-
PDF
- Split View
-
Views
-
Cite
Cite
Aida Karachi, Farhad Dastmalchi, Duane A Mitchell, Maryam Rahman, Temozolomide for immunomodulation in the treatment of glioblastoma, Neuro-Oncology, Volume 20, Issue 12, December 2018, Pages 1566–1572, https://doi.org/10.1093/neuonc/noy072
Close - Share Icon Share
Abstract
Temozolomide is the most widely used chemotherapy for patients with glioblastoma (GBM) despite the fact that approximately half of treated patients have temozolomide resistance and all patients eventually fail therapy. Due to the limited efficacy of existing therapies, immunotherapy is being widely investigated for patients with GBM. However, initial immunotherapy trials in GBM patients have had disappointing results as monotherapy. Therefore, combinatorial treatment strategies are being investigated. Temozolomide has several effects on the immune system that are dependent on mode of delivery and the dosing strategy, which may have unpredicted effects on immunotherapy. Here we summarize the immune modulating role of temozolomide alone and in combination with immunotherapies such as dendritic cell vaccines, T-cell therapy, and immune checkpoint inhibitors for patients with GBM.
Despite keen interest in the development of novel therapies for glioblastoma (GBM), most clinical trials have resulted in no survival benefit for patients.1–4 Due to decades of negative results, immunotherapy for GBM has been met with enthusiasm. Immunotherapeutic strategies such as vaccines, T-cell therapy, and immune checkpoint inhibitors have had success in solid tissue cancer.5–9 Recently, these approaches have been under investigation for GBM patients. Unfortunately, initial large immunotherapy trials in subsets of patients with GBM have also been negative.1,10 The explanation for lack of efficacy of different approaches for GBM is multifactorial. A large contributor to these failures is the robust nature of GBM tumor cells11 and patient immunosuppression.12
The current standard treatment for GBM patients includes surgical resection of tumor followed by radiation therapy and temozolomide. Temozolomide, a prodrug alkylating agent, is an oral chemotherapy that crosses the blood–brain barrier. Temozolomide adds a methyl group to purine and pyrimidine in DNA, resulting in damage to cells and ultimately apoptosis.13 However, approximately 55% of GBM patients are resistant to temozolomide because of their methyl guanine methyl transferase (MGMT) DNA repair system.14 MGMT transfers the methyl group from guanine, thereby repairing damaged DNA and counteracting cytotoxic effects of temozolomide on tumor cells. Moreover, even patients who have an initial response to temozolomide fail therapy due to acquired resistance.
Although traditionally used for direct antitumor effects, temozolomide also has immunomodulatory properties. Host immunity is significantly impacted by chemotherapy. The effects of chemotherapy are dependent on the drug mechanism of action, the dose, as well as the host immune environment. In both animal models and cancer patients, chemotherapy has been shown to impact factors such as T-cell proliferation,15 regulatory T-cell (Treg) proportion,16 and monocyte death.17 As immunotherapeutic options for GBM patients are being expanded and investigated, the role of temozolomide as a combinatorial strategy with immunotherapy will become increasingly relevant.
In this review, we will discuss the impact of temozolomide on various components of the immune system, including effector cells, antigen presentation, and immunosuppressive cells. Additionally, we will review how the effects of temozolomide may be leveraged for immunotherapeutic strategies. Each section will first review the pertinent human studies followed by a summary of the preclinical data.
Temozolomide Effects on Cytotoxic T Cells and Antigen Presentation
Temozolomide is well known to induce lymphopenia.18 This phenomenon is even more pronounced in GBM patients being treated with combined temozolomide and radiation.19 Multiple studies have found that GBM patients have a decrease in primarily CD4 T cells and B cells after treatment with radiation and temozolomide.19–21 Importantly, this lymphopenia develops without compensatory increases in cytokines important for lymphocyte proliferation such as interleukin (IL)-7 and IL-15.21 Although temozolomide does not usually decrease total CD8+ T-cell counts, coadministration of radiation and temozolomide decreases CD8+ CD56+ effector T cells in patients with GBM.20 Attempts to reduce lymphopenia in patients by adoptive transfer of lymphocytes after temozolomide treatment have been shown to fail to increase lymphocytic counts,22 demonstrating the long-lasting impact of temozolomide on immune function.
Not surprisingly, the lymphopenia induced by temozolomide is dose dependent. In aggressive dosing schemes used for melanoma, patients were found to have lymphopenia primarily affecting CD4+ T cells and were susceptible to pneumocystis pneumonia or systemic fungal infections.23 Of the patients who developed lymphopenia, 61% had persistently low counts 2 months after discontinuation of temozolomide.
The lymphopenia induced by temozolomide has been reported to have variable effects on overall host response to immunotherapy. Although counterintuitive, lymphodepletion induced by temozolomide has been found to enhance antitumor activity associated with cellular immunotherapy in human studies. Sampson et al showed patients with GBM who received an epidermal growth factor receptor variant III (EGFRvIII) vaccine and temozolomide demonstrated larger humoral and delayed hypersensitivity responses when the temozolomide was dose intense compared with standard dose.24 This increase in antigen-specific immune response was found despite a greater lymphopenia in the dose-intensified group of patients. The same groups recently demonstrated increased antigen-specific immune responses to a cytomegalovirus antigen (pp65) when GBM patients were treated with dose-intensified temozolomide and pp65 dendritic cell vaccines.25
In animal studies, the effects of temozolomide on host immunity have been more variable. Using a glioma and melanoma model, Litterman et al demonstrated that alkylating agents, including temozolomide, resulted in a decrease in lymphocytes and qualitative dysfunction of T and B cells.26 Using a vaccine approach against a neoantigen (ovalbumin), they found that temozolomide affected the high affinity responder lymphocytes that responded strongest to the vaccine as measured by DNA damage. Therefore, temozolomide enriched for lower affinity lymphocytes and it abrogated the survival benefit of vaccination in a murine tumor model.27 Others have found that like the human studies, temozolomide can be synergistic with immunotherapy. Myeloablative doses of temozolomide combined with vaccine immunotherapy increased serum levels of IL-2, resulting in maturation of naïve T cells to effector/memory T cells and expansion of antigen-specific CD8 T cells in murine studies.28 These changes resulted in improved survival of tumor-bearing mice. In addition to expansion of antigen-specific T cells, lymphodepletion can be utilized to remove memory T cells, which contribute to tumor-mediated tolerance against tumor-associated antigens. Thus, by erasing memory T cells, stronger response to tumor antigens can be induced.29 The lymphopenia induced by temozolomide is followed by a period of homeostatic recovery which can be leveraged for immunotherapy.
In addition to direct effects on lymphocytes, temozolomide results in a relatively increased abundance of cytokines and proliferation factors for the remaining lymphocytes. IL-7 and IL-15 are cytokines that increase proliferation of CD8 T cells and augment effector function, and these cytokines usually do not increase with temozolomide. However, in lymphodepleted environments the remaining lymphocytes no longer need to compete for IL-7 and IL-15. In murine experiments, this phenomenon has been shown to increase T-cell proliferation and activation, resulting in robust antitumor immune responses.30
To overcome the systemic effects of temozolomide, some investigators have evaluated the role of intratumoral infusions of temozolomide. Intratumoral temozolomide has not been tested in humans. In a rat glioma model intracranial temozolomide was found to be safe and more efficacious without any toxicity compared with systemic temozolomide.31 Others described administration of polymers containing temozolomide to 9L gliosarcoma rats, which increased survival of tumor-bearing animals without significant lymphopenia.32 Interestingly, intratumoral temozolomide administration may have immune effects that vary from systemic administration. Fritzell et al showed that combined intratumoral temozolomide and granulocyte-macrophage colony-stimulating factor (GM-CSF) immunotherapy sustained CD8 T-cell proliferation and reduced the number of intratumoral suppressor cells.33 The animals treated with intratumoral temozolomide had improved survival compared with those that received systemic temozolomide. This survival benefit was reversed with T-cell depletion. Therefore, the authors hypothesized that intratumoral temozolomide improved survival in a T cell dependent fashion.
In addition to direct effects on T cells, temozolomide affects how T cells are activated by antigen presentation. Temozolomide exposure may result in release of tumor antigens that can then be picked up by dendritic cells and presented to T cells via major histocompatibility complex class I molecules. This process is known as cross-priming. Kim et al showed that combination of temozolomide with tumor antigen pulsed dendritic cells prolonged survival in a GL-261 murine model and enhanced antitumor immune responses.34 This immunological response was due to increased antigen cross-priming mediated by calreticulin surface exposure. Temozolomide has relatively few direct effects on dendritic cells. Briegert et al showed that dendritic cells were resistant to alkylating agents due to their DNA repair system, despite having significantly less MGMT expression.35 Others have shown that dendritic cell maturation and function were enhanced when exposed to low, nontoxic doses of chemotherapy.36 Therefore, temozolomide has the potential to improve antigen presentation to effector T cells.
Overall, human studies and some animal studies demonstrate that temozolomide can be utilized to enhance immune activation when combined with immunotherapy. However, these effects are dependent on the timing and dose of treatment. Additionally, temozolomide may have more synergy with cellular immunotherapy platforms where patients receive exogenous cell products that have not been exposed to temozolomide. Immunotherapeutic platforms such as immune checkpoint inhibitors that depend on endogenous lymphocyte activation may have reduced efficacy when combined with temozolomide due to the effects of standard dose temozolomide on lymphocyte function. Additionally, temozolomide may inhibit immune responses to neoantigens as shown in murine models. The effect of temozolomide-induced immunosuppression on outcomes for GBM patients is less clear.
Temozolomide Effects on the Immunosuppressive Environment
Major subsets of immunosuppressive cells include Tregs, myeloid derived suppressor cells (MDSCs), and macrophages. In GBM patients, these immunosuppressive cells are present in the tumor microenvironment as well as the peripheral blood.37,38 Tregs and MDSCs play key roles in tumor development.39
MDSCs represent a heterogeneous population of immature myeloid cells derived from pluripotent hematopoietic stem cells.40 Malignant glioma secretes monocyte chemoattractant protein 1 or chemokine C-C ligand 2 (CCL2) to enhance monocyte migration to the tumor site.39,41 Within the GBM tumor microenvironment, monocytes convert to MDSCs and immunosuppressive tumor-associated macrophages, which induce tumor progression.42 These cells are associated with poorer outcome in patients with GBM due to an associated reduction in tumor-infiltrating T cells and suppressed T-cell function.43–45 Raychaudhuri et al observed increased levels of MDSCs in patients with GBM compared with healthy control subjects.44 They found that these cells suppressed antitumor T-cell immune responses, which could be restored by blocking MDSCs. Others have also described the presence of MDSCs in patients with GBM which are responsible for dysfunction of CD4 T effector memory cells.46
Since MDSCs play an important role in creation of the immunosuppressive environment, various strategies to target MDSCs within the GBM tumor microenvironment have been studied in murine models. MDSCs lack DNA repair proteins such as X-ray repair cross-complementing protein 1 and poly(ADP-ribose) polymerase 1, leading to vulnerability to death by alkylating agents.47 Temozolomide treatment of human myeloid cells has been shown to induce p53, P-21, and ɣ-H2AX activation, which prompts an intrinsic mitochondrial pathway of apoptosis.48 However, in murine glioma models temozolomide has not been described to reduce macrophage derived chemokines. The combination of anti-CCL2 to block MDSC migration and temozolomide increased survival in a GL-261 tumor model compared with anti-CCL2 alone.49 However, MDSC function was decreased with anti-CCL2 and not temozolomide. The effects of temozolomide on MDSCs has not been corroborated in human patients and further study is necessary.
Another population of cells that may play a role in tumor progression are Tregs. CD4+CD25+FoxP3 represent Tregs which are a subpopulation produced in the thymus or in the peripheral blood from naïve CD4 T cells.50 Tregs contribute to immune tolerance by suppressing reactive T lymphocytes, dendritic cells, macrophages, and natural killer cells.51 Tregs suppress the immune system by either relieving cytotoxic T-cell activity by direct cell-cell contact, IL-2 consumption and cytolysis, or secretion of immunosuppressive cytokines like transforming growth factor-β and IL-10.52 Accumulation of Tregs has been demonstrated in the tumor microenvironment and the peripheral blood of GBM patients.53,54 Sayour et al showed that increased intratumoral Tregs were associated with tumor recurrence and reduced survival in patients with GBM.55 However, other groups have found that Treg presence in tumors is prognostically neutral.56
The effects of temozolomide on Tregs has been an important area of research due to the importance of temozolomide in GBM treatment and the potential of Tregs to reduce efficacy of immunotherapy. Human studies have shown that temozolomide alone is not enough to deplete Tregs. Fadul et al showed a moderate increase in functional Tregs in 25 GBM patients 4 weeks after concomitant standard radiation and temozolomide therapy.20 Others have corroborated these results.57 In mice and patients with GBM, Mitchell et al showed that temozolomide increased the proportion of Tregs, which could be reversed with IL-2 receptor blockade.58 Patients with GBM who had blockade of IL-2 receptor (CD25) post temozolomide treatment had reduced Tregs and improved immune responses to vaccine treatment.58,59 In murine experiments, Fecci et al utilized immune checkpoint blockade to create resistance to Treg-induced immunosuppression.60 They found that cytotoxic T lymphocyte antigen 4 antibody treatment restored CD4+ T-cell counts and that these cells were resistant to Treg suppression.54
However, the dosing of temozolomide may impact Treg numbers and function. In murine studies, others have shown that a very low dose of metronomic temozolomide (0.5 mg/kg/day for 21 days) led to a selective Treg depletion in a rat GBM model, as well as inhibiting Treg immunosuppressive activity.16 Therefore, the timing and dose of treatment are important for the effects on Tregs.61 Treg depletion enhances the efficacy of immunotherapy in both humans and mice. However, this cannot be achieved with temozolomide alone.
Temozolomide in Combination with Immunotherapy
Temozolomide was established as the standard care of GBM after the seminal trial demonstrating concurrent and adjuvant temozolomide with radiation therapy improved median survival of GBM patients compared with radiation alone.62 This study resulted in widespread use of temozolomide for GBM. Due to the limitations of temozolomide, various combinatorial strategies have been tested in GBM patients. Unfortunately, combinations of temozolomide with other chemotherapy have not resulted in improved outcomes. Kim et al combined temozolomide with other alkylating agents (nimustine or carmustine and lomustine) to intensify treatment but the study was stopped due to high frequency of toxicity.63 Combination of temozolomide with small-molecule inhibitors has also been disappointing. Inhibitor of protein kinase C64 and EGFR tyrosine kinase65 have been tested in phase II trials without compelling results. Additionally, bevacizumab, which inhibits vascular endothelial growth factor, did not show benefit when added to radiotherapy-temozolomide for patients with newly diagnosed GBM.2,3
In addition to combination therapy, variations in dosing temozolomide have been tested with mixed results. Intensification of the dose has been the most intuitive next step for temozolomide. A phase II clinical trial of continuous dose-dense temozolomide demonstrated safety in recurrent GBM patients.66 A subsequent study showed that prolonged administration of temozolomide with concomitant radiotherapy for newly diagnosed GBM, 9 cycles or more, improved progression-free and overall survival, without adding toxicity.67 Disappointingly, the phase III clinical trial showed that dose-dense temozolomide did not improve efficacy in patients with newly diagnosed GBM compared with standard dose, regardless of methylation status.68 Alternatively, lower, more continuous doses (metronomic) have also been tested for GBM patients. This dosing regimen has been described for use in patients who are elderly and may not tolerate standard temozolomide doses.69 Some hypothesize that metronomic dosing may prevent emergence of temozolomide-resistant cancer cells as well as have improved targeting of cancer vasculature.70 Moreover, animal studies have shown that metronomic temozolomide dosing results in a reduction of immunosuppressive Tregs.16 In GBM patients, only small phase I and phase II studies have been performed with metronomic temozolomide alone which demonstrate safety.71,72
The most compelling role for temozolomide moving forward is as an immunomodulator for GBM patients receiving immunotherapy.73 Temozolomide has the benefit of having direct antitumor effects in addition to significant effects on host immunity. Several studies have demonstrated the potential role of temozolomide for immunomodulation. Sampson et al demonstrated that combination of standard or dose-intensified temozolomide with EGFRvIII targeted peptide vaccine enhanced EGFRvIII-specific immune responses.24 Compensated homeostatic cytokines after lymphodepleted temozolomide cause enhanced immune responses by reduction of the T-cell activation threshold and proliferation induction. Although both standard and dose-intense temozolomide were capable of eliminating EGFRvIII-expressing tumor cells in GBM patients, dose-intensified temozolomide produced higher humoral and delayed-type hypersensitivity responses in EGFRvIII targeted immunotherapy.24 Interestingly, the homeostatic proliferation following temozolomide results in an increase in effector lymphocytes that potentiates antitumor responses despite a concomitant increase in Tregs.
A randomized study evaluating a dendritic cell pulsed pp65 (cytomegalovirus antigen) RNA vaccine site with tetanus/diphtheria toxoid demonstrated potential efficacy of immunotherapy when combined with standard dose temozolomide. GBM patients were treated with standard adjuvant temozolomide and vaccination. The tetanus/diphtheria toxoid resulted in enhanced dendritic cell migration due to CCL3 upregulation and ultimately significantly improved survival.74 In another study, newly diagnosed GBM received pp65 mRNA pulsed dendritic cell vaccines mixed with GM-CSF and dose-intensified temozolomide, which increased pp65-specific cellular responses significantly.25 These studies demonstrate that although temozolomide induces lymphopenia, a vaccine strategy enhances endogenous antigen-specific T-cell proliferation and function during the homeostatic recovery period.
In another phase I/II trial, temozolomide was combined with a vaccine of fused dendritic cells and glioma cells from surgical specimens for newly diagnosed and recurrent GBM. The investigators noted the surgical specimens from recurrent GBM patients had a higher expression of tumor-associated antigens (WT-1, gp100, and MAGE-A3). Moreover, these peptides were more likely to be accumulated in the cellular cytoplasm in recurrent specimens compared with being in the nucleus in newly diagnosed GBM samples. The authors hypothesized that temozolomide treatment may result in cytoplasmic accumulation of immunogenic peptides that would enhance response to immunotherapy.75
As an alternative to dose-intensified temozolomide, metronomic temozolomide may have benefits beyond just reduced toxicity. Cominelli et al demonstrated that metronomic temozolomide regimens were associated with a preferential increase in survival in patients with EGFRvIII amplification/overexpression compared with other patients.76 This difference was not seen in patients treated with standard temozolomide. The improved antitumor efficacy in EGFRvIII-overexpressing GBM with metronomic temozolomide occurred through inhibition of nuclear factor-kappaB transcriptional activity. Therefore, metronomic temozolomide seems to alter transcriptional activity in glioma cells, which may render them more sensitive to certain immunotherapeutic strategies. In animal studies, Ouyang et al found that metronomic temozolomide enhanced the antitumor efficacy of cytosine-phosphate-guanine oligonucleotide immunotherapy due to an increase in tumor-specific cytotoxic T cells in a resistant murine GBM model.73
On the other hand, some have hypothesized that immunotherapy may sensitize patients to chemotherapy. Wheeler et al retrospectively compared GBM patients who were treated with dendritic cell vaccination followed by chemotherapy with those who received either treatment alone and found improved progression-free and overall survival.77 These studies were performed prior to temozolomide becoming standard treatment for GBM patients. Additionally, no proposed mechanism or further studies have elucidated these findings.
Overall, temozolomide has both direct antitumor effects and immune modulatory properties (Fig. 1). The timing and dose of temozolomide significantly alter the effects seen on immune cells and the tumor microenvironment. The effects that temozolomide will have on response to newer therapies such as immune checkpoint inhibitors is currently unknown. Early studies have other treatment modalities such as radiation impact response to immune checkpoint inhibition. The effects of dosing and timing of temozolomide and immune checkpoint inhibition are an ongoing focus of our laboratory. Combinatorial strategies using temozolomide and immunotherapy will require thoughtful consideration to ensure optimal outcomes.
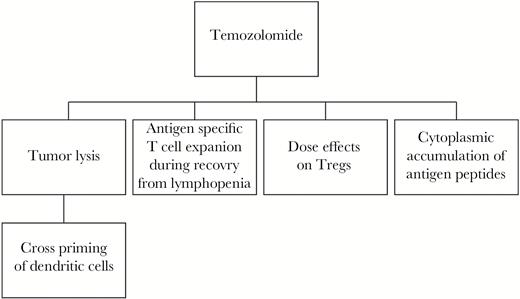
Immune effects of temozolomide as an immunomodulatory agent. Temozolomide is able to affect immune function in brain tumor patients through both effects on tumor cells and direct effects on immune cells. The tumor cell death that results from temozolomide allows release of tumor antigens that can be presented by dendritic cells (DCs). Additionally, temozolomide treatment results in cytoplasmic accumulation of antigen peptides in tumor cells, allowing for better recognition by immune cells. The effects of temozolomide on host immunity includes lymphopenia. This phenomenon can be leveraged. The homeostatic lymphocyte recovery after the temozolomide-induced lymphopenia is a window during which antigen-specific T cells can be rapidly expanded. Additionally, temozolomide may deplete or expand regulatory T cells (Tregs) which are immunosuppressive depending on the dosing regimen.
Conclusion
Temozolomide has been shown to cause lymphopenia, increase the proportion of Tregs, and potentially enhance dendritic cell function. Human studies have demonstrated that cellular immunotherapy can be delivered successfully with temozolomide. Moreover, temozolomide enhances antigen-specific T-cell proliferation and function due to the homeostatic recovery period following the lymphopenia. The role of temozolomide with newer immunotherapeutic platforms such as immune checkpoint inhibitors is unknown. Future studies can focus on how to exert different immunomodulatory changes based on temozolomide timing and dose of delivery. Thoughtful combinatorial strategies with immunotherapy may improve outcomes in patients with GBM.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS K08NS099484; principal investigator, M.R.).
Conflict of interest statement. None declared.




