-
PDF
- Split View
-
Views
-
Cite
Cite
Morgan L Prust, Kourosh Jafari-Khouzani, Jayashree Kalpathy-Cramer, Pavlina Polaskova, Tracy T Batchelor, Elizabeth R Gerstner, Jorg Dietrich, Standard chemoradiation in combination with VEGF targeted therapy for glioblastoma results in progressive gray and white matter volume loss, Neuro-Oncology, Volume 20, Issue 2, February 2018, Pages 289–291, https://doi.org/10.1093/neuonc/nox217
Close - Share Icon Share
Chemotherapy and radiation have known neurotoxic effects, including cognitive decline.1–3 Standard chemoradiation for glioblastoma is associated with progressive brain atrophy.4 Potentially additive or synergistic effects of radiation, chemotherapy, or anti-angiogenic therapy are less well understood. Investigation into the pattern and time-course of neurotoxicity is needed to guide future neuroprotective strategies. We present longitudinal neuroimaging data from glioblastoma patients treated with chemoradiation and cediranib, a vascular endothelial growth factor (VEGF) inhibitor.
Data were obtained through an institutional review board–approved protocol at our institution (NCT00756106), in which patients received 6 weeks of focal fractionated radiation and daily temozolomide. One month after chemoradiation, temozolomide resumed (150–200 mg/m2) for 6 months. Cediranib (30 mg daily) was started at treatment onset and continued until disease progression or toxicity.
MRI scans (3.0T), including 1 mm3 multi-echo magnetization-prepared rapid acquisition with gradient echo and fluid attenuated inversion recovery (FLAIR), were obtained weekly during chemoradiation, then monthly. Images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) from radiographically tumor-free contralateral hemispheres using Matlab according to segmentation methods already described.4 Patients with bi-hemispheric disease were excluded.
GM, WM, and CSF volumes and percent change from baseline were plotted over time and assessed with linear mixed effects models, controlling for age, sex, and tumor FLAIR volume.
Evaluated were 34/40 subjects (6 excluded for bilateral disease; mean age, 55 y, range 22–74; 12 female, 22 male; mean KPS, 93, range 70–100; mean follow-up time, 260 days, range 28–777).
Subjects displayed loss of absolute GM volume relative to baseline imaging (F = −3.28, P = 0.001; Fig. 1A) and mean percent GM volume loss of 5.1% (F = −4.10, P ≤ 0.001; Fig. 1B). There was significant absolute WM volume loss (F = −3.54, P ≤ 0.001; Fig. 1A), with average percent WM loss of 3.2% (F = 3.57, P ≤ 0.001; Fig. 1B). Absolute CSF volumes increased (F = 18.47, P < 0.001; Fig. 1A), with mean percent volume increase of 16.4% (F = 19.47, P ≤ 0.001; Fig. 1B). These effects were independent of age, sex, or tumor volume.
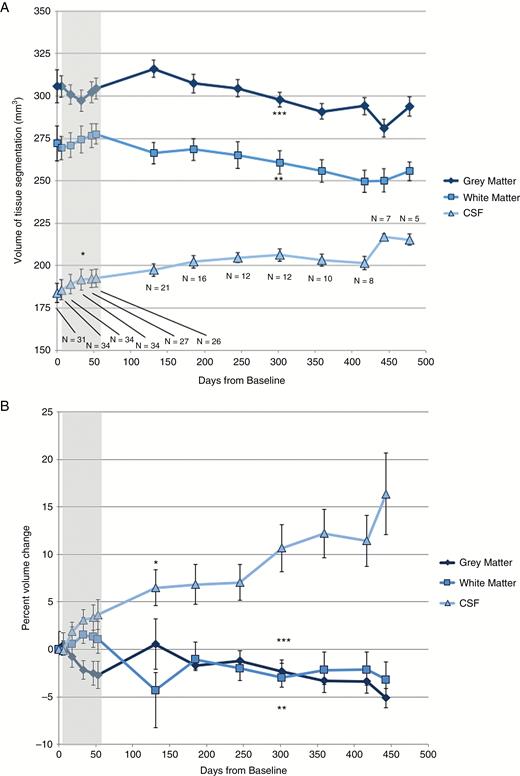
(A) Absolute GM, WM, and CSF volumes and (B) percent volume change from baseline over days since treatment onset. Sample sizes are indicated at each time point. Gray region indicates time points during chemoradiation. Points at which the linear mixed effects model becomes significant are indicated by *(CSF), **(WM), and ***(GM), with all subsequent time points on each curve also meeting statistical significance.
We present evidence of progressive brain atrophy in glioblastoma patients receiving standard chemoradiation combined with cediranib. Atrophy was evident in GM and WM loss and expansion of CSF space. While treatment-associated GM loss and ventriculomegaly have been shown in glioblastoma,4 the present study’s larger sample size and extended follow-up time allowed us to further characterize development of delayed WM loss. Our findings expand on prior work documenting progressive cerebral atrophy over the course of standard chemoradiation for glioblastoma, and suggest that volume loss continues beyond one year after treatment onset, with no evidence of reversibility after cessation of radiation and chemotherapy.
An important limitation in comparison of the present findings to prior work is the exposure of patients in the present cohort to cediranib. This novel anti-angiogenic agent has proven to improve survival in a subset of glioblastoma patients,5 presumably through alteration of tumor perfusion dynamics, though its potential for neurotoxicity is unknown, and it is unclear if it alters the rate or degree of cerebral atrophy in glioblastoma patients. As such, generalization of the current findings to patients receiving standard glioblastoma therapy should be interpreted with caution.
The mechanisms of treatment-associated atrophy remain incompletely understood, though they appear to involve progenitor cell damage, neurovascular injury, and impaired neurogenesis.6,7 Longitudinal imaging studies with serial neurocognitive testing are needed to correlate cerebral atrophy with neurocognitive performance and to assess whether early radiographic changes predict long-term atrophy and functional impairment. While chemoradiation remains central to glioma management, its prolonged neurotoxic effects underscore the need for neuroprotective strategies to optimize long-term outcomes, especially in patients with expected prolonged survival.
Funding
This work was conducted with support from the National Cancer Institute and NIH, R01CA129371 and K24CA125440A (T.T.B.), the American Brain Foundation (J.D.), the American Cancer Society (J.D.), internal funds from the Department of Radiology at the Massachusetts General Hospital (E.R.G., K.J-K, J.K-C, and P.P.), and philanthropic support from the Amy Gallagher Foundation (to J.D.).
Conflict of interest statement. M.L.P., K.J-K., J.K-C., P.P., E.R.G.: no relevant disclosures. T.T.B.: AstraZeneca, Millennium, Advance Medical, Agenus, Champions Biotechnology, Kirin Pharmaceuticals, Merck & Co., Inc., Novartis, Proximagen, and Roche, and Pfizer. J.D.: Monteris Pharmaceuticals, Amy Gallagher Foundation.




