-
PDF
- Split View
-
Views
-
Cite
Cite
Timothy F Cloughesy, Jan Drappatz, John de Groot, Michael D Prados, David A Reardon, David Schiff, Marc Chamberlain, Tom Mikkelsen, Annick Desjardins, Jerry Ping, Jaymes Holland, Ron Weitzman, Patrick Y Wen, Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients with prior antiangiogenic therapy, Neuro-Oncology, Volume 20, Issue 2, February 2018, Pages 259–267, https://doi.org/10.1093/neuonc/nox151
Close - Share Icon Share
Abstract
Cabozantinib is a potent, multitarget inhibitor of MET and vascular endothelial growth factor receptor 2 (VEGFR2). This open-label, phase II trial evaluated cabozantinib in patients with recurrent or progressive glioblastoma (GBM).
Patients were initially enrolled to a starting cabozantinib dose of 140 mg/day, but the starting dose was amended to 100 mg/day because of safety concerns. Treatment continued until disease progression or unacceptable toxicity. The primary endpoint was objective response rate, assessed by an independent radiology facility using modified Response Assessment in Neuro-Oncology criteria. Additional endpoints included duration of response, 6-month and median progression-free survival, overall survival, glucocorticoid use, and safety.
Among 222 patients enrolled, 70 had received prior antiangiogenic therapy. Herein, we report results in this subset of 70 patients. The objective response rate was 4.3%, and the median duration of response was 4.2 months. The proportion of patients alive and progression free at 6 months was 8.5%. Median progression-free survival was 2.3 months, and median overall survival was 4.6 months. The most common adverse events reported in all patients, regardless of dose group, included fatigue (74.3%), diarrhea (47.1%), increased alanine aminotransferase (37.1%), headache (35.7%), hypertension (35.7%), and nausea (35.7%); overall, 34 (48.6%) patients experienced adverse events that resulted in dose reductions.
Cabozantinib treatment appeared to have modest clinical activity with a 4.3% response rate in patients who had received prior antiangiogenic therapy for GBM.
NCT00704288 (https://www.clinicaltrials.gov/ct2/show/NCT00704288)
GBM is a common CNS tumor with limited treatment options. Antiangiogenic therapy with bevacizumab, a VEGF pathway inhibitor, is a standard of care for patients with recurrent disease, and therapies are needed for patients who have progressed on this treatment. Cabozantinib is an orally bioavailable inhibitor of tyrosine kinases including VEGFR2, MET, and AXL. The MET pathway has been implicated in resistance to bevacizumab therapy and the pathogenesis of GBM. The current study reports the results of cabozantinib treatment in 70 patients who received prior antiangiogenic therapy. Cabozantinib treatment showed only modest clinical activity in this patient population.
Glioblastoma (GBM) is a fatal disease that constitutes 45.6% of brain and CNS malignancies; is more common in adults, especially males; and peaks between 75 and 84 years of age.1 Despite current first-line treatment options, disease recurrence is high, with approximately one-third of patients surviving for 1 year, and <5% remaining alive at 5 years.1 Current treatment options for recurrent GBM are limited and may include surgery, reirradiation, chemotherapy, tumor treating fields, and bevacizumab.2
GBM tumors are highly vascularized, and therapies that inhibit angiogenic signals, such as vascular endothelial growth factor (VEGF), have been shown to suppress tumor growth in vivo.3 Single-agent bevacizumab is approved in the United States for treatment of recurrent GBM based on durable objective response rates (ORRs).4 Phase II trials that evaluated bevacizumab in patients with recurrent GBM demonstrated median progression-free survival (PFS) of approximately 4 months and median overall survival (OS) in the range of 8 to 9 months.5,6 While bevacizumab is a standard of care for recurrent GBM, the phase II Dutch BELOB trial reported 9-month OS rates of 38% for bevacizumab, 43% for lomustine, 59% for bevacizumab/lomustine 90 mg/m2, and 88% for bevacizumab/lomustine 110 mg/m2 in patients with a first recurrence, suggesting that single-agent bevacizumab is not superior to lomustine and that the combination warrants further investigation.7 However, a phase III trial of bevacizumab plus lomustine compared with lomustine alone in patients with GBM at first recurrence did not show improved OS for the combination compared with lomustine alone: median OS was 9.1 months versus 8.6 months, respectively.8
Although bevacizumab-based therapy is commonly used for treatment of recurrent GBM, prognosis remains poor, and patients either do not respond or develop treatment-acquired resistance.9 Patients who experience disease progression while receiving bevacizumab rarely respond to further salvage therapy.9 Currently, there is no standard treatment for patients following progression on bevacizumab, and other cancers post bevacizumab progression appear resistant to selective targeting of the VEGF pathway. Therapies that target multiple pathways involved in GBM pathogenesis are needed.10–13 In vivo and in vitro studies have shown that the MET proto-oncogene/hepatocyte growth factor (MET/HGF) pathways are important mediators in the development of GBM.14–17 In particular, high levels of MET are observed in recurrent GBM, and MET overexpression is associated with poor response to treatment and shorter PFS and median OS.16,18 Preclinical data suggest that MET overexpression may be responsible for the development of bevacizumab resistance and that targeting MET may offer a mechanism to prevent or overcome resistance.17,19 The receptor tyrosine kinase AXL may also be involved in GBM pathogenesis based on preclinical studies and has been associated with a poor prognosis in patients with GBM.20–23
Cabozantinib is a multitarget tyrosine kinase inhibitor with potent activity against MET and VEGF receptor 2 (VEGFR2), as well as other kinases that have been implicated in tumor pathobiology (AXL, KIT, RET, TIE2, and FLT3).24 In preclinical studies, cabozantinib treatment resulted in dose-dependent inhibition of tumor growth in breast, lung, and glioma models and decreased tumor and endothelial cell proliferation as well as reduced tumor vasculature in mouse models.24,25 In a mouse xenograft model of human GBM, cabozantinib treatment significantly increased median OS compared with the placebo-treated control (32 vs 20 days, respectively; P < 0.0001).26
This phase II trial examined the efficacy and safety of 2 starting doses of cabozantinib (140 mg/day and 100 mg/day) in 222 patients with progressive or recurrent GBM. As molecular changes in disease may contribute to the development of resistance to antiangiogenic therapy, we conducted separate subgroup analyses for patients based on prior antiangiogenic therapy. Here we report the results from the subgroup of 70 patients who had received previous antiangiogenic therapy before study entry. Results for patients naive to antiangiogenic therapy are presented in the companion article (Wen et al).
Materials and Methods
Patients
Eligible adult patients (≥18 y old) had progressive or recurrent GBM that was confirmed by gadolinium (Gd)-enhanced MRI performed within 14 days of the first dose of cabozantinib. Some entry criteria differed among patients due to amendments to the protocol (see Supplementary material). Patients were required to have received prior radiation therapy and temozolomide (for patients enrolled at the 100 mg/day dose), and could have also received prior VEGF or VEGFR2 inhibitors. Eligible patients had a Karnofsky performance status ≥60% and adequate hematologic, renal, and liver function. Patients were excluded if they had received prior anticancer therapy within specified time limits before the first dose of cabozantinib, including anticancer therapy within 28 days (including investigational agents and biologic agents except bevacizumab), bevacizumab within 14 days, or mitomycin C or nitrosoureas within 42 days. All patients provided informed written consent. The protocol was approved by ethics committees or institutional review boards at each investigator’s institution. This trial was conducted in accordance with the principles of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline for Good Clinical Practice, the World Medical Association Declaration of Helsinki, and Title 21 of the United States Code of Federal Regulations.
Study Design
This phase II, multicenter, open-label, single-agent study evaluated 2 doses of cabozantinib in patients with recurrent or progressive GBM in first or second relapse. Patients were defined to be enrolled upon receipt of the first dose of cabozantinib. Cabozantinib was orally administered once daily at a starting dose of 140 mg/day (freebase weight); however, because rates of dose reduction and interruption at 140 mg/day were deemed to be high, and initial evaluations suggested continued efficacy with doses that were reduced to ≤100 mg/day, the starting dose was amended to 100 mg/day, and a cohort for initial qualitative assessment of the safety and tolerability of the dose was added (15 antiangiogenic pretreated patients planned). Subsequently, another planned cohort of 45 antiangiogenic pretreated patients was added at the 100 mg/day dose. Patients were treated until disease progression or unacceptable toxicity. To manage adverse events (AEs), dose could be reduced to 100 mg (for those enrolled at 140 mg/day), 60 mg, and 40 mg; dose could be reduced to lower than 40 mg after consultation with the sponsor. Further details of the study design and protocol amendments are provided in the Supplementary material.
Endpoints and Assessments
The primary endpoint was ORR assessed by an independent radiology facility (IRF). Key secondary endpoints included duration of response, median PFS, OS, and evaluation of glucocorticoid use. Radiographic assessments by Gd-contrast MRI were performed at screening and generally every 6 to 8 weeks thereafter (see Supplementary material) and were assessed by the investigator for patient management and by the IRF, BioClinica (formerly CoreLab Partners [Princeton, NJ]). Tumor response and progression were determined by modified Response Assessment in Neuro-Oncology (RANO) criteria, which included the components of radiographic assessment and glucocorticoid use. For implementation in this study, the primary modifications to the RANO criteria for time point responses include defining operational conventions for changes in glucocorticoid dose and removal of the clinical deterioration component to reduce subjectivity and facilitate IRF assessment. Neurologic and clinical status did not contribute to the response or progression criteria. Tumor assessments for determination of response and progression for the primary efficacy analyses were performed by an IRF. The minimum lesion size required for measurable disease by IRF was 10 mm × 5 mm, reflecting the implementation of RANO in this study before publication of the now standard 10 mm × 10 mm criterion for measurable disease.
Safety and tolerability assessments included monitoring AEs, performing standard clinical laboratory tests (including hematology, serum chemistry, and urinalysis) and physical examinations, and recording electrocardiograms. Severity of AEs was assessed by using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3. Serious AEs (SAEs) were defined in accordance with the ICH Guidelines for Clinical Safety Data Management: Definitions and Standards for Expedited Reporting, Topic E2A.
Statistical Analysis
Results for patients who received prior antiangiogenic therapy are presented here. Efficacy and safety analyses include all such patients enrolled unless otherwise specified. ORR was the proportion of subjects experiencing a confirmed complete or partial response per modified RANO criteria at any time during the study. ORR was computed among patients with measurable disease at baseline per modified RANO criteria and is presented with 2-sided 95% CIs. Forty-five patients were planned to evaluate ORR in patients with prior antiangiogenic therapy with the following hypotheses (1-sided nominal alpha of 0.1 and power of >80%): H0: ORR = 5% and HA: ORR = 15%. The primary endpoint was to be analyzed in the last group of patients enrolled at the 100 mg/day dose; however, for simplicity, all patients who received prior antiangiogenic therapy at the 100 mg/day dose were retrospectively combined for the efficacy analyses presented here. See Supplementary material for more details on the planned analyses.
PFS was calculated as the time from first cabozantinib dose to the earlier of documented disease progression per modified RANO or death from any cause. OS was calculated as the time from first cabozantinib dose to death from any cause. Median PFS and OS and the associated 2-sided 95% CIs were computed using the Kaplan–Meier method. All statistical analysis was performed using SAS version 8.2 or higher.
Results
Patient Characteristics
Of 222 patients enrolled between June 2008 and June 2012, 70 (32%) had received ≥1 prior antiangiogenic therapy (Table 1). All subsequent results refer to this subgroup. Baseline demographics and clinical characteristics were similar between the 140 mg/day (n = 12) and 100 mg/day (n = 58) dose groups (Table 1). The study population included mostly male patients (61.4%) with a median age of 52.5 years (range, 23–71 y). Overall, the mean number of previous anticancer therapies for GBM was 1.8 ± 0.64, and 57 patients (81.4%) had received prior bevacizumab. The median duration of treatment was 5.7 weeks (range, 2.0–186.3) in the 140 mg/day group and 7.6 weeks (range, 1.4–47.9) in the 100 mg/day group.
| Characteristic . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Age, y | ||
| Median (range) | 53 (23–71) | 52 (24–68) |
| Sex | ||
| Male | 7 (58.3) | 36 (62.1) |
| Female | 5 (41.7) | 22 (37.9) |
| Race | ||
| White | 11 (91.7) | 54 (93.1) |
| Asian | 0 | 1 (1.7) |
| Other/not reported | 1 (8.3) | 3 (5.2) |
| Karnofsky performance status | ||
| 90–100 | 2 (16.7) | 36 (62.1) |
| 70–80 | 10 (83.3) | 20 (34.5) |
| ≤60 | 0 | 2 (3.4) |
| Years since initial diagnosis | ||
| Median (range) | 1.07 (0.6–3.5) | 1.16 (0.5–7.2) |
| GBM type | ||
| Primary | 11 (91.7) | 55 (94.8) |
| Secondary | 1 (8.3) | 3 (5.2) |
| Prior radiotherapy for GBM | ||
| Yes | 11 (91.7) | 58 (100.0) |
| No | 1 (8.3) | 0 |
| Prior lines of systemic therapy | ||
| 1 | 3 (25.0) | 19 (32.8) |
| 2 | 9 (75.0) | 35 (60.3) |
| ≥3 | 0 | 4 (6.9) |
| Prior antiangiogenic therapy | ||
| Bevacizumab* | 9 (75.0) | 48 (82.8) |
| Other** | 3 (25.0) | 10 (17.2) |
| Steroid use at baseline*** | ||
| Yes | 4 (33.3) | 23 (39.7) |
| No/unknown | 8 (66.7) | 35 (60.3) |
| Characteristic . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Age, y | ||
| Median (range) | 53 (23–71) | 52 (24–68) |
| Sex | ||
| Male | 7 (58.3) | 36 (62.1) |
| Female | 5 (41.7) | 22 (37.9) |
| Race | ||
| White | 11 (91.7) | 54 (93.1) |
| Asian | 0 | 1 (1.7) |
| Other/not reported | 1 (8.3) | 3 (5.2) |
| Karnofsky performance status | ||
| 90–100 | 2 (16.7) | 36 (62.1) |
| 70–80 | 10 (83.3) | 20 (34.5) |
| ≤60 | 0 | 2 (3.4) |
| Years since initial diagnosis | ||
| Median (range) | 1.07 (0.6–3.5) | 1.16 (0.5–7.2) |
| GBM type | ||
| Primary | 11 (91.7) | 55 (94.8) |
| Secondary | 1 (8.3) | 3 (5.2) |
| Prior radiotherapy for GBM | ||
| Yes | 11 (91.7) | 58 (100.0) |
| No | 1 (8.3) | 0 |
| Prior lines of systemic therapy | ||
| 1 | 3 (25.0) | 19 (32.8) |
| 2 | 9 (75.0) | 35 (60.3) |
| ≥3 | 0 | 4 (6.9) |
| Prior antiangiogenic therapy | ||
| Bevacizumab* | 9 (75.0) | 48 (82.8) |
| Other** | 3 (25.0) | 10 (17.2) |
| Steroid use at baseline*** | ||
| Yes | 4 (33.3) | 23 (39.7) |
| No/unknown | 8 (66.7) | 35 (60.3) |
*Four patients in the 140 mg/day group and 21 patients in the 100 mg/day group received bevacizumab as part of initial therapy.
**Other antiangiogenic therapies: vandetanib (5 patients); cediranib (4 patients); CT-322 (2 patients); sunitinib (1 patient); investigational VEGF trap (1 patient). Two patients in the 140 mg/day group and 4 patients in the 140 mg/day group received other antiangiogenic therapy as initial therapy.
***Received at least 7 days of systemic steroids within 30 days before the first dose of cabozantinib.
| Characteristic . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Age, y | ||
| Median (range) | 53 (23–71) | 52 (24–68) |
| Sex | ||
| Male | 7 (58.3) | 36 (62.1) |
| Female | 5 (41.7) | 22 (37.9) |
| Race | ||
| White | 11 (91.7) | 54 (93.1) |
| Asian | 0 | 1 (1.7) |
| Other/not reported | 1 (8.3) | 3 (5.2) |
| Karnofsky performance status | ||
| 90–100 | 2 (16.7) | 36 (62.1) |
| 70–80 | 10 (83.3) | 20 (34.5) |
| ≤60 | 0 | 2 (3.4) |
| Years since initial diagnosis | ||
| Median (range) | 1.07 (0.6–3.5) | 1.16 (0.5–7.2) |
| GBM type | ||
| Primary | 11 (91.7) | 55 (94.8) |
| Secondary | 1 (8.3) | 3 (5.2) |
| Prior radiotherapy for GBM | ||
| Yes | 11 (91.7) | 58 (100.0) |
| No | 1 (8.3) | 0 |
| Prior lines of systemic therapy | ||
| 1 | 3 (25.0) | 19 (32.8) |
| 2 | 9 (75.0) | 35 (60.3) |
| ≥3 | 0 | 4 (6.9) |
| Prior antiangiogenic therapy | ||
| Bevacizumab* | 9 (75.0) | 48 (82.8) |
| Other** | 3 (25.0) | 10 (17.2) |
| Steroid use at baseline*** | ||
| Yes | 4 (33.3) | 23 (39.7) |
| No/unknown | 8 (66.7) | 35 (60.3) |
| Characteristic . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Age, y | ||
| Median (range) | 53 (23–71) | 52 (24–68) |
| Sex | ||
| Male | 7 (58.3) | 36 (62.1) |
| Female | 5 (41.7) | 22 (37.9) |
| Race | ||
| White | 11 (91.7) | 54 (93.1) |
| Asian | 0 | 1 (1.7) |
| Other/not reported | 1 (8.3) | 3 (5.2) |
| Karnofsky performance status | ||
| 90–100 | 2 (16.7) | 36 (62.1) |
| 70–80 | 10 (83.3) | 20 (34.5) |
| ≤60 | 0 | 2 (3.4) |
| Years since initial diagnosis | ||
| Median (range) | 1.07 (0.6–3.5) | 1.16 (0.5–7.2) |
| GBM type | ||
| Primary | 11 (91.7) | 55 (94.8) |
| Secondary | 1 (8.3) | 3 (5.2) |
| Prior radiotherapy for GBM | ||
| Yes | 11 (91.7) | 58 (100.0) |
| No | 1 (8.3) | 0 |
| Prior lines of systemic therapy | ||
| 1 | 3 (25.0) | 19 (32.8) |
| 2 | 9 (75.0) | 35 (60.3) |
| ≥3 | 0 | 4 (6.9) |
| Prior antiangiogenic therapy | ||
| Bevacizumab* | 9 (75.0) | 48 (82.8) |
| Other** | 3 (25.0) | 10 (17.2) |
| Steroid use at baseline*** | ||
| Yes | 4 (33.3) | 23 (39.7) |
| No/unknown | 8 (66.7) | 35 (60.3) |
*Four patients in the 140 mg/day group and 21 patients in the 100 mg/day group received bevacizumab as part of initial therapy.
**Other antiangiogenic therapies: vandetanib (5 patients); cediranib (4 patients); CT-322 (2 patients); sunitinib (1 patient); investigational VEGF trap (1 patient). Two patients in the 140 mg/day group and 4 patients in the 140 mg/day group received other antiangiogenic therapy as initial therapy.
***Received at least 7 days of systemic steroids within 30 days before the first dose of cabozantinib.
Efficacy
All patients (n = 70) had measurable disease at baseline as assessed by the IRF and were included in the response assessment. The results for ORR did not meet the predefined statistical target for success. Objective responses were observed in 3 (4.3%) patients, 33 (47.1%) had stable disease, and 18 (25.7%) had progressive disease as best response (Table 2). Sixteen patients (23%) were unevaluable for tumor response because they did not have an evaluable postbaseline tumor assessment. In the 3 patients with objective responses, the durations of response were 1.2, 4.2, and 12.3 months. Among these patients, 1 had received prior bevacizumab, and 2 had received other antiangiogenic therapies. In patients with ≥1 evaluable postbaseline tumor assessment (n = 62), 41 (66%) experienced reductions in tumor volume (Fig. 1). The median follow-up for scans was 1.5 months (range, 0.03–42.6) in the 140 mg/day group and 1.8 months (range, 0.03–11.0) in the 100 mg/day group.
| Parameter . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Objective response rate | 1 (8.3) | 2 (3.4) |
| Best overall response | ||
| Confirmed partial response | 1 (8.3) | 2 (3.4) |
| Stable disease | 6 (50.0) | 27 (46.6) |
| Progressive disease | 2 (16.7) | 16 (27.6) |
| Unevaluable or missing** | 3 (25.0) | 13 (22.4) |
| Duration of objective response, mo | 12.3+ | 1.2+, 4.2 |
| Parameter . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Objective response rate | 1 (8.3) | 2 (3.4) |
| Best overall response | ||
| Confirmed partial response | 1 (8.3) | 2 (3.4) |
| Stable disease | 6 (50.0) | 27 (46.6) |
| Progressive disease | 2 (16.7) | 16 (27.6) |
| Unevaluable or missing** | 3 (25.0) | 13 (22.4) |
| Duration of objective response, mo | 12.3+ | 1.2+, 4.2 |
+Censored at the date of the last tumor assessment.
*All patients with measurable disease at baseline were included in the response assessment.
**Unevaluable by modified RANO or no postbaseline tumor assessments.
| Parameter . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Objective response rate | 1 (8.3) | 2 (3.4) |
| Best overall response | ||
| Confirmed partial response | 1 (8.3) | 2 (3.4) |
| Stable disease | 6 (50.0) | 27 (46.6) |
| Progressive disease | 2 (16.7) | 16 (27.6) |
| Unevaluable or missing** | 3 (25.0) | 13 (22.4) |
| Duration of objective response, mo | 12.3+ | 1.2+, 4.2 |
| Parameter . | Patients, n (%) . | |
|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |
| Objective response rate | 1 (8.3) | 2 (3.4) |
| Best overall response | ||
| Confirmed partial response | 1 (8.3) | 2 (3.4) |
| Stable disease | 6 (50.0) | 27 (46.6) |
| Progressive disease | 2 (16.7) | 16 (27.6) |
| Unevaluable or missing** | 3 (25.0) | 13 (22.4) |
| Duration of objective response, mo | 12.3+ | 1.2+, 4.2 |
+Censored at the date of the last tumor assessment.
*All patients with measurable disease at baseline were included in the response assessment.
**Unevaluable by modified RANO or no postbaseline tumor assessments.
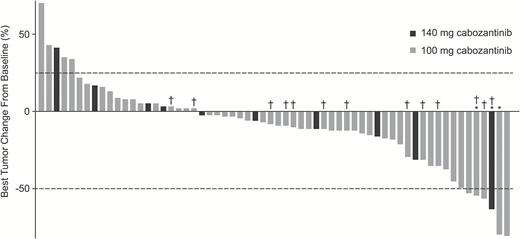
Best changes from baseline in IRF measurements of tumor lesions using modified RANO criteria in patients who had measurable disease at baseline and ≥1 postbaseline radiographic scan. Lines indicate the threshold for response and progression per RANO criteria, ≥50% decrease and ≥25% increase, respectively. Partial responses were confirmed in 1 patient in the 140 mg/day group and 2 patients in the 100 mg/day group. *Confirmed partial response. †No prior bevacizumab therapy.
Median PFS was 2.3 months overall, 3.3 months in the 140 mg/day group, and 2.3 months in the 100 mg/day group (Fig. 2A). Median OS was 4.6 months overall, 4.1 months (95% CI, 1.4–16.7 mo) in the 140 mg/day group, and 4.6 months (95% CI, 2.9–5.6 mo) in the 100 mg/day group (Fig. 2B). At baseline, 33 patients (47.1%) reported systemic steroid treatment. Among patients who reported any glucocorticoid use at baseline, there was a trend toward decreasing glucocorticoid requirements over time (Fig. 3).
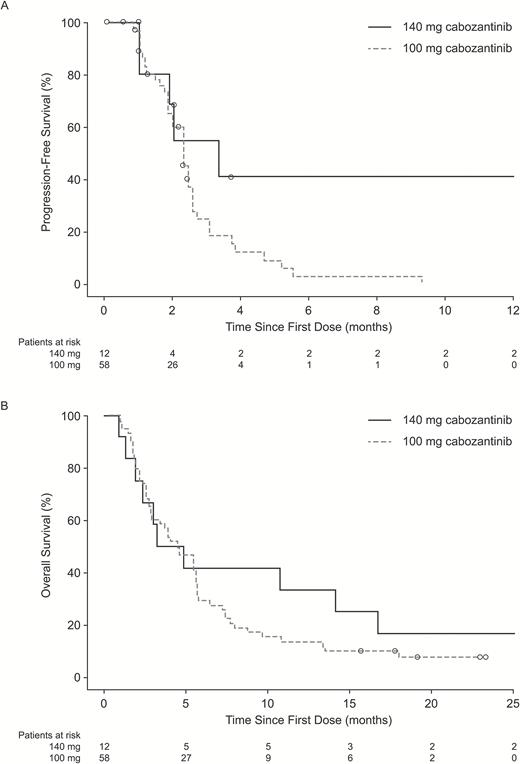
Kaplan–Meier estimates of (A) progression-free survival and (B) overall survival by dose group on the basis of central assessment of radiographic images. Circles = censored records.

Average daily glucocorticoid dose up to last treatment date among patients who reported any glucocorticoid use at baseline.
Safety
The most common treatment-emergent AEs included fatigue (74.3%), diarrhea (47.1%), increased alanine aminotransferase (37.1%), headache (35.7%), hypertension (35.7%), nausea (35.7%), constipation (34.3%), increased aspartate aminotransferase (30.0%), dysphonia (28.6%), increased lactate dehydrogenase (27.1%), proteinuria (27.1%), and memory impairment (25.7%) (Table 3).
Treatment-emergent adverse events (TEAEs) reported in ≥15% of patients in the 100 mg group
| Adverse Event* . | Patients, n (%) . | |||
|---|---|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |||
| All Grades . | Grade 3/4 . | All Grades . | Grade 3/4 . | |
| Any TEAE | 12 (100.0) | 12 (100.0) | 58 (100.0) | 42 (72.4) |
| Fatigue | 8 (66.7) | 3 (25.0) | 44 (75.9) | 12 (20.7) |
| Diarrhea | 8 (66.7) | 2 (16.7) | 25 (43.1) | 2 (3.4) |
| ALT increased | 4 (33.3) | 2 (16.7) | 22 (37.9) | 4 (6.9) |
| Headache | 4 (33.3) | 1 (8.3) | 21 (36.2) | 5 (8.6) |
| Nausea | 5 (41.7) | 0 | 20 (34.5) | 0 |
| Hypertension | 6 (50.0) | 2 (16.7) | 19 (32.8) | 4 (6.9) |
| Constipation | 5 (41.7) | 1 (8.3) | 19 (32.8) | 1 (1.7) |
| AST increased | 5 (41.7) | 2 (16.7) | 16 (27.6) | 3 (5.2) |
| Blood LDH increased | 3 (25.0) | 0 | 16 (27.6) | 1 (1.7) |
| Dysphonia | 5 (41.7) | 0 | 15 (25.9) | 0 |
| Memory impairment | 3 (25.0) | 0 | 15 (25.9) | 0 |
| Convulsion | 2 (16.7) | 1 (8.3) | 15 (25.9) | 3 (5.2) |
| Proteinuria | 5 (41.7) | 0 | 14 (24.1) | 2 (3.4) |
| Confusional state | 3 (25.0) | 0 | 14 (24.1) | 5 (8.6) |
| Cognitive disorder | 2 (16.7) | 1 (8.3) | 13 (22.4) | 0 |
| Decreased appetite | 4 (33.3) | 0 | 12 (20.7) | 1 (1.7) |
| Gait disturbance | 3 (25.0) | 1 (8.3) | 12 (20.7) | 3 (5.2) |
| PPES | 3 (25.0) | 0 | 11 (19.0) | 3 (5.2) |
| Rash | 3 (25.0) | 0 | 11 (19.0) | 0 |
| Depression | 1 (8.3) | 0 | 11 (19.0) | 0 |
| Speech disorder | 1 (8.3) | 1 (8.3) | 10 (17.2) | 4 (6.9) |
| Thrombocytopenia | 0 | 0 | 9 (15.5) | 5 (8.6) |
| Adverse Event* . | Patients, n (%) . | |||
|---|---|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |||
| All Grades . | Grade 3/4 . | All Grades . | Grade 3/4 . | |
| Any TEAE | 12 (100.0) | 12 (100.0) | 58 (100.0) | 42 (72.4) |
| Fatigue | 8 (66.7) | 3 (25.0) | 44 (75.9) | 12 (20.7) |
| Diarrhea | 8 (66.7) | 2 (16.7) | 25 (43.1) | 2 (3.4) |
| ALT increased | 4 (33.3) | 2 (16.7) | 22 (37.9) | 4 (6.9) |
| Headache | 4 (33.3) | 1 (8.3) | 21 (36.2) | 5 (8.6) |
| Nausea | 5 (41.7) | 0 | 20 (34.5) | 0 |
| Hypertension | 6 (50.0) | 2 (16.7) | 19 (32.8) | 4 (6.9) |
| Constipation | 5 (41.7) | 1 (8.3) | 19 (32.8) | 1 (1.7) |
| AST increased | 5 (41.7) | 2 (16.7) | 16 (27.6) | 3 (5.2) |
| Blood LDH increased | 3 (25.0) | 0 | 16 (27.6) | 1 (1.7) |
| Dysphonia | 5 (41.7) | 0 | 15 (25.9) | 0 |
| Memory impairment | 3 (25.0) | 0 | 15 (25.9) | 0 |
| Convulsion | 2 (16.7) | 1 (8.3) | 15 (25.9) | 3 (5.2) |
| Proteinuria | 5 (41.7) | 0 | 14 (24.1) | 2 (3.4) |
| Confusional state | 3 (25.0) | 0 | 14 (24.1) | 5 (8.6) |
| Cognitive disorder | 2 (16.7) | 1 (8.3) | 13 (22.4) | 0 |
| Decreased appetite | 4 (33.3) | 0 | 12 (20.7) | 1 (1.7) |
| Gait disturbance | 3 (25.0) | 1 (8.3) | 12 (20.7) | 3 (5.2) |
| PPES | 3 (25.0) | 0 | 11 (19.0) | 3 (5.2) |
| Rash | 3 (25.0) | 0 | 11 (19.0) | 0 |
| Depression | 1 (8.3) | 0 | 11 (19.0) | 0 |
| Speech disorder | 1 (8.3) | 1 (8.3) | 10 (17.2) | 4 (6.9) |
| Thrombocytopenia | 0 | 0 | 9 (15.5) | 5 (8.6) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; LDH, lactate dehydrogenase; MedDRA, Medical Dictionary for Regulatory Activities; PPES, palmar-plantar erythrodysesthesia syndrome.
*MedDRA v. 15.0 Preferred Terms (converted to US spelling), CTCAE v. 3.0 grading.
Treatment-emergent adverse events (TEAEs) reported in ≥15% of patients in the 100 mg group
| Adverse Event* . | Patients, n (%) . | |||
|---|---|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |||
| All Grades . | Grade 3/4 . | All Grades . | Grade 3/4 . | |
| Any TEAE | 12 (100.0) | 12 (100.0) | 58 (100.0) | 42 (72.4) |
| Fatigue | 8 (66.7) | 3 (25.0) | 44 (75.9) | 12 (20.7) |
| Diarrhea | 8 (66.7) | 2 (16.7) | 25 (43.1) | 2 (3.4) |
| ALT increased | 4 (33.3) | 2 (16.7) | 22 (37.9) | 4 (6.9) |
| Headache | 4 (33.3) | 1 (8.3) | 21 (36.2) | 5 (8.6) |
| Nausea | 5 (41.7) | 0 | 20 (34.5) | 0 |
| Hypertension | 6 (50.0) | 2 (16.7) | 19 (32.8) | 4 (6.9) |
| Constipation | 5 (41.7) | 1 (8.3) | 19 (32.8) | 1 (1.7) |
| AST increased | 5 (41.7) | 2 (16.7) | 16 (27.6) | 3 (5.2) |
| Blood LDH increased | 3 (25.0) | 0 | 16 (27.6) | 1 (1.7) |
| Dysphonia | 5 (41.7) | 0 | 15 (25.9) | 0 |
| Memory impairment | 3 (25.0) | 0 | 15 (25.9) | 0 |
| Convulsion | 2 (16.7) | 1 (8.3) | 15 (25.9) | 3 (5.2) |
| Proteinuria | 5 (41.7) | 0 | 14 (24.1) | 2 (3.4) |
| Confusional state | 3 (25.0) | 0 | 14 (24.1) | 5 (8.6) |
| Cognitive disorder | 2 (16.7) | 1 (8.3) | 13 (22.4) | 0 |
| Decreased appetite | 4 (33.3) | 0 | 12 (20.7) | 1 (1.7) |
| Gait disturbance | 3 (25.0) | 1 (8.3) | 12 (20.7) | 3 (5.2) |
| PPES | 3 (25.0) | 0 | 11 (19.0) | 3 (5.2) |
| Rash | 3 (25.0) | 0 | 11 (19.0) | 0 |
| Depression | 1 (8.3) | 0 | 11 (19.0) | 0 |
| Speech disorder | 1 (8.3) | 1 (8.3) | 10 (17.2) | 4 (6.9) |
| Thrombocytopenia | 0 | 0 | 9 (15.5) | 5 (8.6) |
| Adverse Event* . | Patients, n (%) . | |||
|---|---|---|---|---|
| 140 mg/day (n = 12) . | 100 mg/day (n = 58) . | |||
| All Grades . | Grade 3/4 . | All Grades . | Grade 3/4 . | |
| Any TEAE | 12 (100.0) | 12 (100.0) | 58 (100.0) | 42 (72.4) |
| Fatigue | 8 (66.7) | 3 (25.0) | 44 (75.9) | 12 (20.7) |
| Diarrhea | 8 (66.7) | 2 (16.7) | 25 (43.1) | 2 (3.4) |
| ALT increased | 4 (33.3) | 2 (16.7) | 22 (37.9) | 4 (6.9) |
| Headache | 4 (33.3) | 1 (8.3) | 21 (36.2) | 5 (8.6) |
| Nausea | 5 (41.7) | 0 | 20 (34.5) | 0 |
| Hypertension | 6 (50.0) | 2 (16.7) | 19 (32.8) | 4 (6.9) |
| Constipation | 5 (41.7) | 1 (8.3) | 19 (32.8) | 1 (1.7) |
| AST increased | 5 (41.7) | 2 (16.7) | 16 (27.6) | 3 (5.2) |
| Blood LDH increased | 3 (25.0) | 0 | 16 (27.6) | 1 (1.7) |
| Dysphonia | 5 (41.7) | 0 | 15 (25.9) | 0 |
| Memory impairment | 3 (25.0) | 0 | 15 (25.9) | 0 |
| Convulsion | 2 (16.7) | 1 (8.3) | 15 (25.9) | 3 (5.2) |
| Proteinuria | 5 (41.7) | 0 | 14 (24.1) | 2 (3.4) |
| Confusional state | 3 (25.0) | 0 | 14 (24.1) | 5 (8.6) |
| Cognitive disorder | 2 (16.7) | 1 (8.3) | 13 (22.4) | 0 |
| Decreased appetite | 4 (33.3) | 0 | 12 (20.7) | 1 (1.7) |
| Gait disturbance | 3 (25.0) | 1 (8.3) | 12 (20.7) | 3 (5.2) |
| PPES | 3 (25.0) | 0 | 11 (19.0) | 3 (5.2) |
| Rash | 3 (25.0) | 0 | 11 (19.0) | 0 |
| Depression | 1 (8.3) | 0 | 11 (19.0) | 0 |
| Speech disorder | 1 (8.3) | 1 (8.3) | 10 (17.2) | 4 (6.9) |
| Thrombocytopenia | 0 | 0 | 9 (15.5) | 5 (8.6) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; LDH, lactate dehydrogenase; MedDRA, Medical Dictionary for Regulatory Activities; PPES, palmar-plantar erythrodysesthesia syndrome.
*MedDRA v. 15.0 Preferred Terms (converted to US spelling), CTCAE v. 3.0 grading.
A total of 34 patients (48.6%) experienced AEs that resulted in dose reductions, and the median time to first dose reduction was 40.5 days (range, 13–173 days). Dose reductions were reported in 5 patients (41.7%) in the 140 mg/day group and in 29 patients (50.0%) in the 100 mg/day group. Dose interruptions due to AEs occurred in 29 patients (41.4%). The median average dose was 123 mg/day (range, 78.3–140 mg/day) in the 140 mg/day group, and 92.6 mg/day (range, 48.1–100 mg/day) in the 100 mg/day group. Overall, 12 patients (17.1%) discontinued treatment due to AEs: 5 (41.7%) in the 140 mg/day group and 7 (12.1%) in the 100 mg/day group.
Grade 3 or 4 AEs were reported in 12 (100.0%) and 42 (72.4%) patients in the 140 mg/day and 100 mg/day groups, respectively. Serious AEs were reported in 35 patients (50%): 6 patients (50%) in the 140 mg/day group and 29 patients (50%) in the 100 mg/day group. Common SAEs observed in patients treated in either dose group included convulsion (5.7%, n = 4), alanine aminotransferase increased (4.3%, n = 3), confusional state (4.3%, n = 3), and disease progression (4.3%, n = 3). Sixty-three deaths were reported: 10 (83.3%) in the 140 mg/day group and 53 (91.4%) in the 100 mg/day group. Deaths were attributed primarily to progressive disease. Grade 5 AEs were reported in 6 patients (8.6%) in the 100 mg/day group and no patients in the 140 mg/day group. Grade 5 AEs consisted of disease progression (3 patients) and intracranial tumor hemorrhage, general physical health deterioration, and increased intracranial pressure with herniation (1 patient each). None of these grade 5 AEs were assessed as related to study treatment.
Discussion
Additional therapies for patients with GBM whose disease has progressed while receiving antiangiogenic agents are needed. There is no standard of care for GBM patients who have progressed on bevacizumab,5,9,27,28 and there are only scant reports of responses or prolonged stable disease following progression while on other antiangiogenic agents.29 This phase II trial examined the efficacy and safety of cabozantinib in 70 patients with recurrent GBM who had progressed on prior antiangiogenic therapy. The study did not meet the predefined statistical target for success. Approximately two-thirds of patients receiving cabozantinib had a reduction in tumor lesions and 50% of patients achieved stable disease as best response; however, the ORR was 4.3%, median PFS was 2.3 months, and median OS was 4.6 months (95% CI, 3.0–5.6 mo). These results are similar to the median PFS of 1.3 months reported in a retrospective analysis of patients who received an alternative bevacizumab regimen after progressing on bevacizumab9 and a separate retrospective pooled analysis that reported medians for PFS and OS of 2.8 months and 5.9 months, respectively, in patients who continued on bevacizumab after progression.27 Similarly, median PFS was approximately 1 month for dasatinib,30 rilotumumab,31 or sunitinib32 therapy in patients with recurrent GBM after progression on bevacizumab.
Reported AEs were similar to those observed with other antiangiogenic agents, including bevacizumab, cediranib, and pazopanib, in this patient population.5,6,10,11 Adverse events were managed with supportive care and dose reductions or interruptions, and the overall duration of treatment was >5 weeks. The rate of discontinuations due to AEs and the rate of grade 3/4 AEs were higher in the 140 mg/day group than in the 100 mg/day group, although SAEs and dose reductions were similar in the 2 groups. Both higher and lower starting doses of cabozantinib have been used in other indications. A cabozantinib dose of 140 mg/day has been used for the treatment of medullary thyroid cancer,33 while a lower dose of 60 mg/day has been used for advanced renal cell carcinoma.34
GBM may be one of the most comprehensive molecularly characterized solid tumors,35 but molecular evaluations after GBM progression are far less robust. Nonetheless, clinical and preclinical data implicate a number of possible mechanisms of resistance with antiangiogenic therapies in GBM,36 and MET activation has been established as one of the principal mechanisms,19 suggesting a need for multitarget inhibitors in this setting.37 While cabozantinib targets multiple oncogenic pathways (MET, VEGFR2, AXL, KIT, RET, TIE2, and FLT3),24,38 it appears to have only modest clinical activity in patients with progressive or recurrent GBM who have received prior antiangiogenic therapy. More promising activity was observed with cabozantinib in the subset of 152 patients not previously treated with antiangiogenic therapy (ORR of 15% for the combined dosing cohorts and a median PFS of 3.7 months in both cohorts; see companion article by Wen et al). Overall, patient baseline characteristics were similar in the naive and antiangiogenic treated patients with the exception of prior therapies. Additional studies that include molecular characterization of disease may help to better characterize differences between naive and antiangiogenic treated patients with GBM.
This was the first investigational trial utilizing an experimental therapy in the treatment of patients with GBM who had progressed on prior antiangiogenic therapy. The population of patients with GBM progressing on bevacizumab is an area of unmet need warranting further clinical investigation. Challenges in study design include how to better define the study population (eg, prior antiangiogenic therapies used, prior response obtained) and how to best define a meaningful response (eg, ORR, PFS, survival).
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
Research funding and financial support for medical editorial assistance were provided by Exelixis, Inc (South San Francisco, California).
The conflict of interest statement follows:
Employment:J. Holland: Exelixis; J. Ping: Exelixis; R. Weitzman: Exelixis
Leadership Position: None
Stock Ownership: A. Desjardins: Istari Oncology; J. Drappatz: Exelixis; J. Holland: Exelixis; J. Ping: Exelixis; R. Weitzman: Exelixis
Honoraria: J. de Groot: Merck; J. Drappatz: Angiochem, Geron; D. Reardon: Roche/Genentech, Merck, Amgen, Stemline, Novartis; D. Schiff: Merck
Consultant and/or Advisory Role: T. Cloughesy: Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab, MedQIA; J. de Groot: Celldex, Deciphera Pharmaceuticals, Foundation Medicine, Inc., AstraZeneca, Novartis, Genentech; M.D. Prados: Novartis; D. Reardon: Roche/Genentech, Merck, Amgen, Stemline, Novartis; M Chamberlain: Eli Lilly, Genentech/Roche, Nativis, NeurOnc, Pharmacokinesis; D. Schiff: DSMC member for Celldex, Vascular Biogenics; consultant/advisory board for Tau Pharmaceutical, Genentech, Sigma Tau, Midatech, Heron; T. Mikkelsen: Celgene, Roche/Genentech; A. Desjardins: Genentech USA, Inc, PTC Therapeutics; P. Wen: Agios, Genentech/Roche, Cavion, Cortice Biosciences, Foundation Medicine, Insys, Monteris, Novartis, Vascular Biogenics, VBI Vaccines.
Research Funding:J. de Groot: Eli Lilly, Sanofi-Aventis, EMD-Serono, AstraZeneca, Novartis, Deciphera Pharmaceuticals; D. Schiff: Novocure, Angiochem; P. Wen: Acerta, Agios, AstraZeneca, Ely Lilly, Exelixis, Genentech/Roche, Karyopharm, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics; M.D. Prados: Genentech, Novartis; J. Drappatz: Angiochem, AbbVie, Genentech, Celldex; A. Desjardins: Genentech USA, PTC Therapeutics, Celldex
Patents, Royalties, Other Intellectual Property:A. Desjardins: PVSRIPO (modified poliovirus—clinical)
Speakers’ Bureau:D. Reardon: Roche/Genentech, Merck; P. Wen: Merck; M Chamberlain: Genentech
Expert Testimony:T. Cloughesy: Roche
Travel, Accommodations, Expenses:J. de Groot: Genen tech, Deciphera, Celldex; T. Mikkelsen: Celgene, Roche/Genentech; J. Ping: Exelixis; M Chamberlain: Genentech; J. Drappatz: Angiochem, AbbVie, Geron, Genentech
Other Relationship:J. de Groot: DSMB for VBL Therapeutics and Novella
Prior Publication: Previous analysis of data presented in part at 2010 ASCO Annual Meeting (oral presentation); abstract published as Wen PY et al. J Clin Oncol. 2010;28(15 suppl): abstr 2006).
Acknowledgments
We thank Stephanie K. Doerner, of Scientific Strategy Partners, Inc, for her medical editorial assistance with this manuscript. The authors are fully responsible for all content and editorial decisions for this manuscript.
References
Author notes
J.D. current affiliation: University of Pittsburgh Medical Center, Hillman Cancer Centers, UPMC Cancer Pavilion, Pittsburgh, Pennsylvania.




