoreactivity and ultimately recurred as HG-MPNSTs. (C) The sensitivity and specificity of β–III-spectrin immunostaining for high-grade MPNSTs is shown. (D–K) Positive β–III-spectrin immunostaining in a representative (D) HG-MPNST, which was not observed in (E) normal peripheral nerve, (F) leiomyosarcomas, (G) undifferentiated pleiomorphic sarcomas, (H) synovial sarcomas, or (I) fibrosarcomas. More challenging cases are depicted in (J) “negative” LG-MPNST with weak to moderate staining in <50% of cells and (K) “positive” LG-MPNST with moderate to strong staining in 90% of cells. Scale bar, 40 μm. Atypical neurofibroma: tumor with cellular atypia alone. LG-MPNST: tumor with cellular atypia, a low mitotic index (<10/10 HPF), and sometimes increased cellularity. HG-MPNST: tumor with high cellularity, nuclear atypia, and brisk mitotic activity (≥10/10 HPF).
In summary, all HG-MPNSTs exhibited strong β–III-spectrin immunoreactivity in >90% of cells. This degree of staining was also observed in 57% of LG-MPNSTs, but not in atypical neurofibromas or other sarcomas. Taken together, these data suggest that in combination with other genetic and immunochemical markers, positive β–III-spectrin immunostaining could become an ancillary diagnostic test in the evaluation of soft-tissue sarcomas. To determine its use as a predictive marker for transformation to HG-MPNST, work going forward is currently focused at developing a clinic-grade monoclonal antibody, validating these findings using samples from other institutions, defining correlations with other markers (eg, loss of H3K27 trimethylation), and correlating β–III-spectrin immunostaining with clinical outcome. This study was performed under active human studies protocols approved by the institutional review board.
Funding
This work is funded by a SARC (Sarcoma Alliance for Research through Collaboration) career development award and the Doris Duke Charitable Foundation. A.C.H. is funded by a SARC career development award and a Francis Collins Award from the Neurofibromatosis Therapeutic Acceleration Program. This work was also partially supported by funding from Schnuck Markets, Inc (to D.H.G.).
Conflict of interest statement. None.




