-
PDF
- Split View
-
Views
-
Cite
Cite
Richard S. Brown, Ryan A. Harnish, Kathleen M. Carter, James W. Boyd, Katherine A. Deters, M. Brad Eppard, An Evaluation of the Maximum Tag Burden for Implantation of Acoustic Transmitters in Juvenile Chinook Salmon, North American Journal of Fisheries Management, Volume 30, Issue 2, April 2010, Pages 499–505, https://doi.org/10.1577/M09-038.1
Close - Share Icon Share
Abstract
A substantial percentage of the Pacific salmon Oncorhynchus spp. and steelhead O. mykiss smolts that emigrate to the ocean each year are smaller than 110 mm (fork length). However, relatively few researchers have implanted acoustic transmitters in fish of this size, and none have reported minimum fish lengths below 110 mm for which the tag burden did not negatively influence growth or survival. The influence of a surgically implanted acoustic microtransmitter and a passive integrated transponder (PIT) tag on the growth and survival of hatchery‐reared juvenile Chinook salmon was examined over a period of 30 d. Growth and survival were compared between treatment (tagged) and control (untagged) fish within three size‐groups (80–89, 90–99, and 100–109 mm). The acoustic microtransmitter and PIT tag implanted in our study had a combined weight of 0.74 g; the combined tag burden for implanted fish ranged from 4.5% to 15.7%. The results indicated that growth and survival among implanted juvenile Chinook salmon were size dependent. Significant differences in growth rate and survival were observed between treatment and control fish in the 80–89‐mm group. The survival of implanted fish smaller than 11.1 g (tag burden, >6.7%) and the growth of fish smaller than 9.0 g (tag burden, >8.2%) were negatively affected by the implantation or presence of an acoustic microtransmitter and PIT tag. The results of this study will aid researchers in determining the minimum fish size suitable for use in acoustic telemetry studies that estimate the short‐term (30‐d) survival and growth of juvenile salmonids.
Biotelemetry studies are being used increasingly to monitor the survival and behavior of migratory juvenile salmonids (Jepsen et al. 1998; Skalski et al. 1998, 2001; Hockersmith et al. 2003; Plumb et al. 2006; Scruton et al. 2007). An important assumption in biotelemetry studies is that the tagged sample is representative of the general population. Therefore, it is imperative that the implantation or presence of a transmitter does not negatively influence the performance or survival of the implanted fish (i.e., tag or tagging effect). If a tag effect exists, inferences from implanted individuals to the population of interest may be invalid.
Tag burden (i.e., the weight of a transmitter relative to the weight of a fish) can influence the behavior and survival of implanted salmonids, and numerous researchers have demonstrated the importance of tag burden in biotelemetry studies (Peake et al. 1997; Adams et al. 1998a, 1998b; Lacroix et al. 2004; Zale et al. 2005; Welch et al. 2007). Typically, low tag burdens are more suitable than higher burdens with respect to fish performance. The swimming ability, predator avoidance, growth, tag retention, or survival of implanted fish may be affected if the tag burden is too great (Peake et al. 1997; Anglea et al. 2004; Lacroix et al. 2004; Brown et al. 2006; Welch et al. 2007; Chittenden et al. 2009; Hall et al. 2009).
A substantial percentage of the Pacific salmon Oncorhynchus spp. and steelhead O. mykiss smolts that emigrate to the ocean each year are smaller than 110 mm (fork length). However, relatively few researchers have implanted acoustic transmitters in salmonids of this size. Using real or dummy acoustic transmitters, Brown et al. (2006) implanted juvenile sockeye salmon O. nerka (length = 90–133 mm) and Chinook salmon O. tshawytscha, and Chittenden et al. (2009) implanted coho salmon O. kisutch (length = 95–110 mm) (Table 1). However, the growth and survival results from both studies were variable among species. Additionally, neither study reported an estimate of minimum fish length within the size range tested for which tag burden did not negatively influence growth or survival. Taking another approach, Zale et al. (2005) estimated the minimum‐size fish that can be tagged without influencing performance by examining the growth of small adult cutthroat trout O. clarkii implanted with dummy radio transmitters along a gradient of sizes. They noted regression analysis was more sensitive than comparing treatment means at detecting subtle tag effects on growth.
Results of studies conducted to determine the effects of surgically implanting acoustic transmitters on the mortality of juvenile salmonids. The studies are listed in order of tag burden, from lowest to highest
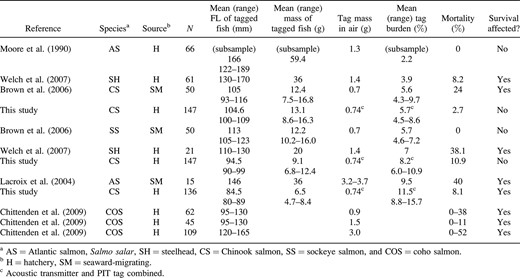
Results of studies conducted to determine the effects of surgically implanting acoustic transmitters on the mortality of juvenile salmonids. The studies are listed in order of tag burden, from lowest to highest

The goal of our study was to estimate the maximum tag burden that can be tolerated by juvenile salmon (80–109 mm) without influencing fish performance. Because an examination of all aspects of fish performance in a single study would be a monumental feat, we focused on growth and survival as proxies of tag burden of Chinook salmon surgically implanted with an acoustic microtransmitter (0.64 g) and a passive integrated transponder (PIT) tag. Results of this study will aid in determining the suitability of acoustic telemetry to estimate short‐term (30‐d) survival and growth of juvenile salmonids.
Methods
Fish acquisition, holding, and surgical protocols
Chinook salmon eggs were obtained from Priest Rapids Hatchery (Mattawa, Washington). The eggs were hatched and juvenile salmon reared at the Pacific Northwest National Laboratory's Aquatic Research Laboratory before experimentation. To initiate experimentation, fish were placed in an anesthetic bath of tricaine methanesulfonate (MS‐222) at 80–100 mg/L of water and PolyAqua (Kordon Aquarium Products, Hayward, California) at 0.15 mL/L of water until they reached stage‐4 anesthesia (Summerfelt and Smith 1990). While immobile, fish were measured (FL; mm), weighed (g), and grouped into three 10‐mm size‐groups of 147–150 treatment (tagged) and 121–142 control (untagged) fish (Table 2). While still anesthetized, treatment fish were surgically implanted with an expired Juvenile Salmon Acoustic Telemetry System (JSATS) acoustic transmitter (mean weight of 0.64 g in air, 0.36 g in water; 0.28 mL volume; Sonic Concepts, Inc., Bothell, Washington) and a PIT tag (weight of 0.10 g in air, 0.06 g in water; 0.04 mL volume) following implantation methods similar to those described in Brown et al. (2006). Frequently, PIT tags are implanted in conjunction with acoustic transmitters in juvenile salmon in the Snake and Columbia rivers to enable their detection in bypass facilities and fish ladders at hydroelectric dams. Fish not implanted with a PIT tag may be barged or trucked downstream past dams, compromising fish passage research. Therefore, to more closely reflect actual fish passage study conditions, a PIT tag was implanted in the treatment fish in our study.
Study results for fish that were surgically implanted with an acoustic transmitter and a PIT tag (treatment) or left untagged (control), by size category
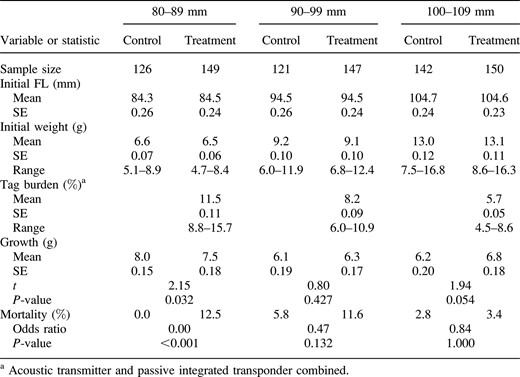
Study results for fish that were surgically implanted with an acoustic transmitter and a PIT tag (treatment) or left untagged (control), by size category

Because individual growth and mortality provides a more precise metric for assessing tag and tagging effects than estimates calculated from a group of fish, we opted for assessing individual fish. Each control fish was injected in the right and left adipose eyelids with various color combinations of visible implant elastomer (VIE; Northwest Marine Technology, Inc., Shaw Island, Washington) and by clipping the very distal end of either the right or left pelvic fin while the fish was anesthetized. Multiple markings were necessary due to the large number of fish included in the experiment. Treatment fish were individually identified by their PIT tag; they also received a fin clip and had the VIE injection needle inserted into the adipose eyelids but without injection of the elastomer. This ensured that the treatment effect was the implantation and presence of an acoustic transmitter and PIT tag. Treatment and control fish were subjected to similar anesthetic times.
After marking, fish were allowed to recover in buckets or tanks of oxygenated water until they achieved equilibrium. They were then transferred to one of six circular tanks (378 or 770 L) that were maintained between 17°C and 18.5°C for the duration of the 30‐d study period. Treatment and control fish from a given size‐group (80–89, 90–99, or 100–109 mm) were placed into the same tank (two tanks per size‐group). Fish were subjected to a photoperiod of 12 h light: 12 h dark and fed Biodiet moist pellets (Bio‐Oregon, Longview, Washington) ad libitum. Tanks were checked daily for mortalities and expelled tags. Individuals that died or expelled acoustic transmitters or PIT tags were identified by PIT tag code (treatment) or marking pattern (control), and the date of mortality or expulsion was recorded. At the end of the 30‐d study period, all fish were euthanized with an overdose of MS‐222 (250 mg/L of water), identified, and their final lengths and weights were recorded.
Statistical analysis of mortality
Because of a tank effect, mortality was greatest for the 90–99‐mm size‐group, and linear regression analyses using fish from all three size‐groups was not informative. Therefore, the probability of mortality was calculated for treatment and control fish of each 10‐mm size‐group by dividing the number of mortalities by the number of fish in a given size‐group. A Fisher exact test was used to determine if mortality differences between treatment and control fish in each 10‐mm size‐group were significant (α = 0.05). To determine if differences in mortality existed between treatment and control fish within a given size‐group, mortality data were pooled by initial weight into 1‐g bins. The probability of mortality was then computed for treatment and control groups for each 1‐g bin and examined graphically to obtain a more precise estimate of the minimum weight at which surgical implantation of an acoustic transmitter and PIT tag adversely affected survival. Standard errors of the mortality probability estimates for each 1‐g bin were estimated using the binomial variance and assuming the normal approximation to the binomial distribution. Treatment fish that expelled an acoustic transmitter or PIT tag were omitted from all analyses.
Statistical analysis of growth
Growth (g) was calculated for each fish that survived to the end of the 30‐d study period by subtracting the initial weight from the final weight. Mean growth (g) was then calculated for treatment and control fish in each 10‐mm size‐group (80–89, 90–99, and 100–109 mm). A two‐sample t‐test was used to determine if the mean growth differed significantly (α = 0.05) between treatment and control fish in each 10‐mm size‐group. To determine if differences in growth existed between treatment and control fish within a given size‐group, growth data were pooled by initial weight into 1‐g bins. The mean growth was then calculated for treatment and control groups for each 1‐g bin and examined graphically to obtain a more precise estimate of the minimum weight at which surgical implantation of an acoustic transmitter and PIT tag adversely affected growth.
Results
Mortality
During the 30‐d study, mortality differed significantly (P < 0.001) between treatment and control fish in the 80–89‐mm size‐group (Table 2); of the implanted fish in this size‐group, 12.5% died, versus 0% of control fish. The highest overall mortality rate occurred in the 90–99‐mm group; 5.8% of control fish from this group and 11.6% of treatment fish died. However, there was no significant difference in mortality rate between control and treatment fish in the 90–99‐mm and 100–109‐mm groups (P > 0.05).
Although differences in the probability of mortality between control and treatment fish in the 90–99‐mm group were not significant, an apparent threshold existed within this size‐group. Plotted on a line graph, the probabilities of mortality for treatment and control fish in the 90–99‐mm group intersect at an initial weight of 11.1 g (Figure 1), indicating that implanted fish larger than this size survived as well as, or better than, untagged fish. For the size of transmitters and fish used during this study, this threshold is equivalent to a tag burden (acoustic transmitter and PIT tag combined) of 6.7% and a minimum fork length of about 99 mm.
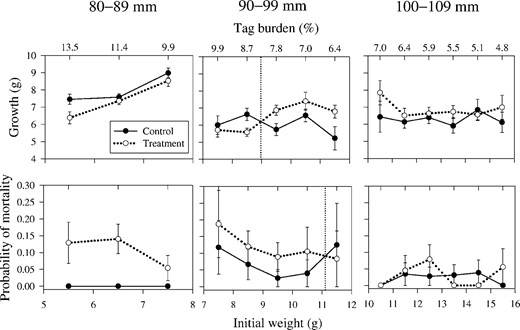
Growth and probability of mortality for juvenile Chinook salmon of different lengths that were implanted with an acoustic transmitter and a PIT tag (treatment) or left untagged (control), by initial weight. The points at which implanted fish survived or grew as well as or better than untagged fish are indicated by the perpendicular dashed lines. The error bars represent standard errors.
Growth
Growth was significantly (P = 0.03) lower for treatment fish than for control fish in the 80–89‐mm size‐group (Table 2). Although differences in growth of control and treatment fish in the 90–99‐mm and 100–109‐mm groups were not significant (P > 0.05), an apparent threshold existed within the 90–99‐mm group. Plotted on a line graph, growth of treatment and control fish in the 90–99‐mm group intersect at an initial weight of 9.0 g (Figure 1), indicating that implanted fish larger than this size grew as much as, or more than, untagged fish. For the size of transmitters and fish used during this study, this threshold is equivalent to a tag burden (acoustic transmitter and PIT tag combined) of 8.2% and a minimum fork length of about 94 mm.
Tag Expulsion
Only 4% of the treatment fish initially implanted with acoustic transmitters and PIT tags expelled their transmitter, PIT tag, or both. Of the 149 fish from the 80–89‐mm size‐group, 10 (6.7%) expelled their transmitter, 2 (1.3%) expelled their PIT tag, and 1 (0.7%) expelled both. None of the 147 fish in the 90–99‐mm group expelled their acoustic transmitters, and 1 (0.7%) expelled its PIT tag. Of the 150 fish from the 100–109‐mm group, 3 (2.0%) expelled their transmitter, and 1 (0.7%) expelled its PIT tag.
Discussion
We found that acoustic transmitters and PIT tags had negative effects on the survival of juvenile Chinook salmon when tag burdens were 6.7% or more, which accords with the recommended maximum tag burdens for juvenile coho salmon (7%; Chittenden et al. 2009) and Atlantic salmon (8%; Lacroix et al. 2004). Other studies evaluating survival of juvenile salmonids experiencing similar tag burdens have reported much higher mortality than we observed. For example, mortality of implanted juvenile steelhead (120–130 mm FL; mean tag burden, 6.5%) was 50%; however, this result may have been influenced by small sample size (N = 15) and longer study period (29 weeks; Welch et al. 2007) than in our study. Brown et al. (2006) observed 24% mortality of implanted seaward‐migrating juvenile Chinook salmon (93–116 mm FL; mean tag burden, 5.6%), which was significantly higher than the mortality of untagged control fish. Brown et al. (2006) hypothesized that poor condition of test fish (tail rot and scale loss) contributed to high mortality. Variation in survival among studies assessing maximum tag burdens for juvenile salmonids may stem from differences in fish origin, fish health, and study duration.
Mortality was greatest for fish in the 90–99‐mm group in our study. An unknown amount of the mortality observed for this size‐group was disease related, which introduced a tank effect into our results. However, because treatment and control fish were held in the same tank, any tank effect on mortality was manifested in both the control and treatment fish. Therefore, it is unlikely that the tank effect influenced our results.
The growth of our implanted Chinook salmon was negatively influenced when the tag burden was greater than 8.2%. Other researchers have found that similar tag burdens did not lower the growth of juvenile coho salmon (7–8% burden; Chittenden et al. 2009) and sockeye and Chinook salmon (4.3%–9.7% burden; Brown et al. 2006) relative to that of control fish. In addition, Lacroix et al. (2004) did not find decreased growth owing to the implantation of dummy acoustic transmitters in Atlantic salmon (mean burden, 8.5–10.1%) during the first 6 months of holding.
Tag expulsion was relatively low (2%) in the largest size‐group but considerably higher (9%) among the smallest fish tested (i.e., the 80–89‐mm group). This varies from the results of Brown et al. (2006) and Chittenden et al. (2009), neither of whom observed expulsion among similar‐size fish with similar tag burden. However, other researchers examining larger salmonids have found tag expulsion among juvenile salmonids with even lower tag burdens (Lacroix et al. 2004; Welch et al. 2007). Thus, although growth and survival of juvenile Chinook salmon may not be influenced by a tag burden of approximately 7%, researchers should expect some tag expulsion to occur.
Although valuable information can be obtained from research conducted with hatchery‐reared fish in a laboratory environment, caution should be taken in extrapolating the results to wild or seaward‐migrating fish. A comparison of our results with those from a similar study indicates that implanted seaward‐migrating fish may experience higher mortality rates (24.0% in Brown et al. 2006) than implanted hatchery fish (2.8% this study) of similar size (mean, 105 mm FL) and with similar tag burdens (mean, 5.6%–5.7%). Compared with hatchery‐raised fish, seaward‐migrating wild fish may have lower condition factors, higher pathogen loads, and experience higher levels of stress during capture, confinement, transportation, and handling (Woodward and Strange 1987; Peake et al. 1997; Congleton et al. 2000; Arkoosh et al. 2004); thus, results from studies using hatchery fish may not accurately reflect the effect of transmitter implantation on wild seaward‐migrating fish.
Recently, smaller acoustic transmitters (JSATS Model SS208; weight, 0.43 g in air) than those used in this study have become available and may provide opportunity for tagging fish less than 8 g without adversely affecting growth and survival. Tagging of smaller individuals would allow managers and researchers to implant a sample of fish that would be more representative of the general population than was previously possible.
We suggest that future research be conducted using wild seaward‐migrating fish to determine the minimum fish size and maximum tag burden appropriate for the particular objectives of a given study. Although growth and survival of implanted fish are important aspects, several other factors also should be considered (e.g., swimming ability, predator avoidance, and buoyancy) to determine the effects of acoustic microtransmitter and PIT‐tag implantation on fishes (Brown et al. 1999; Jepsen et al. 2002; Jepsen et al. 2004). Because water temperature has been linked to survival of implanted fish (Clapp et al. 1990; Knights and Lasee 1996; Peake et al. 1997; Walsh et al. 2000), temperature conditions imposed in future studies should mimic those encountered by fish in the wild. As suggested by Zale et al. (2005) and Thorstad et al. (2009), conclusive evidence of transmitter effects will require comparative field studies that involve implanting a wide size range of juvenile salmonids with transmitters and measuring their rates of migration, growth, predation, and survival.
Acknowledgments
This study was funded by the U.S. Army Corps of Engineers, Portland District. The Pacific Northwest National Laboratory is operated by Battelle for the U.S. Department of Energy under contract DE‐AC05‐76RL01830. With appreciation, we acknowledge the technical contributions to the project made by the following people from the Pacific Northwest National Laboratory: Brian Bellgraph, Geoffrey McMichael, Jessica Carter, David Geist, Tom Carlson, Abby Welch, Marie Theriault, Garrett McKinney, Jennifer Panther, Katie Ovink, Katie Murray, Gayle Dirkes, Tirell Monter, Ian Welch, Julie Miller, Brooke Sakara, Chris Eilers, Scott Abernethy, Craig McKinstry, and Andrea Currie. We also thank John Skalski of the University of Washington. Our thanks go also to Brad Ryan, Eric Hockersmith, and Michelle Rub of NOAA–Fisheries.



